Abstract
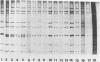
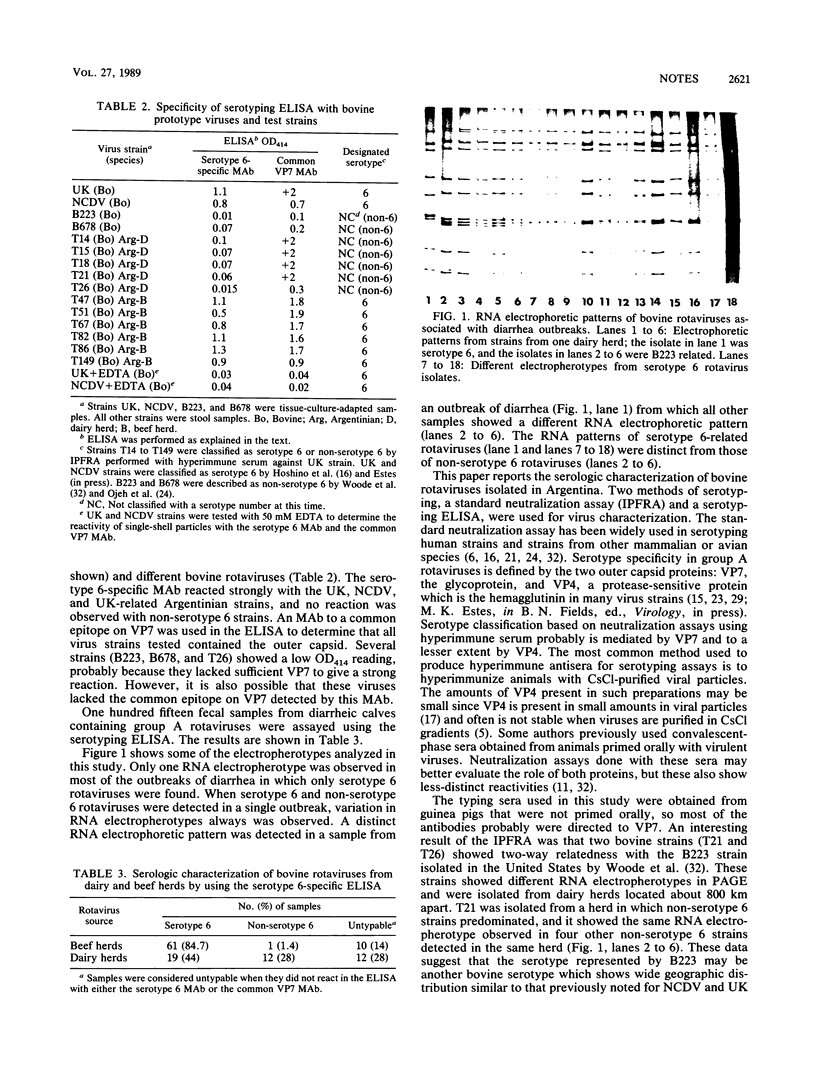
Bovine rotaviruses isolated from beef and dairy herds in Argentina were serotyped by the immunoperoxidase focus reduction assay as previously described (G. Gerna, M. Battaglia, G. Milenesi, N. Passarani, E. Percivalle, and E. Cattaneo, Infect. Immun. 43:722-729, 1984). Three strains from beef herds were related to the UK and NCDV bovine rotavirus strains defined as serotype 6 (Y. Hoshino, R. G. Wyatt, H. B. Greenberg, J. Flores, and A. Z. Kapikian, J. Infect. Dis. 149:694-702, 1984). Two other strains from dairy herds were classified as bovine viruses related to the bovine B223 strain reported by Woode and co-workers (G. N. Woode, N. E. Kelso, T. F. Simpson, S. K. Gaul, L. E. Evans, and L. Babiuk, J. Clin. Microbiol. 18:358-364, 1983) in the United States. A serotyping antibody-capture enzyme-linked immunoassay to detect serotype 6 rotavirus using a serotype 6-specific monoclonal antibody was developed and evaluated for strain characterization. Characterization of 72 group A rotavirus-positive fecal samples from beef herds and 43 fecal samples from dairy herds showed a predominance of serotype 6 rotavirus in beef herds but both serotype 6 and non-serotype 6 rotaviruses in dairy herds. Analysis of genomic double-stranded RNA by polyacrylamide gel electrophoresis showed that when outbreaks were caused by one serotype only a single electropherotype was present in all samples.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellinzoni R. C., Blackhall J., Baro N., Auza N., Mattion N., Casaro A., La Torre J. L., Scodeller E. A. Efficacy of an inactivated oil-adjuvanted rotavirus vaccine in the control of calf diarrhoea in beef herds in Argentina. Vaccine. 1989 Jun;7(3):263–268. doi: 10.1016/0264-410X(89)90241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinzoni R. C., Mattion N., La Torre J. L., Scodeller E. A. Incidence of rotavirus in beef herds in Argentina. Res Vet Sci. 1987 Mar;42(2):257–259. [PubMed] [Google Scholar]
- Brüssow H., Marc-Martin S., Eichhorn W., Sidoti J., Fryder V. Characterization of a second bovine rotavirus serotype. Arch Virol. 1987;94(1-2):29–41. doi: 10.1007/BF01313723. [DOI] [PubMed] [Google Scholar]
- Bulgin M. S., Anderson B. C., Ward A. C., Evermann J. F. Infectious agents associated with neonatal calf disease in southwestern Idaho and eastern Oregon. J Am Vet Med Assoc. 1982 May 15;180(10):1222–1226. [PubMed] [Google Scholar]
- Burns J. W., Greenberg H. B., Shaw R. D., Estes M. K. Functional and topographical analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. J Virol. 1988 Jun;62(6):2164–2172. doi: 10.1128/jvi.62.6.2164-2172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Hoshino Y., Bell L. M., Groff J., Hess G., Bachman P., Offit P. A. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol. 1987 Sep;25(9):1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Unicomb L. E., Pitson G. A., Bishop R. F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987 Mar;25(3):509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S. Variation in neutralization epitopes of human rotaviruses in relation to genomic RNA polymorphism. Virology. 1987 Aug;159(2):209–216. doi: 10.1016/0042-6822(87)90457-0. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Lazdins I., Tregear G. W., Holmes I. H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc Natl Acad Sci U S A. 1986 May;83(10):3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Identification of rotaviruses of different origins by the plaque-reduction test. Am J Vet Res. 1980 Jan;41(1):151–152. [PubMed] [Google Scholar]
- Gaul S. K., Simpson T. F., Woode G. N., Fulton R. W. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982 Sep;16(3):495–503. doi: 10.1128/jcm.16.3.495-503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Battaglia M., Milenesi G., Passarani N., Percivalle E., Cattaneo E. Serotyping of cell culture-adapted subgroup 2 human rotavirus strains by neutralization. Infect Immun. 1984 Feb;43(2):722–729. doi: 10.1128/iai.43.2.722-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., di Matteo A., Parea M., Orsolini P., Battaglia M. Identification of two subtypes of serotype 4 human rotavirus by using VP7-specific neutralizing monoclonal antibodies. J Clin Microbiol. 1988 Jul;26(7):1388–1392. doi: 10.1128/jcm.26.7.1388-1392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Saif L. J., Sereno M. M., Chanock R. M., Kapikian A. Z. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J Virol. 1988 Mar;62(3):744–748. doi: 10.1128/jvi.62.3.744-748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Liu M., Offit P. A., Estes M. K. Identification of the simian rotavirus SA11 genome segment 3 product. Virology. 1988 Mar;163(1):26–32. doi: 10.1016/0042-6822(88)90230-9. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Benfield D. A., Greenberg H. B. Characterization of homotypic and heterotypic VP7 neutralization sites of rhesus rotavirus. Virology. 1988 Aug;165(2):511–517. doi: 10.1016/0042-6822(88)90595-8. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. M., Mackow E. R., Greenberg H. B. Molecular determinant of rotavirus neutralization and protection. Adv Virus Res. 1989;36:181–214. doi: 10.1016/s0065-3527(08)60585-0. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi T., Akatani K., Ikegami N., Katsushima N., Nakagomi O. Occurrence of changes in human rotavirus serotypes with concurrent changes in genomic RNA electropherotypes. J Clin Microbiol. 1988 Dec;26(12):2586–2592. doi: 10.1128/jcm.26.12.2586-2592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeh C. K., Snodgrass D. R., Herring A. J. Evidence for serotypic variation among bovine rotaviruses. Arch Virol. 1984;79(3-4):161–171. doi: 10.1007/BF01310809. [DOI] [PubMed] [Google Scholar]
- Reynolds D. J., Morgan J. H., Chanter N., Jones P. W., Bridger J. C., Debney T. G., Bunch K. J. Microbiology of calf diarrhoea in southern Britain. Vet Rec. 1986 Jul 12;119(2):34–39. doi: 10.1136/vr.119.2.34. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Ojeh C. K., Campbell I., Herring A. J. Bovine rotavirus serotypes and their significance for immunization. J Clin Microbiol. 1984 Sep;20(3):342–346. doi: 10.1128/jcm.20.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Terzolo H. R., Sherwood D., Campbell I., Menzies J. D., Synge B. A. Aetiology of diarrhoea in young calves. Vet Rec. 1986 Jul 12;119(2):31–34. doi: 10.1136/vr.119.2.31. [DOI] [PubMed] [Google Scholar]
- Sorrentino A., Scodeller E. A., Bellinzoni R., Muchinik G. R., La Torre J. L. Detection of an atypical rotavirus associated with diarrhoea in Chaco, Argentina. Trans R Soc Trop Med Hyg. 1986;80(1):120–122. doi: 10.1016/0035-9203(86)90209-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Hoshino Y., Nishikawa K., Green K. Y., Maloy W. L., Morita Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Cross-reactive and serotype-specific neutralization epitopes on VP7 of human rotavirus: nucleotide sequence analysis of antigenic mutants selected with monoclonal antibodies. J Virol. 1988 Jun;62(6):1870–1874. doi: 10.1128/jvi.62.6.1870-1874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K., Morita Y., Sakurada N., Saeki Y., Morita O., Hasegawa S. Validity of an enzyme-linked immunosorbent assay with serotype-specific monoclonal antibodies for serotyping human rotavirus in stool specimens. Microbiol Immunol. 1988;32(7):699–708. doi: 10.1111/j.1348-0421.1988.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw P. W., Ellens D. J., Talmon F. P., Zimmer G. N., Kommerij R. Rotavirus infections in calves: efficacy of oral vaccination in endemically infected herds. Res Vet Sci. 1980 Sep;29(2):142–147. doi: 10.1016/S0034-5288(18)32654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]