Summary
Objectives
To isolate and characterize an asparaginyl endopeptidase from the carcinogenic liver fluke, Opisthorchis viverrini, and evaluate its expression profile, biochemical activity, and potential as an immunodiagnostic antigen.
Methods
The full length mRNA encoding an asparaginyl endopeptidase (family C13), Ov-aep-1, was isolated by immunoscreening of a cDNA bacteriophage library of adult O. viverrini using sera from patients infected with O. viverrini. Investigation of Ov-aep-1 transcripts in developmental stages of the parasite, and phylogenetic analysis, immunohistochemical localization, and recombinant protein expression and enzymology were employed to characterize the Ov-AEP-1 protein. Immunoblotting was used to assess the potential of this enzyme for immunodiagnosis of human opisthorchiasis.
Results
Ov-AEP-1 is characteristic of the C13 cysteine protease family. Ov-aep-1 transcripts were detected in adult and juvenile worms, eggs, and metacercariae. Phylogenetic analysis indicated that Ov-AEP-1 is closely related to homologous proteins in other trematodes. Recombinant Ov-AEP-1 was expressed in bacteria in inclusion bodies and refolded to a soluble form. Excretory–secretory (ES) products derived from adult O. viverrini and refolded recombinant Ov-AEP-1 both displayed catalytic activity against the diagnostic tripeptide substrate, Ala–Ala–Asn-aminomethylcoumarin. Rabbit antiserum raised to recombinant Ov-AEP-1 identified the native AEP-1 protease in both somatic extract and ES products of adult worms. Anti-Ov-AEP-1 IgG immunolocalized the anatomical site of expression to the gut of the fluke, implying a physiological role in digestion of food or activation of other digestive enzymes. Recombinant Ov-AEP-1 was recognized by serum antibodies from patients with opisthorchiasis but not other helminth infections, with a sensitivity and specificity of 85% and 100%, respectively. The positive and negative predictive values are 100% and 67%, respectively.
Conclusions
The liver fluke, O. viverrini, has a gut-localized asparaginyl endopeptidase. Refolded recombinant Ov-AEP-1 is catalytically active and has potential for immunodiagnosis of human opisthorchiasis.
Keywords: Asparaginyl endopeptidase, Legumain, Opisthorchis viverrini, Liver fluke, Cholangiocarcinoma, Immunodiagnosis, Protease
Introduction
The liver fluke, Opisthorchis viverrini, is an important human pathogen endemic in Southeast Asia, predominantly Northeast Thailand.1 Infection with this parasite causes opisthorchiasis, which is associated with a number of hepatobiliary abnormalities, including cholangitis, obstructive jaundice, hepatomegaly, cholecystitis, and cholelithiasis.2 Experimental and epidemiological evidence strongly implicates O. viverrini infection in the etiology of cholangiocarcinoma, cancer of the bile ducts.1,3–5 Indeed, O. viverrini is the only metazoan pathogen that is considered a class I carcinogen.4 It has been implied that inflammation of the bile ducts caused by O. viverrini infection and induction of endogenous nitric oxide are important factors for carcinogenesis.6,7 Moreover, cell proliferation induced by O. viverrini and exposure to exogenous carcinogens such as nitrates, nitrites, and even N-nitroso compounds found in several fermented or preserved foods have been implicated as factors involved with parasite-associated neoplastic processes.1 O. viverrini infection induces several pathological changes including epithelial desquamation, epithelial and adenomatous hyperplasia, goblet cell metaplasia, inflammation, periductal fibrosis, and granuloma formation.8 The pathogenesis of O. viverrini-mediated hepatobiliary changes may be due to mechanical irritation caused by the liver fluke suckers and/or its excretory–secretory (ES) products.2,9,10
Proteolytic enzymes are major components of helminth ES products, where they play essential roles in digestion of food proteins, immunomodulation, and host tissue invasion.11 Asparaginyl endopeptidases are cysteine proteases (MEROPS clan CD, family C13) that hydrolyze peptides and proteins on the carboxyl side of asparagine residues. These enzymes are often referred to as ‘legumain-like’ in reference to the first member of the family that was described from beans. Legumains were then discovered in Schistosoma mansoni12,13 and later reported from other parasitic helminths including Fasciola spp14,15 and Haemonchus contortus.16 Schistosome asparaginyl endopeptidases (AEPs) are expressed in the gastrodermis where they may trans-activate other proteases including the papain-like cathepsin B cysteine protease, SmCB1.17,18 Prior to the first reports of genes encoding plant legumains, the S. mansoni AEP was called Sm32, and was of interest due to its potential as a serodiagnostic antigen for schistosomiasis.19
In this report, we describe the identification of a cDNA encoding asparaginyl endopeptidase from O. viverrini and its expression in the gut of adult worms and in eggs. We also describe the preparation and purification of a recombinant form of the protease and investigation of its potential as a serodiagnostic antigen for human opisthorchiasis.
Materials and methods
Immunoscreening of an adult Opisthorchis viverrini cDNA library
A cDNA library of adult O. viverrini was constructed using the SMART™ library construction kit (Clontech, Mountain View, CA, USA) as described elsewhere.20 Immunoscreening of the cDNA library was performed with the picoBlue™ immunoscreening kit in accordance with the manufacturer’s instructions (Stratagene, La Jolla, CA, USA). Membranes were probed with a pool of sera from people infected with O. viverrini and diagnosed with cholangiocarcinoma. Moreover, these sera were pooled from samples exhibiting elevated antibody titers against ES antigen, as previously described.21 Sera used in this study were obtained with the approval of the Ethics Committee of Khon Kaen University (HE451132). Positive phage plaques were selected for conversion to phagemids. Nucleotide sequences of the immunopositive recombinant clones were analyzed using standard automated sequencing methodologies. Sequences were edited and translated to deduced amino acid sequences using BioEdit.22 Homology searches were performed using Blast search at NCBI (http://www.ncbi.nlm.nih.gov/Blast/). Open reading frames (ORFs) were further analyzed for signal peptides/anchors using SignalP-NN prediction and SignalP-HMM prediction at http://www.cbs.dtu.dk/services/SignalP/.
Phylogenetic analysis
The phylogenetic relationship between Ov-AEP-1, Ov-AEP-2, and other AEPs was analyzed based on amino acid sequence. The full length ORFs of the Ov-AEPs were aligned with AEPs from other species using ClustalW.23 A phylogenetic tree was constructed with distance matrix using the neighbor-joining method with 1000 bootstrap samplings in Phylip software package version 3.6.3.
Reverse transcription PCR
Reverse transcription (RT)-PCR was used to evaluate developmental expression of Ov-aep-1 in life cycle stages of O. viverrini. cDNAs of adult, juvenile, egg, and metacercariae were prepared from fresh sample or samples stored in RNAlater (Ambion, Foster city, CA, USA) using Nucleospin® RNA II (Macherey-Nagel, Düren, Germany). RT-PCR was carried out using a Robust II RT-PCR kit (Finnzymes, Espoo, Finland). Ov-aep-1-specific primers (5′-GGGCAAGGTGTTCAATGACT for the forward primer and 5′-CAAGTGCCAATGATTGTGTC for the reverse primer) were designed to span nucleotides 286–720 (the active site His and Cys residues in the ORF) and amplify a 433-bp target. PCR was performed with Taq polymerase (Invitrogen, Carlsbad, CA, USA) with 35 cycles of 94 °C for 1 min, 55 °C for 1 min, 72 °C for 2 min, followed by a final extension at 72 °C for 10 min. A positive control targeting O. viverrini actin, a constitutively expressed housekeeping gene, and a negative control in which reverse transcriptase was substituted with water were included. PCR products were analyzed by 0.8% agarose gel electrophoresis.
Production and purification of recombinant Ov-AEP-1
The entire ORF of Ov-AEP-1 minus the predicted signal peptide was amplified by PCR using oligonucleotide primers: forward primer: 5′-ACGCGCGCTCGAGGCATGGTTAGGCGCTGTT; reverse primer: 5′-ACGGCGCCCTCGAGCTAGGAACAGACTTCATGAAC. Primers contained Xho I and Nde I sites (underlined), respectively, to facilitate ligation into the expression plasmid, pET-15b (Novagen, Madison, WI, USA). PCR products were gel purified (Qiagen, Hilden, Germany), ligated into pGEM-T (Promega, Madison, WI, USA), and the ligation products employed to transform Escherichia coli strain JM109 (Promega, Madison, WI, USA). Recombinant plasmids were purified using a kit (Qiagen, Hilden, Germany), after which they were digested with Xho I and Nde I. The excised fragments were separated through 1% agarose and purified by gel extraction. The inserts were then cloned into the Xho I and Nde I sites of pET-15b that had been linearized with these enzymes. The resulting plasmid was designated pOVAEP1. The insert sizes of plasmids were confirmed by restriction digestion and PCR using the T7 promoter primer and the gene-specific reverse primer. Plasmids were sequenced using the T7 primer to confirm their identity and in-frame fusion to the hexaHis-tag encoded by the pET15b vector.
The recombinant protein was expressed in E. coli strain BL21(DE3) (Novagen Madison, WI, USA). E. coli strain BL21(DE3) were transformed with pOVLGM1 by heat shock at 42 °C after which transformed cells were plated on LB agar supplemented with ampicillin (50 µg/ml) and incubated at 37 °C overnight. Single colonies were picked and cultured in 100 ml LB medium with ampicillin (50 µg/ml) at 37 °C until the OD600 reached 0.6. Recombinant protein expression was induced by addition of isopropyl-beta-d-thiogalactopyranoside (IPTG) to 1 mM final concentration for 3 h at 37 °C with shaking at 300 rpm. To purify the recombinant protein, cells were chilled on ice and collected by centrifugation at 5000 g for 15 min at 4 °C. Cells were then resuspended in 10 ml of binding buffer (5 mM imidazole, 0.5 M NaCl, 20 mM Tris–HCl, pH 7.9), and then lysed by freeze/thawing two times followed by sonication (25 amps, 5 s burst and 5 s rest, for 5 min) at 4 °C. The lysates were clarified by centrifugation as described above and supernatants collected. Lysed cells were also resuspended in 10 ml of binding buffer containing 8 M urea and sonicated again. The supernatants (both denatured and non-denatured) containing recombinant proteins were purified by affinity chromatography using His-Trap FF nickel columns (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) fitted to a liquid chromatography system (AKTA Prime, GE Healthcare, Piscataway, NJ, USA). The recombinant hexaHis-tagged proteins were eluted with a 10–50 mM imidazole gradient in binding buffer, with or without 8 M urea. Protein purity was evaluated at various stages of purification by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and staining of gels with Coomassie Brilliant Blue and/or Western blotting using an anti-hexaHis-Tag antibody (Invitrogen, Carlsbad, CA, USA).
Refolding of denatured Ov-AEP-1
Fractions containing hexaHis-tagged Ov-AEP-1 were pooled and processed for refolding. To obtain bioactive recombinant protein, the protein isolated in its denatured form was refolded by stepwise dialysis (1 M urea increments every 24 h) against phosphate buffered saline (PBS), pH 7.4. After removal of urea the refolded protein was concentrated (Eppendorf concentrator 5301) to approximately 0.3 mg/ml. The protein concentration was determined by absorbance at 280 nm and fractions were analyzed by SDS-PAGE.
Production and assessment of anti-Ov-AEP-1 rabbit antiserum and purification of IgG
Antiserum against affinity-purified Ov-AEP-1 was produced by immunization of a male New Zealand White rabbit. Before immunization, pre-immune serum was collected (10 ml) from blood taken from the ear vein. For the first injection, 1.0 mg of refolded recombinant Ov-AEP-1 was emulsified in an equal volume of Freund’s complete adjuvant and injected subcutaneously with equal volumes being administered to the buttock, neck, and fore leg. The immunization was repeated two more times at 14-day intervals using Freund’s incomplete adjuvant to emulsify the recombinant Ov-AEP-1. The rabbit was bled from the ear vein two weeks after the final immunization and the serum IgG titer was determined against purified recombinant Ov-AEP-1 protein by enzyme-linked immunosorbent assay (ELISA) using standard protocols.21 Western blotting was also performed to evaluate the recognition of recombinant Ov-AEP-1 and the native protein in parasite extracts from different developmental stages of O. viverrini. Parasite extracts were obtained as described.10,24 Recombinant protein was transferred to nitrocellulose membrane using a Mini Trans Blot electrode (Bio-Rad, Hercules, CA, USA). Membranes were blocked overnight at 4 °C or room temperature for 1 h in PBS containing 0.03% Tween 20 (PBS/T) and 5% non-fat milk powder in PBS/T (blocking buffer). Membranes were probed for 1 h with primary antibody (rabbit anti-Ov-AEP-1 serum diluted 1:100 in 2% non-fat milk powder in PBS/T) at room temperature. Horseradish peroxidase (HRP)-labeled goat anti-rabbit antibody (Invitrogen Carlsbad, CA, USA) diluted 1:2000 in antibody buffer was applied and the membrane was incubated for 1 h at room temperature. Following removal of the secondary antibody solution, the membrane was washed 3 × 10 min in PBS/T, then incubated in chemiluminescence substrate (ECL, Amersham Biosciences, USA). Signals were detected using X-ray film (Kodak, USA).
IgG from rabbit antiserum was affinity purified on protein A sepharoseTM CL-4B (Zymed, USA), eluted from the resin in glycine pH 3.0, and neutralized in Tris pH 8.0. Concentration of eluted IgG was determined spectrophotometrically at OD 280 nm.
Assessment of recombinant Ov-AEP-1 and Opisthorchis viverrini ES products for AEP activity
Refolded Ov-AEP-1 and ES products of adult O. viverrini (ES products prepared as described previously24) were analyzed for asparaginyl endopeptidase activity using the fluorogenic tripeptide substrate, Z-Ala–Ala–Asn-aminomethylcoumarin (Sigma-Aldrich, St. Louis, MO, USA).25 Release of aminomethylcoumarin from hydrolyzed substrate was measured in a fluorescence spectrometer (FLUOstar, BMG Labtech, Offenburg, Germany). The pH optima for cleavage of O. viverrini ES products and recombinant Ov-AEP-1 were determined by incubating the proteins with substrate at 37 °C for up to 12 h in buffers ranging from pH 3.0 to 8.0 with increments of 0.5 pH units (0.1 M sodium acetate, pH 3.0–6.0; 0.1 M sodium phosphate, pH 6.5–7.5; 0.1 M Tris–HCl, pH 8.0). The final substrate concentration was maintained at 5.0 µM. Catalytic activity was expressed as relative fluorescence units. Iodoacetamide at 5 mM final concentration was included in some reactions as an inhibitor of AEP activity.26
Immunolocalization
Adult O. viverrini recovered from infected hamsters, as well as adult worms in situ in hamster bile ducts, were mounted in paraffin (Sakura Tissue-Tek® TEC™) and sectioned as described.9 The sections were deparaffinized in xylene three times for 5 min each. After deparaffinization, slides were dehydrated in decreasing concentrations of ethanol; absolute ethanol three times, 5 min each, 95% ethanol two times, 3 min each, 70% ethanol one time, 3 min, and a final rinse with tap water for 1–2 min. Slides were then washed in PBS twice, 5 min each, then rinsed with tap water for 5 min. Slides were exposed to 30% H2O2 in methanol for 30 min to block non-specific endogenous peroxidase activity. Sections were then blocked for non-specific binding with normal horse serum diluted 1:20 in PBS/NaN3 for 30 min at room temperature in a humid chamber. The parasite sections were probed with anti-Ov-AEP-1 IgG or preimmunization IgG from the same rabbit at a dilution of 1:100 in PBS/NaN3 in a humid chamber at 4 °C overnight. Sections were then incubated in secondary antibody (HRP-goat anti-rabbit IgG) diluted 1:200 in PBS for 1 h at room temperature. To develop color, slides were submerged in freshly prepared DAB solution for 5 min, after which the reaction was stopped with tap water and slides counterstained with Mayer hematoxylin for 1 min. The slides were washed with tap water and dehydrated as follows: 70% alcohol once for 3 min; 95% alcohol twice, 3 min each; final dehydration in absolute alcohol three times, 3 min each. Slides were then examined and photographed with the aid of 10× and 20× objectives fitted to an Olympus model BX40 compound microscope connected with a digital camera (Nikon DXA1200C).
Serologic recognition of Ov-AEP-1 by sera from human opisthorchiasis cases
An immunoblot assay was developed to identify anti-Ov-AEP-1 serum antibodies in patients with active O. viverrini infection. Recombinant Ov-AEP-1 was resuspended in denaturing/reducing sample buffer, boiled for 5 min, and electrophoresed on a 15% SDS-PAGE gel. The proteins were transblotted onto nitrocellulose membrane by a semi-dry system (Bio-Rad). The membrane was then cut into strips, each strip containing 4 µg recombinant fusion protein. The strips were washed with PBST for 5 min then blocked for 1 h with blocking buffer. Strips were then incubated with sera from patients infected with diverse helminth parasites, including Taenia spp tapeworms, minute intestinal flukes (lecithodendrids), hookworm, Echinostoma spp, and O. viverrini. These human sera were collected individually from several areas of Northeast Thailand according to an approved protocol (Khon Kaen University Institutional Review Board No. HE500119). Positive infection was diagnosed based on detection of eggs from fecal examinations using the formalin–ethyl acetate concentration technique. Sera from four individuals from an urban area of the province of Khon Kaen (and one pooled sample) who were determined to be free of helminth infections after fecal examination, and were negative for antibodies to O. viverrini ES antigens as described,21,24 were used as negative controls. All human sera were diluted 1:50 in 2% skim milk in PBST buffer and incubated at room temperature for 2 h with shaking. Strips were washed with PBST five times for 5 min each followed by incubation for 2 h with HRP-goat anti-human IgG diluted 1:1000 in antibody buffer. The strips were washed again with PBST five times for 5 min each and color reactions were developed by the addition of 3,3′-diaminobenzidine (DAB) substrate.
Results
At least two mRNAs encoding asparaginyl endopeptidases are present in the Opisthorchis viverrini transcriptome
The full-length mRNA sequence encoding an AEP was isolated by immunoscreening an O. viverrini cDNA library with a pool of sera from patients with both O. viverrini infection and cholangiocarcinoma. The original clone isolated from the library was designated Ov-aep-1. The Ov-aep-1 cDNA comprised 1302 bp, encoding a deduced protein of 408 amino acids with predicted molecular mass of 47 kDa. The sequence of Ov-aep-1 has been assigned GenBank accession number DQ402101. For recombinant protein expression, specific primers flanking the entire ORF (minus signal peptide) were designed to amplify the ORF of Ov-aep-1 by PCR using the cDNA library as template. The resulting amplicon was subsequently cloned into pET15b. During this sub-cloning stage, a second (larger) amplicon was generated by PCR. The ORF of this second cDNA was 95% identical to Ov-AEP-1, with most of the difference being due to a unique insertion of 25 amino acids near the C-terminus. This clone was designated Ov-aep-2. Ov-AEP-1 had a predicted signal peptide with a cleavage site between residues 17 and 18 (Figure 1). We did not obtain the full-length 5′ sequence of Ov-aep-2 so the presence of a signal peptide in the ORF was not confirmed. Both Ov-AEP-1 and Ov-AEP-2 exhibited the conserved residues of AEPs (Figure 1). Two potential N-linked glycosylation sites (NXS/T) were detected in both Ov-AEP-1 and Ov-AEP-2 at Asn-68 and Asn-173 (AEP-1 numbering scheme) (Figure 1).
Figure 1.
Multiple sequence alignment of Ov-AEP-1 with other members of the clan CD, C13 peptidase family. Identical residues in more than 50% of sequences are in black boxes; conserved substitutions are in gray boxes. The conserved active site histidine and cysteine residues are highlighted. The putative N-glycosylation sites are shown on the top of the alignments.
Opisthorchis viverrini asparaginyl endopeptidase closely related to asparaginyl endopeptidase from platyhelminths
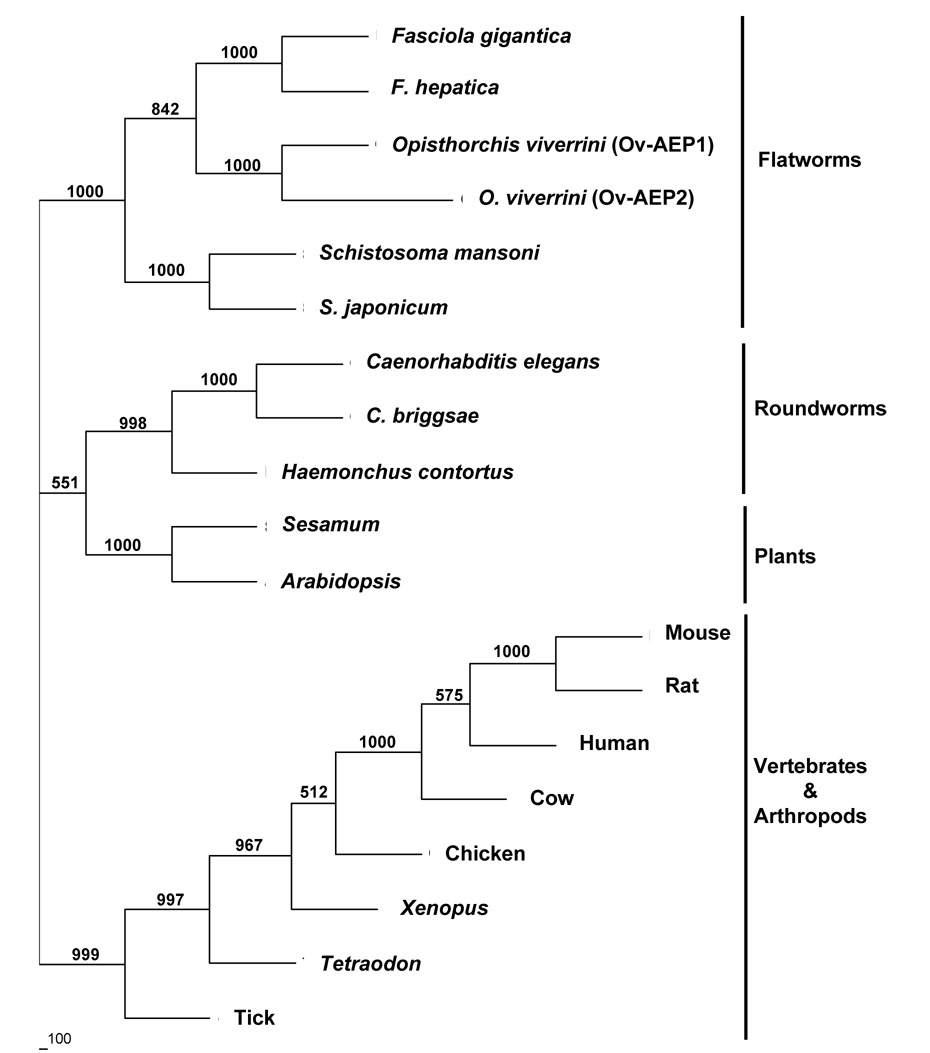
The amino acid sequence of Ov-AEP-1 was compared to AEPs from other eukaryotes including platyhelminths, nematodes, plants, and vertebrates. Multiple sequence alignments used to construct the phylogenetic tree revealed complete conservation of the active site His and Cys residues through these divergent species. The neighbor-joining phylogenetic tree showed that Ov-AEP-1 formed a highly robust clade (100% bootstrap support) with AEPs from other flukes, particularly the closely related Fasciola and Schistosoma species (Figure 2). Other members of the C13 family formed clades mostly on a taxonomic basis; distinct and strongly supported clades were formed by nematodes, plants, and vertebrates. One exception was AEP from the tick, Ixodes ricinus (GenBank accession number AAS94231) (Arthropoda), which formed a very robust clade (99.9% bootstrap support) with vertebrate AEPs.
Figure 2.
Neighbor-joining phylogenetic tree showing the evolutionary relationship of Ov-AEP-1 with other C13 peptidases from animals and flowering plants. Numbers on branches refer to bootstrap values; the scale bar at the bottom represents 100 changes. The accession numbers of legumain sequences used in the phylogenetic analysis are as follows: ABD64147 (Opisthorchis viverrini), ABQ02437 (Fasciola gigantica), CAC85636 (Fasciola hepatica), P09841 (Schistosoma mansoni), P42665 (Schistosoma japonicum), CAB01126 (Caenorhabditis elegans), CAE75506 (Caenorhabditis briggsae), CAJ45481 (Haemonchus contortus), AAF89679 (Sesamum), AAM60827 (Arabidopsis), CAA04439 (mouse), AAH87708 (rat), CAG33687 (human), AAI11118 (cow), XP_421328 (chicken), AAH56842 (Xenopus), CAG13252 (Tetraodon), and AAS94231 (Ixodes ricinus; tick).
Ov-aep-1 mRNA is expressed in all developmental stages of the liver fluke
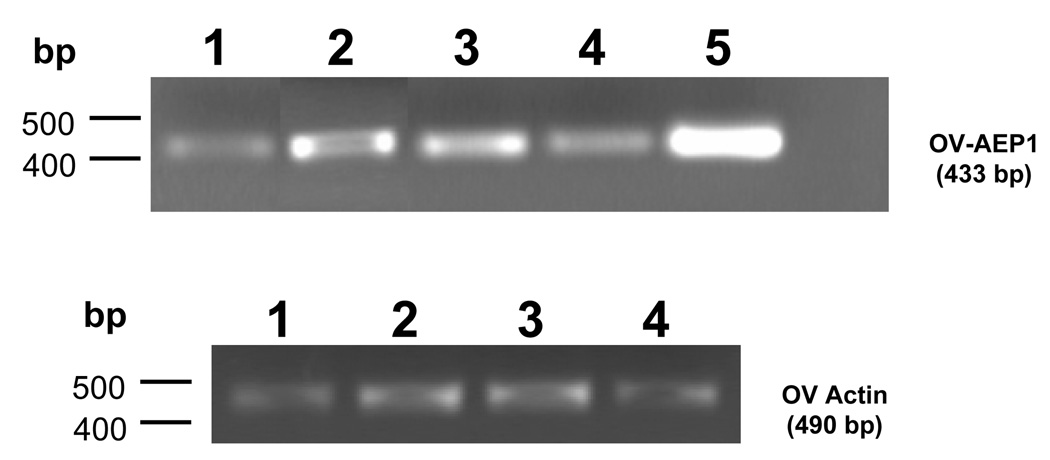
The expression pattern of the Ov-aep-1 mRNA in developmental stages of O. viverrini was investigated by RT-PCR. Amplicons were detected in RNA from egg, metacercaria, juvenile worm, and adult worm (Figure 3A). The actin mRNA was constitutively expressed in all these stages, constituting a control for cDNA integrity and PCR fidelity (Figure 3B). No amplicons were detected in the negative controls where water replaced template DNA or where water was substituted for reverse transcriptase.
Figure 3.
Expression of Ov-aep-1 in developmental stages of O. viverrini, as determined by RT-PCR. The following cDNA templates were included: lane 1, egg; lane 2, metacercaria; lane 3, juvenile worm; lane 4, adult worm; lane 5, adult worm cDNA library. The lower panel shows expression of the actin mRNA in the developmental stages, included here as a constitutively expressed control transcript.
Recombinant Ov-AEP-1 recovered from inclusion bodies
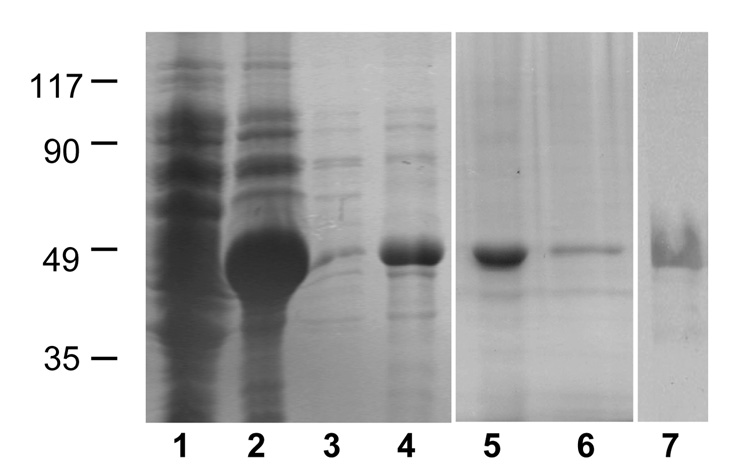
The cDNA encoding the ORF of Ov-aep-1 was amplified by PCR from the O. viverrini library, cloned into bacterial expression plasmid pET-15b, and its identity was confirmed by sequencing. Recombinant Ov-AEP-1 (rAEP-1) was produced in E. coli BL21(DE3) as a crystalline fusion protein that was dissolved in denatured form in 6 M urea in order to recover the protease (Figure 4). The expressed enzyme migrated as a single band in SDS-PAGE of 47 kDa, in agreement with theoretical mass predictions. The urea-denatured rAEP-1 was refolded into PBS to yield a soluble product with approximately 0.3 mg/ml (10% recovery). The purified denatured and refolded recombinant proteins were both recognized on immunoblots by anti-hexaHis monoclonal antibody (Figure 4), confirming their identities as Ov-AEP-1.
Figure 4.
Affinity purification of recombinant Ov-AEP-1 expressed in Escherichia coli as assessed by Coomassie Blue stained SDS-PAGE gel. Lane 1, uninduced E. coli cell pellet; lane 2, IPTG-induced cell pellet; lane 3, supernatant from pellet solubilized in binding buffer; lane 4, supernatant from pellet solubilized in 8 M urea in binding buffer; lane 5, affinity-purified Ov-AEP-1 in 6 M urea; lane 6, equal loading of refolded purified recombinant Ov-AEP-1 in PBS buffer; lane 7, immunoblot of recombinant Ov-AEP-1 protein probed with anti-hexaHis-tag antibody.
Ov-AEP-1 is secreted by adult Opisthorchis viverrini liver flukes
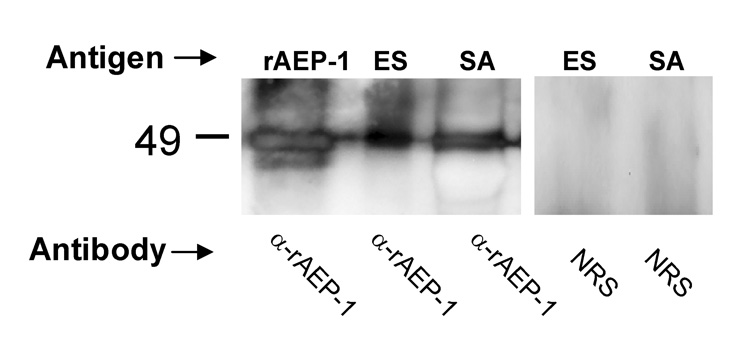
IgG purified from serum from a rabbit immunized with rAEP-1 recognized the recombinant immunogen in Western blot analysis. By contrast, control IgG from the preimmunization rabbit serum did not bind to rAEP-1 (not shown). Anti-rAEP-1 IgG also recognized an antigen of the expected size, ~47 kDa, in soluble parasite extracts and in ES products of adult O. viverrini; the control IgG did not bind to proteins of this size (Figure 5).
Figure 5.
Western blot of recombinant Ov-AEP-1 (rAEP-1), crude somatic (SA), and excretory/secretory (ES) antigens of adult Opisthorchis viverrini probed with purified IgG from the serum of a rabbit immunized with rAEP-1 (α-rAEP-1) or normal rabbit serum (NRS).
Detection of AEP catalytic activity in refolded recombinant Ov-AEP-1 and Opisthorchis viverrini ES products
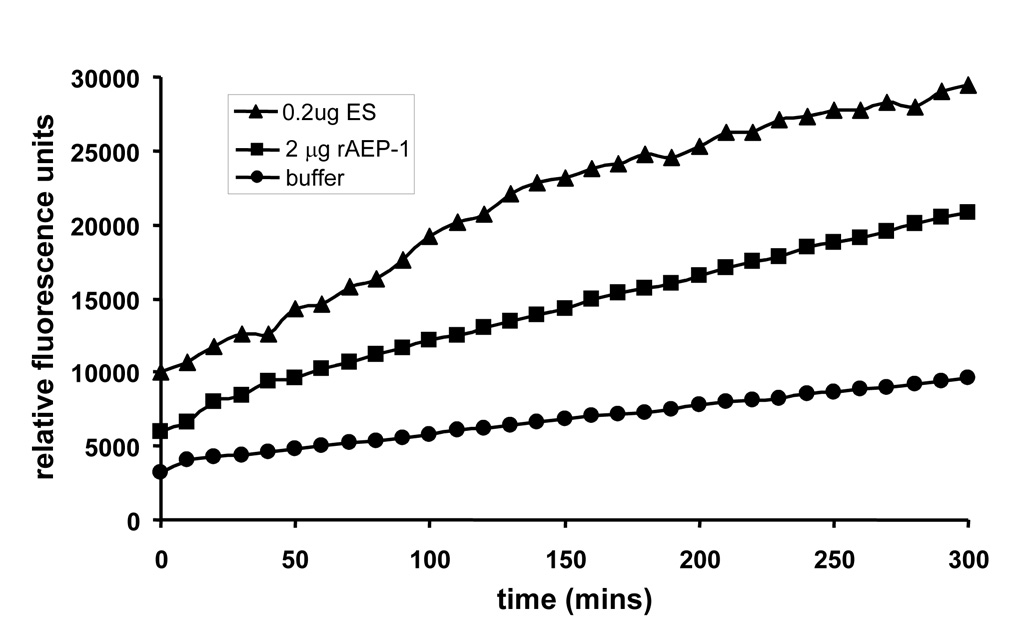
Catalytic activity against the diagnostic substrate Z-Ala–Ala–Asn-aminomethylcoumarin was detected in 0.2 µg of O. viverrini ES products with an optimal pH of 7.0 (Figure 6). Activity was not detected below pH 6.0 (not shown). The activity was completely inhibited in the presence of 5 mM iodoacetamide (not shown). Cleavage of Z-Ala–Ala–Asn-aminomethylcoumarin was also detected with 2.0 µg of rAEP-1 (Figure 6), and this was inhibited completely by iodoacetamide (not shown). However, the specific activity of rAEP-1 was lower than that detected for ES products. Ten-fold less total protein was added to the reaction with ES products (compared to rAEP-1), and moreover, Ov-AEP-1 is only one of many proteins in ES products (J. Mulvenna and A. Loukas, unpublished), indicating that the majority of resolubilized rAEP-1 had not adopted proper conformation, and therefore, only a small proportion of this preparation displayed catalytic activity. Alternatively, all of the refolded material had adopted the same conformation but this was not the same as that of the native protein in ES products and resulted in reduced proteolytic activity.
Figure 6.
Hydrolysis of the fluorogenic peptide Z-Ala–Ala–Asn-aminomethylcoumarin by Opisthorchis viverrini excretory–secretory (ES) products and recombinant Ov-AEP-1 as measured by an increase in relative fluorescence due to release of Z-Ala–Ala–Asn-aminomethylcoumarin over time. The assay was performed at pH 7. 0. Inclusion of the inhibitor iodoacetamide at 5 mM completely inhibited the activity of Ov-AEP-1 (not shown).
Asparaginyl endopeptidase expressed in the parasite gut and in eggs
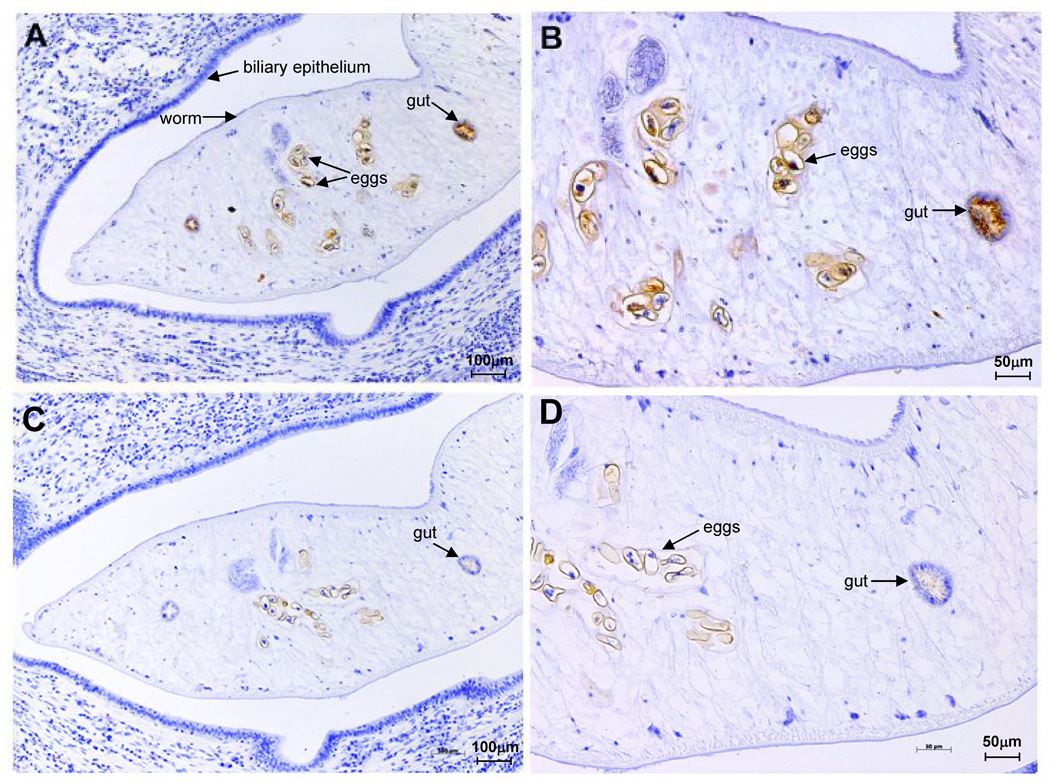
The anatomical site of Ov-AEP-1 expression within sections of adult O. viverrini was investigated by immunolocalization with purified anti-rAEP-1 IgG. Intense staining was observed in the gut of the adult fluke and also in eggs within the uterus of the parasite (Figure 7, A and B). Control sections probed with pre-immunization IgG showed negligible staining (Figure 7, C and D).
Figure 7.
Immunohistochemical localization of legumain in Opisthorchis viverrini adult worms. Anti-Ov-AEP-1 rabbit IgG bound strongly to the gut (panel A) and eggs in the uterus (panel B). Control IgG from the same rabbit prior to immunization did not bind to the same structures (panels C and D). Scale bar are shown.
Ov-AEP-1 may be serodiagnostic for human opisthorchiasis
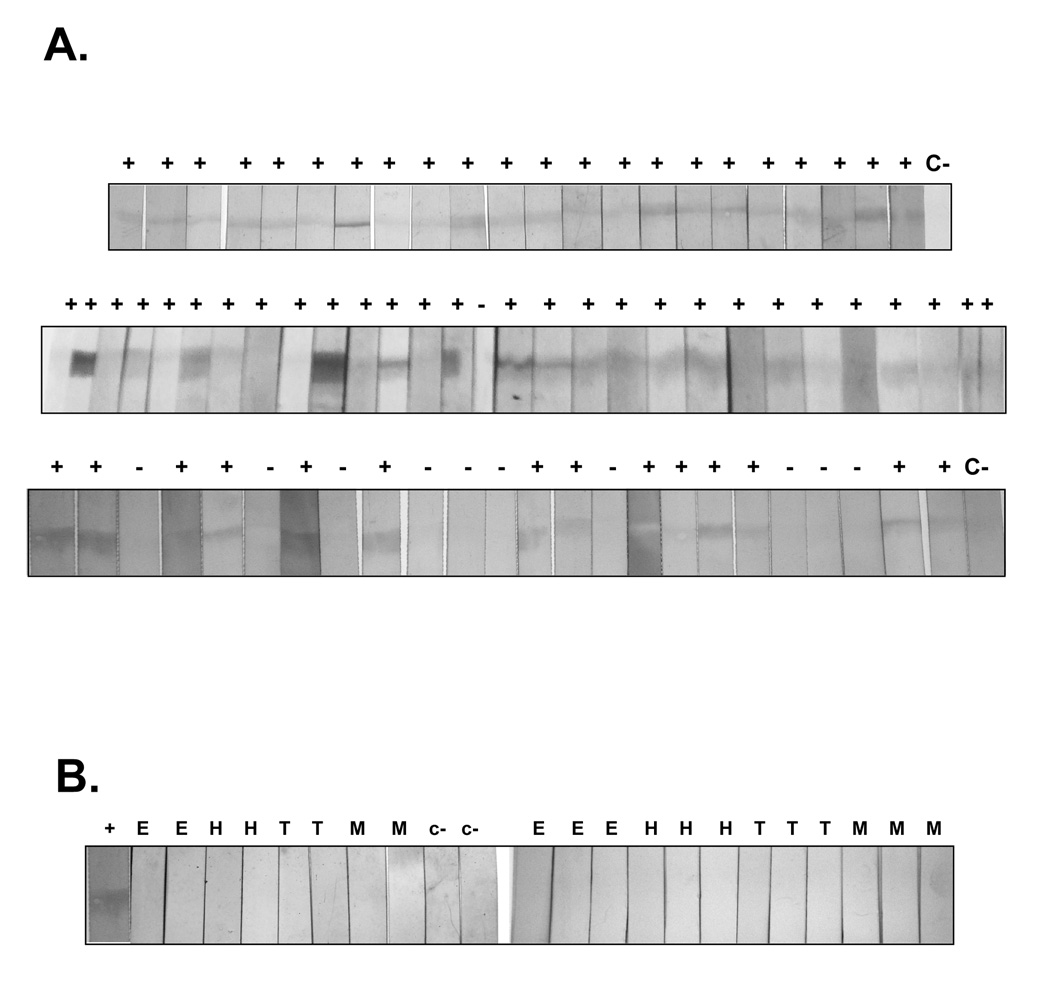
An immunoblot assay was employed to investigate the potential of rAEP-1 for serodiagnosis of human opisthorchiasis. The purified recombinant protein of 47 kDa was recognized by sera from 64 of 75 persons with parasitologically proven O. viverrini infection, i.e., stool positive for O. viverrini eggs (Figure 8A). By contrast, sera from persons confirmed to be stool-negative for O. viverrini eggs but positive for other gastrointestinal helminths (hookworm, minute intestinal fluke, Echinostoma, Taenia) and non-parasitized individuals did not recognize rAEP-1 (Figure 8B). The sensitivity and the specificity of the test therefore were 85% and 100%, respectively. The positive predictive value was 100% and the negative predictive value was 67%.
Figure 8.
Immunoblot analysis of recognition of recombinant Ov-AEP-1 by sera from people with parasitologically proven infection with Opisthorchis viverrini (A). ‘+’ and ‘−’ indicate positive or negative scores, respectively, for the presence of a band at 47 kDa representing recognition of the recombinant antigen. C represents sera pooled from several uninfected control donors. Sera from patients with other parasitic infections did not recognize recombinant Ov-AEP-1 (B). H = hookworm infection; E = echinostomiasis; T = Taenia infection; M = minute intestinal fluke infection.
Discussion
A full-length mRNA transcript encoding Ov-AEP-1 was isolated from adult O. viverrini liver flukes and shown to encode a functionally active legumain-like protease. Furthermore, the recombinant enzyme was recognized by serum antibodies from people infected with this carcinogenic liver fluke. Legumains, or asparaginyl endopeptidases (AEPs), are cysteine proteases of MEROPS clan CD, Family C13, that hydrolyze peptides and proteins on the carboxyl side of asparagine residues.27 They are often found in lysosomes of diverse cell types and are involved in a variety of processing functions, including the trans-processing of other proteins.28 In S. mansoni, SmAE (or Sm32 as it was previously known) was initially thought to be a hemoglobinase but was later shown not to possess hemoglobinolytic activity,29 and instead was a member of the legumain family of proteases, which was proposed to trans-activate other proteins in the schistosome gut.12,30 Sajid and colleagues subsequently demonstrated that SmAE was responsible for processing the S. mansoni cathepsin B hemoglobinase, SmCB1, in the gut of the parasite.17 A comprehensive assessment of the hemoglobinase pathways in S. mansoni using RNA interference and chemical inhibitors of different classes of protease found that silencing of the SmAE mRNA had no effect on hemoglobin digestion, although there was an effect on digestion of serum albumin.31 The authors suggested that this might be due to either an effect on trans-processing of hemoglobinases, or the generation of rare cleavages (at Asn residues) within albumin, thereby further facilitating its subsequent digestion by other proteases.31 Recently it has been shown that SmAE is not essential for cathepsin B1 activation in vivo.18
Two AEPs were recently reported from the giant liver fluke, Fasciola gigantica, and like the schistosome orthologue, both were expressed in the intestinal epithelium but neither was detected in ES products of adult flukes.15 Like these Fasciola AEPs, Ov-aep-1 was expressed in multiple developmental stages of O. viverrini, including egg, metacercaria, juvenile, and adult stages. Due to difficulty in obtaining sufficient tissues, we did not assess Ov-aep-1 expression in miracidia and cercaria stages. Unlike the Fasciola AEPs, however, Ov-AEP-1 was detected in ES products of adult worms, using both specific antibodies and enzymatic techniques. Moreover, using a proteomics approach, Ov-AEP-1 is a component of the ES products of adult O. viverrini (J. Mulvenna and A. Loukas, unpublished). Therefore, most, if not all stages of O. viverrini express the Ov-aep-1 mRNA, suggesting it plays essential roles in processing fluke proteins throughout development. Homologues of schistosome hemoglobinases including cathepsins B and D have been identified in O. viverrini,20 and we aim to assess the ability of Ov-AEP-1 to process these other Opisthorchis proteases in trans in future studies.
Ov-AEP-1 was detected in the digestive tract of adult O. viverrini, accounting for its presence in ES products. SmAE is expressed in the gastrodermal epithelial cells of S. mansoni where erythrocytes are lysed and hemoglobinases act to digest hemoglobin for nutrient acquisition.32 In the human lung fluke, Paragonimus westermani, legumain is expressed in the intestinal epithelium of the adult worm.33 Parasitic helminths other than trematodes also express AEPs in their intestines; the blood-feeding nematode that parasitizes sheep, H. contortus, expresses a legumain on the microvillar surface of the intestinal cells of adult worms,16 co-localizing with the sites of expression of other proteinases involved in blood-feeding.34 Immunolocalization also showed that Ov-AEP-1 was detected in the eggs of O. viverrini, supporting the expression of Ov-aep-1 mRNA in this stage of the life cycle.
Like SmAE, we predict that Ov-AEP-1 is involved in trans-processing other fluke proteins, particularly the gut proteases involved in feeding. We did not detect hemoglobinolytic activity with recombinant Ov-AEP-1 (not shown), despite detecting catalytic activity against the Z-Ala–Ala–Asn-aminomethylcoumarin peptide at neutral pH. Moreover, we did detect potent hemoglobinolytic activity in adult worm extracts at acidic pH values but not at neutral pH (not shown) where Ov-AEP-1 is catalytically active. This is in marked contrast to the ‘hemoglobinase’ sequence from the lung fluke, P. westermani, where the recombinant protein has been reported to digest hemoglobin at acid pH (and not neutral pH).33 Moreover, the P. westermani AEP is apparently sensitive to the inhibitor of C1 family cysteine proteases, E64, while other AEPs are generally not affected by this inhibitor, responding mainly to inhibitors that complex with free-thiols (http://merops.sanger.ac.uk/index.htm).
Immunodiagnostic methods for opisthorchiasis have been developed based on several antigens. A monoclonal antibody specific for an unidentified 89-kDa ES antigen was employed to detect O. viverrini antigen in the feces of infected people, with 57% sensitivity and 68% specificity.35 Also, a recombinant egg shell protein of O. viverrini has been assessed as a diagnostic antigen by enzyme-linked immunoassay, and provides 48–82% sensitivity and 91–97% specificity; however, cross-reactivity with schistosomiasis and fascioliasis occurred with this antigen.36 In comparison, using rAEP-1 we have demonstrated higher sensitivity (85%) and specificity (100%) than these earlier reports, indicating the potential of this antigen for development of a serodiagnostic test for human opisthorchiasis. Furthermore, detection of Ov-AEP-1 in the ES and transcripts of Ov-aep-1 in developmental stages including eggs, metacercariae, and juveniles indicates that it may be feasible to develop a coproantibody diagnostic assay for early and current O. viverrini infection. The potential of antibody- and antigen-based detection methods for opisthorchiasis-associated cholangiocarcinoma also has been established.37,38 In like fashion, if O. viverrini AEP-1 could be developed as a serodiagnostic tool, it would enhance detection of O. viverrini-associated cancer and could contribute to measures aimed at reducing the morbidity and mortality associated with this infection.
Given a putative role for Ov-AEP-1 in trans-processing of digestive proteases in the gut of O. viverrini, it presents as a target for vaccines and drugs against this parasite. By inhibiting the activity of this enzyme, one might predict that a number of downstream pathways dependent on AEP-mediated processing would be affected, particularly digestion of ingested host proteins. Proteases involved in blood-feeding show promise as recombinant vaccines for a number of parasitic helminths,39–42 highlighting the potential of Ov-AEP-1 and other Opisthorchis proteases as intervention targets for treatment, control, and/or diagnosis.20,43
Acknowledgements
This work was supported by Thailand Research Fund grant No. MRG4880045, NIH-NIAID International Collaboration in Infectious Diseases Research award No. AI065871, the Sandler Family Foundation and Faculty of Medicine KKU (grant No. i50206). AL is supported by a senior research fellowship from the National Health and Medical Research Council (Australia).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflict of interest to declare.
References
- 1.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harinasuta T, Riganti M, Bunnag D. Opisthorchis viverrini infection: pathogenesis and clinical features. Arzneimittelforschung. 1984;34:1167–1169. [PubMed] [Google Scholar]
- 3.Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38:4634–4639. [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer/World Health Organization. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC; Schistosomes, liver flukes and Helicobacter pylori. 1994;Vol. 61 [PMC free article] [PubMed]
- 5.Vatanasapt V, Sripa B, Sithithaworn P, Mairiang P. Liver flukes and liver cancer. 1999;33:313–343. [Google Scholar]
- 6.Haswell-Elkins MR, Sithithaworn P, Elkins D. Opisthorchis viverrini and cholangiocarcinoma in Northeast Thailand. Parasitol Today. 1992;8:86–89. doi: 10.1016/0169-4758(92)90241-s. [DOI] [PubMed] [Google Scholar]
- 7.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 8.Sripa B, Kaewkes S. Gall bladder and extrahepatic bile duct changes in Opisthorchis viverrini-infected hamsters. Acta Trop. 2002;83:29–36. doi: 10.1016/s0001-706x(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 9.Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30:735–740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 10.Wongratanacheewin S, Bunnag D, Vaeusorn N, Sirisinha S. Characterization of humoral immune response in the serum and bile of patients with opisthorchiasis and its application in immunodiagnosis. Am J Trop Med Hyg. 1988;38:356–362. doi: 10.4269/ajtmh.1988.38.356. [DOI] [PubMed] [Google Scholar]
- 11.McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in parasitic diseases. Annu Rev Pathol. 2006;1:497–536. doi: 10.1146/annurev.pathol.1.110304.100151. [DOI] [PubMed] [Google Scholar]
- 12.Dalton JP, Hola-Jamriska L, Brindley PJ. Asparaginyl endopeptidase activity in adult Schistosoma mansoni. Parasitology. 1995;111:575–580. doi: 10.1017/s0031182000077052. [DOI] [PubMed] [Google Scholar]
- 13.Caffrey CR, Mathieu MA, Gaffney AM, Salter JP, Sajid M, Lucas KD, et al. Identification of a cDNA encoding an active asparaginyl endopeptidase of Schistosoma mansoni and its expression in Pichia pastoris. FEBS Lett. 2000;466:244–248. doi: 10.1016/s0014-5793(99)01798-6. [DOI] [PubMed] [Google Scholar]
- 14.Tkalcevic J, Ashman K, Meeusen E. Fasciola hepatica: rapid identification of newly excysted juvenile proteins. Biochem Biophys Res Commun. 1995;213:169–174. doi: 10.1006/bbrc.1995.2112. [DOI] [PubMed] [Google Scholar]
- 15.Adisakwattana P, Viyanant V, Chaicumpa W, Vichasri-Grams S, Hofmann A, Korge G, et al. Comparative molecular analysis of two asparaginyl endopeptidases and encoding genes from Fasciola gigantica. Mol Biochem Parasitol. 2007;156:102–116. doi: 10.1016/j.molbiopara.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Oliver EM, Skuce PJ, McNair CM, Knox DP. Identification and characterization of an asparaginyl proteinase (legumain) from the parasitic nematode, Haemonchus contortus. Parasitology. 2006;133:237–244. doi: 10.1017/S0031182006000229. [DOI] [PubMed] [Google Scholar]
- 17.Sajid M, McKerrow JH, Hansell E, Mathieu MA, Lucas KD, Hsieh I, et al. Functional expression and characterization of Schistosoma mansoni cathepsin B and its trans-activation by an endogenous asparaginyl endopeptidase. Mol Biochem Parasitol. 2003;131:65–75. doi: 10.1016/s0166-6851(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 18.Skelly PJ, Krautz-Peterson G. Schistosome asparaginyl endopeptidase (legumain) is not essential for cathepsin B1 activation in vivo. Mol Biochem Parasitol. 2008;159:54–58. doi: 10.1016/j.molbiopara.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinkert MQ, Felleisen R, Link G, Ruppel A, Beck E. Primary structures of Sm31/32 diagnostic proteins of Schistosoma mansoni and their identification as proteases. Mol Biochem Parasitol. 1989;33:113–122. doi: 10.1016/0166-6851(89)90025-x. [DOI] [PubMed] [Google Scholar]
- 20.Laha T, Pinlaor P, Mulvenna J, Sripa B, Sripa M, Smout MJ, et al. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:e189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117:854–860. doi: 10.1002/ijc.21146. Erratum: Int J Cancer 2005;117:1066. [DOI] [PubMed] [Google Scholar]
- 22.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:1167–1169. [Google Scholar]
- 23.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positionspecific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunol. 2000;22:139–145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 25.Hara-Nishimura I. Plant legumain, asparaginyl endopeptidase. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of proteolytic enzymes. London: Elsevier; 2004. pp. 1310–1313. [Google Scholar]
- 26.Brindley PJ, Dalton JP. Schistosome legumain. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of proteolytic enzymes. Elsevier; 2004. pp. 1305–1310. [Google Scholar]
- 27.Chen JM, Rawlings ND, Stevens RA, Barrett AJ. Identification of the active site of legumain links it to caspases, clostripain and gingipains in a new clan of cysteine endopeptidases. FEBS Letters. 1998;441:361–365. doi: 10.1016/s0014-5793(98)01574-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen JM, Dando PM, Barrett AJ. Mammalian legumain. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of proteolytic enzymes. London: Elsevier; 2004. pp. 1302–1305. [Google Scholar]
- 29.Götz B, Klinkert MQ. Expression and partial characterization of a cathepsin B-like enzyme (Sm31) and a proposed ‘haemoglobinase’ (Sm32) from Schistosoma mansoni. Biochem J. 1993;290:801–806. doi: 10.1042/bj2900801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalton JP, Brindley PJ. Schistosome asparaginyl endopeptidase SM32 inhemoglobin digestion. Parasitol Today. 1996;12:125. doi: 10.1016/0169-4758(96)80676-4. [DOI] [PubMed] [Google Scholar]
- 31.Delcroix M, Sajid M, Caffrey CR, Lim KC, Dvorák J, Hsieh I, et al. A multienzyme network functions in intestinal protein digestion by a platyhelminth parasite. J Biol Chem. 2006;281(39):316–329. doi: 10.1074/jbc.M607128200. [DOI] [PubMed] [Google Scholar]
- 32.Sajid M, McKerrow JH. Cysteine proteases of parasitic organisms. Mol Biochem Parasitol. 2002;120:1–21. doi: 10.1016/s0166-6851(01)00438-8. Erratum: Mol Biochem Parasitol 2002;121:159. [DOI] [PubMed] [Google Scholar]
- 33.Choi JH, Lee JH, Yu HS, Jeong HJ, Kim J, Hong YC, et al. Molecular and biochemical characterization of hemoglobinase, a cysteine proteinase, in Paragonimus westermani. Korean J Parasitol. 2006;44:187–196. doi: 10.3347/kjp.2006.44.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skuce PJ, Redmond DL, Liddell S, Stewart EM, Newlands GF, Smith WD, et al. Molecular cloning and characterization of gut-derived cysteine proteinases associated with a host protective extract from Haemonchus contortus. Parasitology. 1999;119:405–412. doi: 10.1017/s0031182099004813. [DOI] [PubMed] [Google Scholar]
- 35.Sirisinha S, Chawengkirttikul R, Haswell-Elkins MR, Elkins DB, Kaewkes S, Sithithaworn P. Evaluation of a monoclonal antibody-based enzyme linked immunosorbent assay for the diagnosis of Opisthorchis viverrini infection in an endemic area. Am J Trop Med Hyg. 1995;52:521–524. doi: 10.4269/ajtmh.1995.52.521. [DOI] [PubMed] [Google Scholar]
- 36.Ruangsittichai J, Viyanant V, Vichasri-Grams S, Sobhon P, Tesana S, Upatham ES, et al. Opisthorchis viverrini: identification of a glycine-tyrosine rich eggshell protein and its potential as a diagnostic tool for human opisthorchiasis. Int J Parasitol. 2006;36:1329–1339. doi: 10.1016/j.ijpara.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Sakolvaree Y, Ybanez L, Chaicumpa W. Parasites elicited cross-reacting antibodies to Opisthorchis viverrini. Asian Pac J Allergy Immunol. 1997;15:115–122. [PubMed] [Google Scholar]
- 38.Akai PS, Pungpak S, Chaicumpa W, Viroj K, Bunnag D, Befus AD. Serum antibody response to Opisthorchis viverrini antigen as a marker for opisthorchiasis-associated cholangiocarcinoma. Trans R Soc Trop Med Hyg. 1994;88:471–474. doi: 10.1016/0035-9203(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 39.Verity CK, McManus DP, Brindley PJ. Vaccine efficacy of recombinant cathepsin D aspartic protease from Schistosoma japonicum. Parasite Immunol. 2001;23:153–162. doi: 10.1046/j.1365-3024.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- 40.Loukas A, Bethony J, Brooker S, Hotez P. Hookworm vaccines: past, present, and future. Lancet Infect Dis. 2006;6:733–741. doi: 10.1016/S1473-3099(06)70630-2. [DOI] [PubMed] [Google Scholar]
- 41.Dalton JP, Neill SO, Stack C, Collins P, Walshe A, Sekiya M, et al. Fasciola hepatica cathepsin L-like proteases: biology, function, and potential in the development of first generation liver fluke vaccines. Int J Parasitol. 2003;33:1173–1181. doi: 10.1016/s0020-7519(03)00171-1. [DOI] [PubMed] [Google Scholar]
- 42.Abdulla MH, Lim KC, Sajid M, McKerrow JH, Caffrey CR. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007;4:e14. doi: 10.1371/journal.pmed.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaewpitoon N, Laha T, Kaewkes S, Yongvanit P, Brindley PJ, Loukas A, et al. Characterization of cysteine proteases from the carcinogenic liver fluke, Opisthorchis viverrini. Parasitol Res. 2008;102:757–764. doi: 10.1007/s00436-007-0831-1. [DOI] [PubMed] [Google Scholar]