Abstract
In an fMRI experiment, participants were exposed to narratives based on true stories designed to evoke admiration and compassion in 4 distinct categories: admiration for virtue (AV), admiration for skill (AS), compassion for social/psychological pain (CSP), and compassion for physical pain (CPP). The goal was to test hypotheses about recruitment of homeostatic, somatosensory, and consciousness-related neural systems during the processing of pain-related (compassion) and non-pain-related (admiration) social emotions along 2 dimensions: emotions about other peoples' social/psychological conditions (AV, CSP) and emotions about others' physical conditions (AS, CPP). Consistent with theoretical accounts, the experience of all 4 emotions engaged brain regions involved in interoceptive representation and homeostatic regulation, including anterior insula, anterior cingulate, hypothalamus, and mesencephalon. However, the study also revealed a previously undescribed pattern within the posteromedial cortices (the ensemble of precuneus, posterior cingulate cortex, and retrosplenial region), an intriguing territory currently known for its involvement in the default mode of brain operation and in self-related/consciousness processes: emotions pertaining to social/psychological and physical situations engaged different networks aligned, respectively, with interoceptive and exteroceptive neural systems. Finally, within the anterior insula, activity correlated with AV and CSP peaked later and was more sustained than that associated with CPP. Our findings contribute insights on the functions of the posteromedial cortices and on the recruitment of the anterior insula in social emotions concerned with physical versus psychological pain.
Keywords: fMRI, insula, morality, posteromedial cortices, social emotions
Social emotions such as admiration and compassion play a critical role in interpersonal relationships and moral behavior (1, 2). They motivate us to either reward (in the case of admiration) or remedy (in the case of compassion) the circumstances of another person (3). The experience of these emotions may also produce a sense of heightened self-awareness that incites our own desire to be virtuous or skillful, or else gratitude for our own good circumstances (4–6).
Admiration can be evoked by witnessing virtuous behavior aimed at reducing the suffering of others [known also as “elevation” (4)] or by displays of virtuosic skill; compassion can be evoked by witnessing situations of personal loss and social deprivation (hereafter, social pain) or by witnessing bodily injury. Notably, each of these emotions pertains to another person's immediate physical circumstances (admiration for skill, compassion for physical pain) or social/psychological circumstances (admiration for virtue, compassion for social pain); and each is either related to pain processing (compassion for physical and for social/psychological pain) or not (admiration for skill and for virtue).
Although understanding the neural underpinnings of these emotions is important to the effort of elucidating the neurobiology of social behavior, the neural basis of admiration has not been investigated and the neural correlates of compassion states, in particular the relationship between compassion for physical pain and social pain, has been studied mainly from the perspective of recognizing someone else's social pain or sadness (e.g., ref. 7) rather than experiencing these emotions. In this study, we induce strong states of admiration and compassion in our participants to investigate the neural underpinnings of feeling these emotion states.
Critical to this argument is the distinction between recognizing another's social or physical situation and emotionally reacting to it. It is well known that basic emotions such as fear, sadness, and happiness and limited social emotions such as moral indignation engage neural systems concerned with sensing and regulating body function (hereafter homeostasis) with varying patterns, and it has been hypothesized that among those systems, the insula plays an especially prominent role (8–13). It is also known that engagement of social emotions and the consequent feeling for another's social/psychological situation are described by poets and lay people alike in visceral and bodily terms and in terms of their heightening effect on one's own self-awareness or consciousness. However, the extent to which we use neural systems related to sensing and regulating our own body and consciousness to react emotionally to the psychological and physical situations of others has not been investigated comprehensively (14), and there is especially little known about how these systems may be involved in positive, approach-oriented emotions such as admiration. If recruitment of body regulation and consciousness systems does occur, how might it differ across varieties of admiration and compassion and between social emotions about others' physical versus social/psychological situations?
Interestingly, neural mechanisms of physical pain (involving especially the dorsal anterior cingulate) have been suggested to underlie the experience of social exclusion or loss (15–18), and recent research has established important shared neural mechanisms between the direct experience of pleasant and unpleasant physical sensations such as pain, noxious odors, and pleasant tastes and the ability to recognize these experiences in others (e.g., 19, 20–28). It has not been established, however, how neural mechanisms used in empathy for another's physical situation may be involved in emotional reactions to another person's psychological state, either admirable or painful. Also, should we find that both compassion for physical pain (CPP) and compassion for social pain (CSP) involve the anterior insula, an important follow-up question relates to the timing of the neural activity in this region. Because CPP is evolutionarily well established (see ref. 29) and develops earlier in children (see ref. 30), it is likely to require minimal cognitive processing before induction, whereas CSP would require more substantial cognitive processing related to cultural factors. We therefore hypothesized that activation in the anterior insula would be induced and dissipated more quickly for CPP than for the other emotions tested, most notably CSP.
In addition to research on physical and social pain, a rich body of literature from the last decade has converged on the involvement of several brain regions in the making of inferences about the mental states of others, prominently the temporoparietal junction (TPJ), the mesial prefrontal cortex, and a territory encompassing 3 contiguous cortical areas—the posterior cingulate cortex, the retrosplenial, area, and the precuneus—which together comprise the posteromedial cortices (PMC). Prefrontal activations have been linked to the process of generating inferences about another person's mental state and psychological characteristics (see ref. 31 for review, and ref. 32), and activations in TPJ have been related to attributing beliefs to another person (e.g., ref. 33, see ref. 34 for review). However, the consistent engagement of the PMC in social tasks (35) and episodic memory (36) has not been clarified. In a parallel line of research drawing on evidence from focal damage and from neuroanatomical and neurophysiological studies, it has been suggested that this region is involved in consciousness, specifically in the construction of the self (see ref. 37 for review). In light of evidence that simulation on one's own self is an important means to understand others (see ref. 38 for review and refs. 39–41), this raises the possibility that the experience of admiration or compassion would differentially engage the PMC. For this reason, we wished to explore patterns of activation in the PMC during feelings of admiration and compassion.
In brief, although the past decade has seen a remarkable effort to elucidate the neural correlates of social cognition, here, we specifically set out to test whether and how areas of the brain involved in homeostatic regulation and consciousness would be engaged during the feeling of different varieties of admiration and compassion, presumably with different patterns. Specifically, we hypothesized:
that admiration and compassion would engage subcortical nuclei in brainstem and hypothalamus, and somatosensensory cortices in interoceptive (anterior insula) and exteroceptive sectors (somatosensory association areas including the superior parietal lobule and supramarginal gyrus);
that admiration and compassion would engage the PMC (posterior cingulate, retrosplenial cortex and precuneus);
that activation in the anterior insula would peak and dissipate more quickly for CPP than for CSP or varieties of admiration.
We used fMRI and behavioral methods in 13 participants (6 women and 7 men) to compare and contrast the neural correlates of experiencing admiration and compassion in 4 distinct conditions: admiration for virtue (AV), admiration for skill (AS), compassion for social pain (CSP), and compassion for physical pain (CPP). For each condition, the experiences were experimentally induced by narratives based on episodes from the lives of real people (none were celebrities; see Methods). Narratives consisted of an experimenter's scripted verbal account supplemented by combinations of audio/video/still images that involved social pain (to induce CSP in participants); bodily injury without social consequences (to induce CPP); virtuous acts (to induce AV); or virtuosic skill without virtuous implications (to induce AS). The control condition required equivalent processing of narratives that were engaging, social, and active but not emotional. Before the imaging experiment, narratives were developed and tested through an extensive piloting procedure. Participants were introduced to the narratives in a prescanning one-on-one interview but were told neither the categories of emotion in the study nor which emotion each particular narrative was meant to elicit. In the scanner, blood-oxygen-level-dependent (BOLD) signal, respiration rate, and heart rate data were collected while participants viewed short reminder versions and were asked to think about the complete narrative that they had been told earlier, to become as emotional as possible, and to report the strength of their emotion via button press. Participants' verbal descriptions of their feelings during pre- and postscan interviews, and strength ratings from button presses during the scan, were used to ensure that only data from trials in which the participant became genuinely consumed with the target emotion were contrasted with data from unequivocally nonemotional control trials. Any emotional narrative that produced mixed emotions in a participant or to which a participant did not react emotionally in the scanner was removed from the analysis for that participant.
Emotional states are constituted by bodily changes in internal milieu (e.g., hormonal), autonomic function (e.g., heart rate), smooth as well as striated musculature, and the expression of specific behaviors (e.g., freezing or flight). Accordingly, psychophysiological data were used in the BOLD analysis to identify the time window of the emotional reaction and to ensure our effects were not explainable solely by arousal. Because emotions include psychophysiological changes, which are thus present in all instances in which an emotion is felt, we did not remove the portion of the BOLD signal that correlates with psychophysiological changes. Within the time window identified by the psychophysiological results, ANCOVA random-effects analysis was used to contrast BOLD data from each target condition with control and emotions about psychological circumstances (AV, CSP) with those about physical circumstances (AS, CPP). Event-related averages (ERAs) and a bootstrap procedure were used to statistically compare time-to-peak and duration of BOLD response among conditions in the anterior insula.
Results
Behavioral and Psychophysiological Results.
All participants reported feeling appropriately emotional both in the preparation session and in the scanner (as judged by button presses during scanning and by interviews) and indicated that the fixation time in the scanner was sufficient to clear their minds between trials. All subjects but 1 attained each of the target emotions in the scanner. Among qualified emotional trials, average reported emotional strength during scanning differed among conditions (F = 16.94, df = 3, P < 0.001); post hoc comparison at α = 0.05 revealed that CSP and CPP had the highest values and were not distinguishable from each other. AV was lower than CSP but not distinguishable from CPP. AS scored lower than the other conditions [for additional details, see Methods and Behavioral Data Analysis in supporting information (SI) Text and Table S1 for a summary of behavioral results]. Average respiration rate (RR) for each condition showed a rise corresponding to trial onset and a return to baseline after ≈12 s. During the 10-s time window used for the BOLD contrasts, average RR was not different between conditions (F = 0.84, df = 4, P < 0.51); average heart rate (HR) was higher than control for AV and CSP, there was a trend for CPP, and AS was indistinguishable from control (F = 3.49, df = 4, P < 0.02; Dunnett t for emotion > control: AV, P < 0.02; AS, P < 0.79; CSP, P < 0.02; CPP, P < 0.13) (for additional details see Image Processing and Psychophysiology in Methods and Psychophysiology in SI Text; see Fig. S1 A and B for RR and HR time courses). These psychophysiological results make it unlikely that the results of BOLD contrasts could be explained solely by differences in arousal between conditions. In particular, the BOLD contrast between AS and control shows significant results in all hypothesized areas, although there are no differences between arousal levels as measured by psychophysiology in these 2 conditions. Furthermore, RR and HR results from AV, CSP, and CPP are highly comparable, yet patterns of brain activation differ among these conditions.
BOLD Results by Condition Compared with Control.
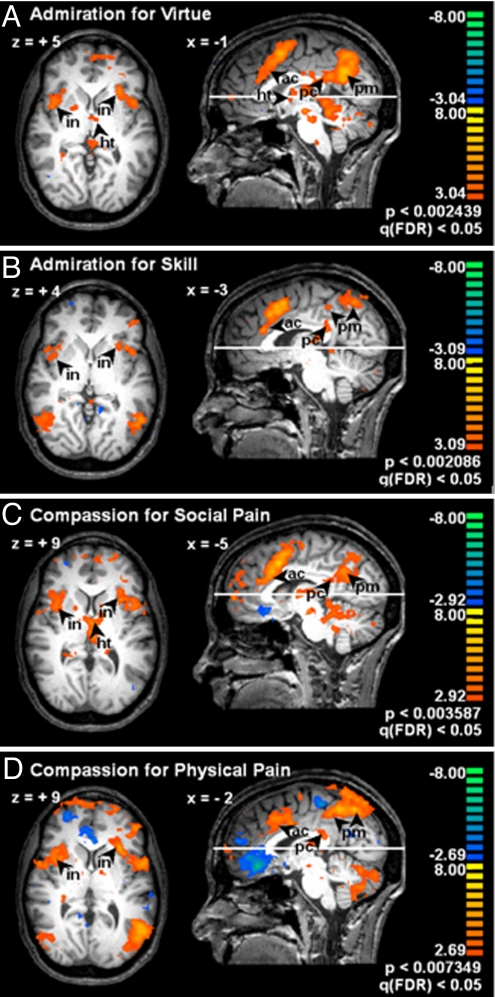
In accord with our prediction that admiration and compassion would engage subcortical regions related to regulating internal organism states, all conditions produced activation of the hypothalamus, as well as the mesencephalon and pontomedullary junction, which include nuclei involved in autonomic regulation. AV and CSP also engaged the medulla. All conditions activated the anterior middle cingulate cortex. All conditions activated cortical regions involved in sensing the body, including anterior insula and supramarginal gyrus. AS and CPP activated the posterior insula. All conditions strongly activated the PMC, but there was a separation between conditions: AV and CSP activated the inferior/posterior sector, whereas AS and CPP activated the superior/anterior sector (see also Fig. 1; see Table S2 for details).
Fig. 1.
Neural correlates of admiration for virtue (A) and skill (B) and compassion for social (C) and physical (D) pain. fMRI data are from 13 subjects, displayed on the brain of 1 subject. Each image shows a target emotion contrasted with control. The position of each transverse slice is marked on the parasagittal images; Talairach coordinates of each slice and of the parasagittal view are annotated. Images are thresholded by using the false discovery rate statistic, q(FDR) < 0.05. The bar to the right of each image provides a color code for t statistics of the respective contrast. Note the bilateral activation in the insula (in), anterior cingulate (ac), and dorsal posterior cingulate (pc) for all emotions. Note the activation in the posteroinferior posteromedial cortices (pm) in AV (A) and CSP (C) compared with anterosuperior sector in AS (B) and CPP (D). Note brainstem and hypothalamus (ht) in A and C.
Of particular interest, relative to the hypothesized regions, there was a partial separation between emotions pertaining to physical circumstances (AS and CPP) versus those pertaining to social/psychological circumstances (AV and CSP). This finding is explored below.
BOLD Results Comparing AV/CSP Versus AS/CPP.
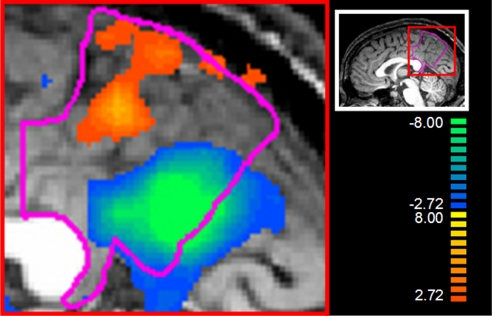
Because of the findings mentioned in the previous section and growing interest in the function of the PMC, we calculated a contrast of AV/CSP (combined) versus AS/CPP (combined). This allowed us to contrast effects of social processing about others' social/psychological situations versus physical situations. This contrast confirmed the functional subdivision in the PMC mentioned above, in that AV/CSP produced more activation in the posterior/inferior portion of the PMC (the sector most related to interoceptive processing), whereas AS/CPP produced more activation in the anterior/superior portion of the PMC (the sector most related to musculoskeletal processing; see Fig. 2). In addition, AV/CSP produced more activation in the anterior cingulate, anterior insula, and hypothalamus, all regions involved in homeostatic regulation. By contrast, AS/CPP produced more activation in the posterior insula and lateral parietal cortices, including the supramarginal gyrus and superior parietal lobule, all regions related to the musculoskeletal system (see Table S3 for more details).
Fig. 2.
Relative activation in the posteromedial cortices (PMC, outlined in pink) for admiration for virtue and compassion for social pain (AV/CSP, blue → green) versus admiration for skill and compassion for physical pain (AS/CPP, orange → yellow). The image is thresholded at q(FDR) < 0.05. The bar to the right provides a color code for t statistics associated with the contrast. The red box frames the location of the magnified view. Note the clear separation between the anterosuperior sector activated by AS/CPP, and the posteroinferior activated by AV/CSP.
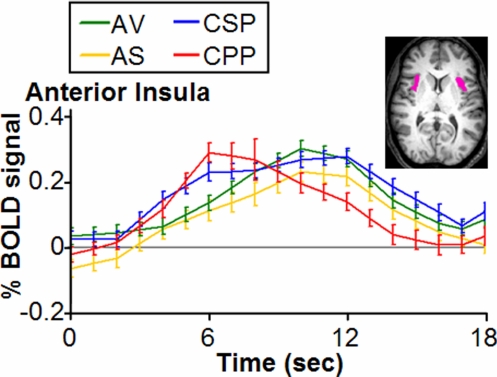
Event-Related Averaging: Comparison of Time Courses.
To test the hypothesis that the activity correlated with CPP would peak earlier and extinguish more quickly than the other emotions, we used double-gamma curve fitting to compare the ERA time courses of the BOLD effects associated with each of the conditions in the anterior insula (see Fig. 3). Duration was calculated from the width of the curves at half-height. Tests for significance were performed in terms of the Z scores of the difference between conditions at a criterion level of 0.05 after correction for multiple comparisons (Bonferroni). CPP peaked more quickly than each of the other emotions and had a shorter duration (see Table S4 for details).
Fig. 3.
Event-related averages for the time courses of admiration and compassion in the anterior insula, with standard errors. Units are percentage change in BOLD signal and time in seconds; time courses are not corrected for hemodynamic delay. For display purposes, BOLD data have been linearly interpolated to 1-s resolution. The volume of interest is displayed in pink. Conditions: AV (green): admiration for virtue; AS (yellow): admiration for skill; CSP (blue): compassion for social pain; CPP (red): compassion for physical pain. Note the rapid rise and dissipation of CPP (red) versus the slower and more sustained rise of CSP (blue), AV (green), and AS (yellow).
Discussion
The ability to empathize with another person's psychological and physical circumstances is a foundation of sociality and moral behavior (42). In most circumstances, empathy is likely to begin with a cognitive appraisal of another's situation, which leads to the induction of a social emotion. In this study, we aimed at inducing strong emotional experiences, either admiring or compassionate, in the participants of the fMRI experiment. One of our aims was to discern whether feelings of admiration and compassion, which are arguably some of the most refined in the human repertoire, would nonetheless recruit neural systems for sensing and regulating body function, as is known to be the case for basic feelings of emotion. Furthermore, we wished to investigate patterns of activation in the posteromedial cortices, a unique set of convergence–divergence cortices (43) known to be involved in self-related processes and the default mode of brain operation (44). Last, we were interested in knowing whether neural activity in the anterior insula associated with compassion for physical pain, arguably a social emotion that requires limited cognitive processing, would peak and dissipate at a rate different from the other emotions tested, in particular compassion for social/psychological pain.
Overall, the finding that homeostatic regulatory mechanisms are engaged in the experience of admiration and compassion supports the hypothesis that social emotions use some of the same basic devices involved in primary emotions (45) and the salience system (46). Relative to the anterior insula and anterior cingulate cortex, our results replicate those of Singer (22) and others (e.g., refs. 16, 21). Our data do not allow us to compare basic and social emotions in terms of degree or pattern of engagement. However, our findings provide insights into the neural differentiation of the social emotions.
First, a notable difference between the experimental conditions was found in the PMC. AV and CSP were associated with strong activation in the inferior/posterior portion of PMC, unlike AS and CPP. By contrast, AS and CPP showed activation in the superior/anterior portion of the PMC, which AV and CSP also showed but to a lesser degree. This distinction is especially intriguing in light of this region's neural connectivity, revealed in a recent neuroanatomical tracing study in the macaque. Parvizi et al. (47) demonstrated that the superior/anterior portion of the PMC is strongly interconnected with lateral parietal cortices, whereas the inferior/posterior portion is closely associated with the anterior middle cingulate cortex, which is itself strongly connected with the insular cortex. Consistent with the anatomical findings, our data suggest a functional subdivision in the PMC regarding emotional processing. Emotions related to someone else's “psychological” state, e.g., social pain or virtue, may preferentially recruit a network involving the inferior/posterior PMC and the anterior middle cingulate, which are affiliated with interoceptive information; by contrast, emotions related to someone else's “physical” state, e.g., painful injury or virtuosic skill, may recruit the sector of PMC most connected with lateral parietal cortices, suggesting a connection to exteroception and musculoskeletal information. Consistent with this interpretation, all emotions engaged the anterior insula, anterior middle cingulate, and lateral parietal cortices; however, in a contrast of AV/CSP (combined) versus AS/CPP (combined), AV/CSP showed more activity in the anterior middle cingulate and anterior insula, and AS/CPP showed more activity in the lateral parietal cortices. AV/CSP also showed more activity in the hypothalamus and mesencephalic reticular formation, both homeostasis-related territories, whereas AS/CPP showed activity in the posterior insula, which is associated with cognitive/motoric processing. Inspection of the data prompted by these findings revealed an equivalent separation at the level of the cerebellum (see ref. 48): AV/CSP activated the anterior/superior cerebellar lobe [Tal: −30, −76, −38, q(FDR) < 0.001], whereas AS/CPP activated the posterior/inferior lobe [Tal: −21, −40, −20, q(FDR) < 0.001]. Overall, these results suggest that the processing of social emotions is organized less around the kind of emotional response, be it compassionate or admiring, than around the contents and context of the situation.
Second, activity in the PMC has also been suggested to underlie the self-processes (37), consistent with its role in processes of consciousness (e.g., refs. 49–51) and its participation in tasks associated with perspective taking and social processing (e.g., refs. 52–54). High levels of activity in the PMC (especially the inferior/posterior sectors) appear to be associated with internally focused attention and introspection (36, 55–57) during resting states (44). Interestingly, compassion for social pain and admiration for virtue, perhaps because they pertain to another's suffering or the alleviation of that suffering, respectively, are often associated with a sense of heightened awareness of one's own condition and its moral implications. For example, several participants volunteered that learning of others' virtue or psychological pain, respectively, incited in them a strong but undifferentiated desire to lead a meaningful life, or gratitude for their own good circumstances. Our participants' report of enhanced self-awareness during AV and CSP may reflect a role for the inferior/posterior PMC in introspecting about someone else's situation related to one's own self. Others have suggested such a process in connection with other brain structures (see ref. 38 for review and ref. 58).
Third, although it is known that the anterior insula is involved in compassion for physical pain, our finding that activity in this region peaked more quickly and for a shorter duration during CPP than during CSP (and AV, AS) suggests that emotions about others' physically painful predicaments co-opt neural mechanisms for personally experienced pain most efficiently and directly, whereas emotions about others' psychological/moral situations build on these same mechanisms but may operate less efficiently and directly. If replicated, this finding could have important implications for the role of culture and education in the development and operation of social and moral systems; in order for emotions about the psychological situations of others to be induced and experienced, additional time may be needed for the introspective processing of culturally shaped social knowledge. The rapidity and parallel processing of attention-requiring information, which hallmark the digital age, might reduce the frequency of full experience of such emotions, with potentially negative consequences.
Taken together, the evidence from neural activity patterns and neural time courses in our experiment suggests a differentiation in the processing of these emotional feelings, in keeping with the complex sociocultural context with which they are associated, building from those related to physical pain and skill to those that transcend immediate involvement of the body to engage the psychological and moral dimensions of a situation. This distinction is seen in the recruitment of neural systems related to the self. Further, although feelings of admiration and compassion recruit the brain's ancient bioregulatory structures as do the primary emotions, when the context for admiration and compassion pertains to social/psychological conditions, the time course of the neural process in the anterior insula is slower than for compassion pertaining to physical pain.
Methods
Participants.
Thirteen right-handed, native English-speaking Americans participated, in accordance with Institutional Review Board requirements (see Participants in SI Text).
Stimuli.
Participants received a one-on-one preparation session in which an experimenter used a combination of audio, video, and still images on a laptop computer to introduce a series of 50 narratives based on episodes from the lives of real people. Quintessential to our experiment was the compelling, realistic, naturalistic feel of the narratives, which unfolded like mini documentaries; narratives that appeared contrived would be less believable and unlikely to produce genuine emotion. For this reason, none of our stimuli involved actors. Instead, the narratives consisted of the experimenter's previously scripted verbal account of each true story supplemented by materials adapted from various sources including television, the internet, documentary films, and radio. Narratives followed a consistent format (i.e., beginning, “This is the story of a woman/man who…” followed by a scripted story and the presentation of additional materials including video and/or still images of the protagonist. Each narrative required between 60 and 90 s to recount and finished with the experimenter asking, “How does this story make you feel?”). The protagonists of all narratives were living, mentally competent individuals who ranged in age from late childhood/early adolescence to adulthood. None were celebrities. Five-second “reminder” versions of these narratives were later shown in the scanner (see below). The narratives were in 5 categories, corresponding to the conditions in the experiment:
Admiration for virtue (AV), which involved people performing highly virtuous, morally admirable acts. The narratives emphasized the virtuous and morally admirable nature of the protagonist, such as dedication to an important cause despite difficult obstacles, and did not include displays of notable skill.
Admiration for skill (AS), which involved people adeptly performing rare and difficult feats, e.g., an athletic or musical performance, with both physical and cognitive components. No physically or socially painful acts were shown, and the skillful feats, although amazing, did not imply a virtuous protagonist or reveal a virtuous act.
Compassion for social pain (CSP), which involved people in states of grief, despair, social rejection, or other difficult psychological circumstances. No physical pain was evident in these narratives, and the troubling circumstances were discerned from the descriptions, rather than being apparent in the images shown.
Compassion for physical pain (CPP), which involved people sustaining a physical injury. The injuries were caused by sports and other mishaps and had no moral or social implications. The injuries were not the result of malevolence, and the participants were reassured that the injuries had no long-term implications. To preclude eliciting disgust, no open wounds were shown.
Control narratives, which involved comparable living, mentally competent people engaged in or discussing how they felt about typical activities under commonplace social circumstances. These circumstances were engaging but not emotion provoking.
See SI Text for descriptions of stimulus selection procedures and examples of stimuli.
Experiment Design.
In order for participants to become genuinely engaged with and emotional about the narratives in the scanner, we designed the imaging experiment to feel as natural and self-paced as possible, rather than rushed. One-on-one piloting established that the behavioral time course of the response to our narratives lasts between 10 and 18 s. For this reason, we set the fMRI trial length to 18 s, so as not to rush participants (for details see Stimulus Selection Procedures in SI Text).
Stimuli shown in the scanner were 5-s reminder versions of the crux of a particular narrative from the preparation session, followed by 13 s of gray screen and 2 s of fixation. To trigger the strongest emotional reaction possible, the most compelling segment of each narrative was constructed by using images of the protagonist, with 1 sentence of verbal information from the preparation session narrative delivered both auditorily and transcribed underneath the image in stationary text. Visual properties of scanner stimuli were equivalent across conditions (see Experiment Design in SI Text).
The imaging portion of the study consisted of a single 1-h session per subject. In addition to an anatomical scan, there were 4 functional runs of ≈9 min each, delivered in 1 of 2 counterbalanced orders, with short rest periods between runs. Each functional run included the presentation of 25 stimuli (5 stimulus categories with 5 items each), pseudorandomly ordered to counterbalance 1-back presentation history. Each of the 50 stimuli was presented twice during the experiment, but never during the same run, for a total of 100 stimulus presentations. Stimuli were separated by 2 s of fixation, during which participants were asked to relax and clear their mind in readiness for the next narrative.
Protocol.
The complete experiment consisted of a preparation session, a scan session, and a debriefing interview. In the preparation session, participants spent approximately 2 h in a quiet, private room reviewing the narratives with an experimenter and discussing how the subject felt about the situation of the person in each narrative. At the beginning of this session, participants were told that they would learn about a series of true stories based on real people's lives, and were asked to be as candid and open as possible about their feelings in relation to each story. To ensure that participants interacted similarly with the control as with the emotional narratives, participants were told that “different people are moved by different kinds of stories,” and that they “might therefore find some of the stories more moving than others.” Participants were not told the categories of emotion in the study, nor were they told what emotion each particular narrative was meant to elicit, although recruitment announcements did mention admiration and compassion. The preparation sessions were videotaped for later reference, and the experimenter noted a brief description of the participants' reaction to each narrative as the session proceeded. Narratives were presented in 1 of 2 pseudorandom orders, with presentation history counterbalanced for 1-back trial history, and no more than 2 stimuli from the same category in a row.
After the preparation session, participants were given a short break and then brought to the scanner. In the scanner, the participants' task was to induce in themselves for each story, as strongly as possible, a similar emotional state to the one they had experienced during the preparation session and to push a button to indicate the strength of the emotion they achieved in the scanner (from 1 to 4 where 1 was for “not particularly emotional,” 2 was for “moderately,” 3 was for “very strongly,” and 4 was for “overwhelmingly emotional”). Participants were asked to report candidly on the strength of their current feelings in the scanner, rather than on the strength of feeling they remembered from the preparation session. Because all of the narratives were designed to include interesting accounts of episodes from people's lives, the word “neutral” was never used to describe participants' emotional state (see Protocol in SI Text for details).
After the scan session, participants were debriefed in the preparation room and asked to describe the emotions they had felt in the scanner for each of the stimuli to which they had responded with a button press indicating moderate, strong, or overwhelming emotion.
Behavioral Data Analysis.
To create the best possible contrasts, only data from good exemplars were included in the analysis. However, to ensure equivalence of the experiment across subjects, all subjects were presented with all of the stimuli in the scanner. Therefore, data were sorted for inclusion/exclusion by using each participant's description of his/her feelings during the preparation and debriefing interviews (judged from video/experimenter notes and used to ensure the quality/label of emotional reaction) and the button press values s/he assigned to his/her emotional reaction in the scanner (to ensure appropriate strength of reaction). This sorting process allowed us to include in the analysis only exemplars that a participant had labeled consistently with our established categories and, of those, only trials that had produced the expected amount of emotion in the scanner (see Behavioral Data Analysis in SI Text for details and interrater reliability analyses with a blind rater).
Psychophysiology.
During scanning, respiration and heart rate data were collected from 10 subjects as an independent measure of arousal over the course of the trial, and to help determine the time window to include in the BOLD contrasts between conditions. (Because of probe slippage, a common problem of psychophysiological data collection inside an fMRI scanner, HR data from only 7 subjects were useable.) Psychophysiological data were normalized and averaged to 2-s intervals to correspond to TR length (see Psychophysiology in SI Text for details).
Image Processing.
After preprocessing and normalization, we estimated the BOLD signal for each participant using a GLM. Individual TRs for each trial type were separately modeled with 9 independent regressors (δ-functions). ANCOVA random effects was used to compare data from TRs corresponding to the time window of maximal psychophysiological effect (see Image Acquisition and Processing in SI Text). Statistical maps were thresholded by using the false discovery rate statistic, q(FDR) < 0.05 (see Localization of Activations in SI Text).
Event-Related Averaging and Analysis of Time Course.
To test the hypothesis that CPP is more quickly induced and shorter-lived in the anterior insula than the other emotions tested, we statistically compared the time courses of the BOLD effects associated with each emotion in this brain region by fitting the ERAs of the z-transformed signal with difference of gamma-functions and implementing a bootstrap procedure to estimate the parameters of interest. See Event-Related Averaging and Analysis of Time Course SI Text for details.
Supplementary Material
Acknowledgments.
We thank B. Tjan, J. Kaplan, Z.-L. Lu, D. Pantazis, and J. Zhuang for valuable advice on fMRI methodology. J. Parvizi consulted on neuroanatomical issues; S. Wong consulted on analyses of psychophysiological data; X. Yang, X. Li, G. Xue, J. L. Poletti Soto, and L. Xiao collaborated on data analysis; J. McArdle advised on behavioral questionnaires; M. Miles performed reliability coding; G. Fox, I. Uricaru, M. Borner, C. Webber, A. N. Zhou, R. Essex, F. Dandekar, StoryCorps assisted with experimental preparations; and Z.-L. Lu and K. Meyer provided helpful comments on the manuscript. This research was supported by National Institutes of Health Grant P01 NS19632 (to A.D. and H.D.), by a grant from the Mathers Foundation (to A.D. and H.D.), and by the Brain and Creativity Institute Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7687.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810363106/DCSupplemental.
References
- 1.Haidt J. The emotional dog and its rational tail: A social intuitionist approach to moral judgment. Psychol Rev. 2001;108(4):814–834. doi: 10.1037/0033-295x.108.4.814. [DOI] [PubMed] [Google Scholar]
- 2.Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293(5537):2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- 3.Davidson RJ, Harrington A, editors. Visions of Compassion: Western Scientists and Tibetan Buddhists Examine Human Nature. New York: Oxford Univ Press; 2002. [Google Scholar]
- 4.Haidt J. Elevation and the positive psychology of morality. In: Keyes CLM, Haidt J, editors. Flourishing: Positive Psychology and the Life Well-Lived. Washington, DC: Am Psychol Assoc; 2003. pp. 275–289. [Google Scholar]
- 5.Algoe SB, Haidt J, Gable SL. Beyond reciprocity: Gratitude and relationships in everyday life. Emotion. 2008;8(3):425–429. doi: 10.1037/1528-3542.8.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett MY, DeSteno D. Gratitude and prosocial behavior: Helping when it costs you. Psychol Sci. 2006;17(4):319–325. doi: 10.1111/j.1467-9280.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 7.Decety J, Chaminade T. Neural correlates of feeling sympathy. Neuropsychologia. 2003;41(2):127–138. doi: 10.1016/s0028-3932(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 8.Damasio AR, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 9.Damasio AR. Looking for Spinoza: Joy, Sorrow and the Feeling Brain. New York: Harcourt; 2003. [Google Scholar]
- 10.Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 12.Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cognit Sci. 2008;12(2):65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Zahn R, et al. The neural basis of human social values: Evidence from functional MRI. Cereb Cortex. 2009;19(2):276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casebeer WD, Churchland PS. The neural mechanisms of moral cognition: A multiple-aspect approach to moral judgment and decision-making. Biol Philos. 2003;18(1):169–194. [Google Scholar]
- 15.Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends Cognit Sci. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131(2):202–223. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- 18.DeWall CN, Baumeister RF. Alone but feeling no pain: Effects of social exclusion on physical pain tolerance and pain threshold, affective forecasting, and interpersonal empathy. J Personality Soc Psychol. 2006;91(1):1–15. doi: 10.1037/0022-3514.91.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Morrison I, Lloyd D, di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: Is empathy a multisensory issue? Cognit Affect Behav Neurosci. 2004;4(2):270–278. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- 20.Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci. 2005;8(7):955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- 21.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage. 2005;24(3):771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 23.Ochsner KN, et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cognit Affect Neurosci. 2008;3(2):144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex. 2007;17(11):2553–2561. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- 25.Saarela MV, et al. The compassionate brain: Humans detect intensity of pain from another's face. Cereb Cortex. 2007;17(1):230–237. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- 26.Benuzzi F, Lui F, Duzzi D, Nichelli PF, Porro CA. Does it look painful or disgusting? Ask your parietal and cingulate cortex. J Neurosci. 2008;28(4):923–931. doi: 10.1523/JNEUROSCI.4012-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. NeuroImage. 2007;34(4):1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Wicker B, et al. Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 29.Hauser MD. Moral minds: How Nature Designed Our Universal Sense of Right and Wrong. New York: Harper Collins; 2006. [Google Scholar]
- 30.Hoffman ML. Empathy and Moral Development: Implications for Caring and Justice. New York: Cambridge Univ Press; 2001. [Google Scholar]
- 31.Mitchell JP. Contributions of functional neuroimaging to the study of social cognition. Curr Dir Psychol Sci. 2008;17(2):142–146. [Google Scholar]
- 32.Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 33.Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proc Natl Acad Sci USA. 2007;104(20):8235–8240. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- 35.Rilling JK, Dagenais JE, Goldsmith DR, Glenn AL, Pagnoni G. Social cognitive neural networks during in-group and out-group interactions. NeuroImage. 2008;41(4):1447–1461. doi: 10.1016/j.neuroimage.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cognit Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Damasio AR, Meyer K. Consciousness: An overview of the phenomenon and of its possible neural basis. In: Laureys S, Tononi G, editors. The Neurology of Consciousness. Elsevier; 2008. pp. 3–14. [Google Scholar]
- 38.Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: The role of cortical midline structures and mirror neurons. Trends Cognit Sci. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Oberman LM, Ramachandran VS. The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychol Bull. 2007;133(2):310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence EJ, et al. The role of ‘shared representations’ in social perception and empathy: An fMRI study. NeuroImage. 2006;29(4):1173–1184. doi: 10.1016/j.neuroimage.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Moll J, de Oliveira-Souza R, Zahn R. The neural basis of moral cognition: Sentiments, concepts, and values. Ann NY Acad Sci. 2008;1124:161–180. doi: 10.1196/annals.1440.005. [DOI] [PubMed] [Google Scholar]
- 43.Hagmann P, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moll J, et al. The moral affiliations of disgust—A functional MRI study. Cognit Behav Neurol. 2005;18(1):68–78. doi: 10.1097/01.wnn.0000152236.46475.a7. [DOI] [PubMed] [Google Scholar]
- 46.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parvizi J, Van Hoesen GW, Buckwalter J, Damasio AR. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA. 2006;103(5):1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44(8):1037–1066. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damasio AR. The Feeling of What Happens. New York: Harcourt; 1999. [Google Scholar]
- 50.Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17(2):1080–1086. [PubMed] [Google Scholar]
- 51.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cognit Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Lamm C, Batson CD, Decety J. The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. J Cognit Neurosci. 2007;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- 53.Schulte-Rüther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy. J Cognit Neurosci. 2007;19(8):1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- 54.Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nat Neurosci. 2001;4(5):546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- 55.Johnson SC, et al. Neural correlates of self-reflection. Brain. 2002;125(8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 56.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kircher TTJ, et al. Towards a functional neuroanatomy of self processing: Effects of faces and words. Cognit Brain Res. 2000;10(1–2):133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cognit Neurosci. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.