Abstract
Persistent hyperglycemia in diabetes causes endothelial cell dysfunction. Exposure to high levels of glucose, which mimics hyperglycemia, induced expression of microRNA 221 (miR-221) but reduced expression of c-kit, the receptor for stem cell factor in human umbilical vein endothelial cells (HUVECs). In addition, high glucose treatment impaired endothelial cell migration. Incubation with the antisense miR-221 oligonucleotide AMO-221 reduced expression of miR-221 and restored c-kit protein expression in HUVECs treated with high levels of glucose. Furthermore, AMO-221 treatment abolished the inhibitory effect of high glucose exposure on HUVECs transmigration. Thus, under hyperglycemic conditions, miR-221 is induced in HUVECs, which consequently triggers inhibition of c-kit and impairment of HUVECs migration. These findings suggest that manipulation of the miR-221-c-kit pathway may offer a novel strategy for treatment of vascular dysfunction in diabetic patients.
Keywords: microRNA, endothelial dysfunction, diabetes, c-kit, SCF
Introduction
Exposure of the vascular endothelium to high levels of glucose causes endothelial dysfunction and further complicates the pathogenesis of diabetes [1–3]. MicroRNAs (miRNAs) are small, non-coding RNAs that regulate gene expression at the post-transcriptional level by specifying translational repression or cleavage of mRNA [4–6]. Many miRNA species have served as novel biomarkers, modulators, and therapeutic targets for disease, and they may regulate intracellular metabolism [7–9]. miRNA-221 (miR-221), a specific miRNA identified in human umbilical vein endothelial cells (HUVECs), participates in the regulation of angiogenesis [10]. miR-221 affects expression of c-kit, the receptor for stem cell factor (SCF), which plays a key role in endothelial progenitor cell migration and homing [10].
Alterations in expression of miRNA help regulate cardiovascular functions [11–17]. However, whether miR-221 plays a role in diabetes-induced endothelial dysfunction is unknown. In this study, we tested the hypothesis that hyperglycemia induces miR-221 expression, c-kit suppression, and consequently endothelial dysfunction.
Materials and methods
Cell culture and reagents
HUVECs were purchased from ScienCell (Carlsbad, CA) and cultured in endothelial cell medium (ScienCell) supplemented with 5% fetal bovine serum, endothelial cell growth supplement (ScienCell), and 100 μg/ml penicillin/streptomycin. Glucose was obtained from Sigma (St. Louis, MO), and human SCF was obtained from R&D (Minneapolis, MN). The anti-c-kit (C-19) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-GAPDH antibody was obtained from Fitzgerald.
Synthesis and transfection of miRNAs antisense inhibitor
We synthesized an antisense miR-221 oligonucleotide inhibitor (AMO-221) based on 5′-AGCUACAUUGUCUGCUGGGUUUC-3′ for mature human miR-221 (Amibon, Austin, Texas). After 24 hours in serum-free medium, cells (1×105 per well) were transfected with AMO-221 by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions.
Micro-RNA isolation and expression
The mirVana™ RT-PCR miRNA Isolation and Detection Kit (Ambion, Inc.) was used. The reactions contain mirVana RT-PCR primer sets specific for miR-221 or 5S rRNA. 5S rRNA was used as an internal control. RT-PCR was performed for 40 cycles. PCR products were confirmed by 3% agarose gel electrophoresis.
Western blot analysis
Cells were washed with phosphate-buffered saline and lysed in lysis buffer. Lysates were subjected to 4% to 15% SDS-PAGE and western blotting with anti-c-kit (1:100) antibodies. Immunopositive bands were visualized by enhanced chemiluminescence (ECL, Amersham). Blots were stripped and reprobed with GAPDH antibody (1:2000) as a control to ensure equal loading.
Assessment of transmigration
We used a transcell system to assess the capacity of HUVECs to transmigrate across a filter into a separate cell-culture chamber. Cells were placed at a concentration of 5×104/well in the upper chamber (BD Biosciences, San Jose, CA), which was placed in a 24-well culture plate (BD Biosciences) containing culture medium and 100 nM SCF to measure the migratory capacity of HUVECs. After 16 hours of incubation at 37°C, the medium was removed, and the insert was transferred to a second plate containing calcein-AM solution. The plates were incubated for 90 minutes at 37°C in a 5% CO2 incubator. Then, the fluorescence of migrated cells was read in a fluorescence plate reader with bottom-reading capabilities at excitation/emission wavelengths of 485/530 nm.
Statistical analysis
Quantitative data were evaluated by analysis of variance (ANOVA) or Student t test when appropriate. Significance was established at a level of P<0.05.
Results
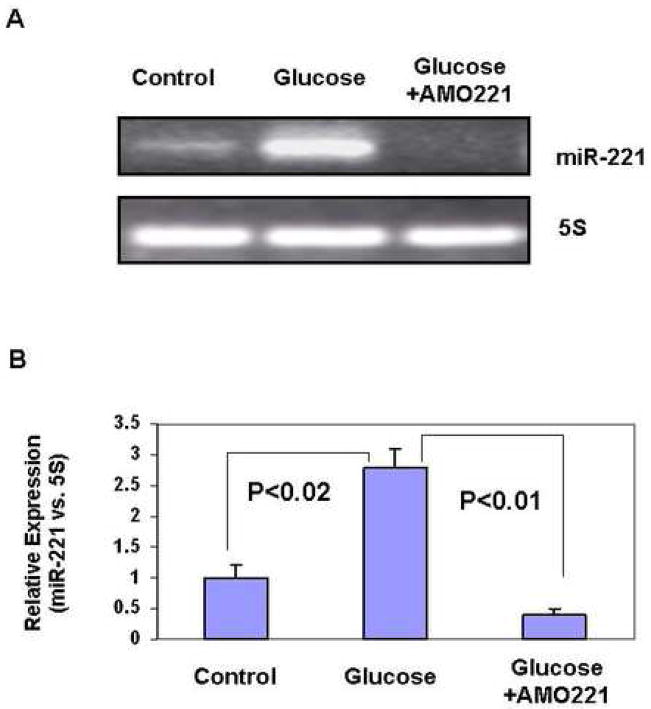
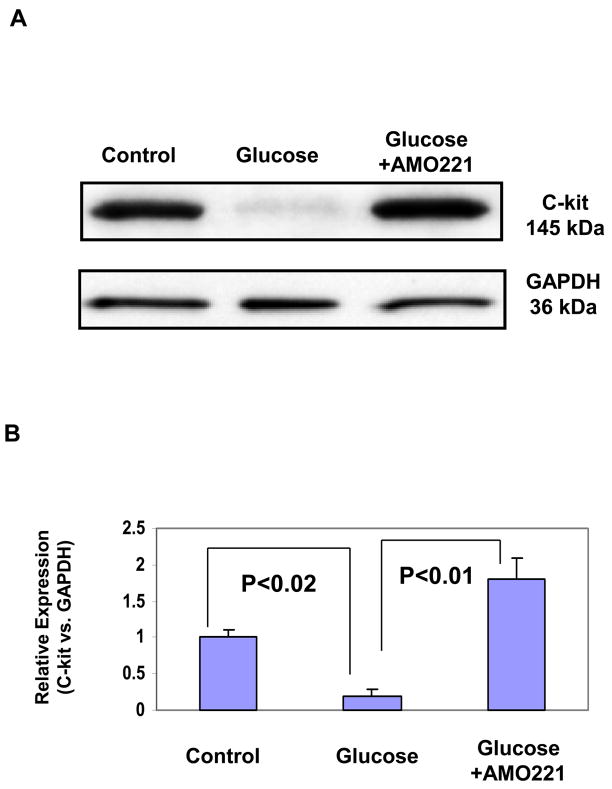
We analyzed miR-221 expression in HUVECs exposed to high levels of glucose and found that conditions of hyperglycemia increased miR-221 expression (Fig. 1A). The glucose-induced expression of miR-221 was inhibited by the antisense inhibitory miRNA oligonucleotide (AMO-221), as determined by the mirVana RT-PCR miRNA detection assay (Fig. 1A). Relative expression of miR-221 was quantified by densitometry (Fig. 1B). Using prediction algorithms, we identified a 3′-untranslated region (3′UTR) of c-kit mRNA as a potential target of miR-221 (Fig. 2A), targeted by the antisense oligonucleotide AMO-221 (Fig. 2B). To determine expression of c-kit protein expression, Western blot was performed with antibodies against c-kit in HUVECs treated with or without high glucose. At baseline, HUVECs expressed the c-kit protein (Fig. 3A). However, under hyperglycemic conditions, c-kit protein expression was markedly reduced, and the treatment with the AMO-221 antisense oligonucleotide against miR-221 reversed the inhibitory effect of high glucose levels on expression of c-kit in HUVECs (Fig. 3A). AMO-221 appeared to selectively target c-kit as it did not alter expression of the house-keeping protein GAPDH in HUVECs (Fig. 3A). Western blot of c-kit was quantified by densitometry (Fig. 3B).
Fig. 1.
Effects of high levels of glucose on miR-221 expression.
(A) RT-PCR analysis shows that high levels of glucose (25 mM) increase miR-221 expression in HUVECs and that the increase in miR-221 expression induced by high glucose levels is attenuated by transfection of the antisense inhibitory miRNA oligonucleotide AMO-221. The miRNA was isolated by using the mirVana miRNA Isolation Kit, and RT-PCR was performed by using the mirVana™ RT-PCR miRNA detection assay containing the primers specific for miR-221 or 5S rRNA. 5S rRNA was used as an internal control. The PCR products were confirmed by 3% agarose gel electrophoresis.
(B) Relative expression of miR-221 was quantified by densitometry. N = 4 for each group.
Fig. 2.
(A) The position of miR-221 target sites along 3′UTR of c-kit is predicted by TargetScan. (B) Sequences of antisense inhibitory miRNA oligonucleotide AMO-221 designed based on the mature human miR-221.
Fig. 3.
Expression of c-kit in HUVECs treated with glucose and the miR-221 antisense inhibitory oligonucleotide AMO-221. (A) HUVECs were exposed to 25 mM glucose with or without AMO-221. After 48 hours, cell lysates were subjected to 4% to 15% SDS-PDGE, transferred to membranes, and blotted with anti-c-kit or anti-GAPDH antibodies (control). (B) Western blot of c-kit was quantified by densitometry. N = 4 for each group.
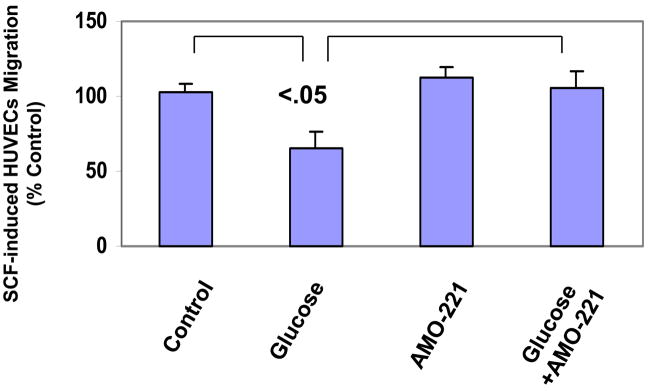
To define the role of miR-221 on c-kit/SCF–regulated endothelial cell function, we used a transcell system to analyze migration of HUVECs treated with the miR-221 inhibitor AMO-221 after exposure to high levels of glucose. Exposure to high glucose levels reduced the transmigration capacity of HUVECs by nearly 40% (Fig. 4). Although treatment with AMO-221 per se had little effect on HUVECs transmigration, AMO-221 treatment of HUVECs almost completely restored the transmigration capacity impaired by exposure to high levels of glucose (Fig. 4).
Fig. 4.
Migration of glucose-treated HUVECs in response to stem cell factor (SCF) in the presence or absence of AMO-221. The pretreated HUVECs were placed in the upper chamber, which was placed in a 24-well culture dish containing culture medium and 100 ng SCF. After 16 hours of incubation at 37°C, the medium was removed, and the insert was transferred to a second plate containing calcein-AM solution. After a 90-minute incubation at 37°C incubator, the fluorescence of migrated cells was read in a fluorescence plate reader with bottom reading capabilities at excitation/emission wavelengths for 485/530 nm. P<0.05, vs. untreated controls.
Discussion
During the development of diabetes, hyperglycemia may cause malfunction of the vasculature. Exposure to high levels of glucose may alter miRNA expression in cardiovascular cells under many pathological conditions. The present study has identified a novel pathway by which hyperglycemic conditions impair c-kit expression and migration of HUVECs, perhaps through induction of miR-221. Migration and homing mediated by c-kit is a key step in angiogenesis and vascular tissue repair and regeneration. Impaired migration of vascular cells may contribute to diabetes-associated pathologic alterations in the vascular wall as reported in studies of animals and patients with diabetes [18, 19] Our finding that downregulation of miR-221 can attenuate high glucose-induced suppression of c-kit and migration in HUVECs confirms recent findings by Poliseno et al.[10] and extends them to a specific disease such as diabetes. Our findings suggest that manipulation of the miR-221-c-kit pathway may offer a novel strategy for treatment of vascular dysfunction in diabetic patients.
Acknowledgments
This work was supported by the American Heart Association (0765149Y to Y Li), MacDonald Foundation (07RDM008 to Y Li), grants from the National Institutes of Health (R01HL69509, YJ Geng) and T5 program of Department of Defense (YJ Geng).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meng X, Li ZM, Zhou YJ, Cao YL, Zhang J. Effect of the antioxidant alpha-lipoic acid on apoptosis in human umbilical vein endothelial cells induced by high glucose. Clin Exp Med. 2008;8:43–49. doi: 10.1007/s10238-008-0155-1. [DOI] [PubMed] [Google Scholar]
- 2.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 3.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111:2073–2085. doi: 10.1161/01.CIR.0000162472.52990.36. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 6.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 7.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 11.Kuehbacher A, Urbich C, Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci. 2008;29:12–15. doi: 10.1016/j.tips.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 13.Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX, Lin SG, Li Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008;376:548–552. doi: 10.1016/j.bbrc.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, Xiao J, Shan H, Wang Z, Yang B. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–3052. doi: 10.1242/jcs.010728. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Plutzky J. Diabetic macrovascular disease: the glucose paradox? Circulation. 2002;106:2760–2763. doi: 10.1161/01.cir.0000037282.92395.ae. [DOI] [PubMed] [Google Scholar]
- 19.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]