Abstract
Background
Hox transcription factors modulate signaling pathways controlling organ morphogenesis and maintain cell fate and differentiation in adults. Retinoid signaling, key in regulating Hox expression, is altered in pulmonary hypoplasia. Information on pattern-specific expression of Hox proteins in normal lung development and in pulmonary hypoplasia is minimal. Our objective was to determine how pulmonary hypoplasia alters temporal, spatial and cellular expression of Hoxa5, Hoxb4 and Hoxb6 proteins compared to normal lung development.
Methods
Temporal, spatial and cellular Hoxa5, Hoxb4 and Hoxb6 expression was studied in normal (untreated) and nitrofen-induced hypoplastic (NT-PH) lungs from gestational day 13.5, 16, 19 fetuses and neonates using western blot and immunohistochemistry.
Results
Modification of protein levels and spatial and cellular Hox expression patterns in NT-PH lungs was consistent with delayed lung development. Distinct protein isoforms were detected for each Hox protein. Expression levels of the Hoxa5 and Hoxb6 isoforms changed with development and further in NT-PH lungs. Compared to normal lungs, Gd19 and neonatal NT-PH lungs had decreased Hoxb6 and increased Hoxa5 and Hoxb4. Hoxa5 cellular localization changed from mesenchyme to epithelia earlier in normal lungs. Hoxb4 was expressed in mesenchyme and epithelial cells throughout development. Hoxb6 remained mainly in mesenchymal cells around distal airways.
Conclusions
Unique spatial and cellular expression of Hoxa5, Hoxb4 and Hoxb6 participates in branching morphogenesis and terminal sac formation. Altered Hox protein temporal and cellular balance of expression either contributes to pulmonary hypoplasia or functions as a compensatory mechanism attempting to correct abnormal lung development and maturation in this condition.
Keywords: Genes, homeobox, lung/growth and development, hypoplasia, pulmonary, respiratory system abnormalities, lung/abnormalities
Introduction
Respiratory failure or compromise in the newborn remains a significant cause of neonatal morbidity and mortality (Hoyert 2006). Pulmonary hypoplasia (PH), one cause of newborn respiratory failure, is a common birth defect seen in 1/800 newborns. PH is a common complication associated with diaphragmatic hernia (CDH), which occurs in approximately 1/2500 newborn infants. The degree of morbidity and potential mortality in pulmonary hypoplasia without or with CDH is mainly attributable to abnormal lung and pulmonary vascular development, which causes progressive difficulty in oxygenation and ventilation with risk of persistent pulmonary hypertension of the newborn. Despite advances in fetal and neonatal medicine, morbidity and mortality from these conditions remain significant (Greer 2003; Gallot 2005).
PH with concomitant CDH is thought to arise from a multiorgan hit that results in a primary defect that affects airway branching morphogenesis and associated vascular development as well as diaphragmatic development (Cilley 1997; Keijzer 2000; Chinoy 2002a; Chinoy 2002b; Gallot 2005). In our validated murine model of pulmonary hyoplasia with CDH, maternal exposure to the herbicide nitrofen (2, 4-dichlorphenyl-p-nitrophenyl ether) during a specific time period on gestational day 8 (Gd8) affects developing embryos such that they develop pulmonary hypoplasia and concurrent CDH in at least 90% of fetuses and neonates. The phenotypic presentation of affected mice pups mimics the characteristics of pulmonary hypoplasia with CDH seen in human infants (Fauza and Wilson 1994; Cilley 1997; Chinoy 2002a). Nitrofen, through its interactions with retinoid signaling and thyroid hormone receptors, alters function of the steroid-thyroid-retinoid nuclear receptor superfamily, with data showing that the more detrimental effect of nitrofen may relate to its effects on the retinoid downstream pathways (Chinoy 2001; Chinoy 2002a; Le 2006). Further, studies involving human infants is supported by these animal models, including our nitrofen model, suggesting that in the development of PH with CDH environmental and genetic factors likely act on the retinoid-steroid-thyroid hormone receptor pathways and subsequently affect other key downstream regulatory pathways of the developing cardiorespiratory system (Manson 1986; Brandsma 1994; Chinoy 2001; Greer 2003; Le 2006; Noble 2007).
The retinoid receptors and other members of their nuclear receptor superfamily (steroid and thyroid hormone receptors), interact by both cis and trans mechanisms with Hox transcription factor proteins. Retinoic acid directly regulates Hox gene expression through binding of retinoic acid to retinoic acid receptors (RARs and RXRs). Retinoic acid binding initiates formation of RAR homo- and hetero-dimers with RXRs as well as binding of retinoic acid receptors to retinoic acid response elements (RAREs) in specific loci within the Hox gene clusters (Krumlauf 1994; Kappen 1996; Deschamps 1999; Hooiveld 1999; Hogan 1999; Volpe 2000; Volpe 2003a). Further, steroid and thyroid response elements have been identified in promoter regions of Hox genes. Our group and others have shown that thyroid and steroid hormones also regulate Hox gene expression in developing lung and other organs (Awgulewitsch 1990; Chinoy 1998; Archavachotikul 2002; Volpe 2003a; Daftary and Taylor 2006).
Individual Hox genes from the four Hox chromosomal clusters are expressed in specific anterior to posterior and organ-specific patterns during development, several being expressed at different time points in developing and adult lung (Krumlauf 1994; Bogue 1994; Bogue 1996; Kappen 1996; Cardoso 1996; Volpe 1997; Mark 1997; Mollard and Dziadek 1997; Hogan 1999; Packer 2000; Golpon 2001; Wang 2006). Experimentally induced Hox gene mutations mimic known human diseases suggesting the ability to use animal models to understand the developmental mechanisms controlled by Hox genes in the developing human (Mark 1997; Hombria and Lovegrove 2003; Volpe 2003b). We have previously shown in our murine model of PH with CDH that expression of the Hox gene Hoxb5 persists in late lung development in a pattern similar to earlier stages of development, however the expression pattern of other Hox genes in PH is not known (Volpe 1997; Chinoy 2002a; Chinoy 2003b). Certain other Hox genes, including Hoxa5, Hoxb4 and Hoxb6 are expressed in thoracic and lung mesenchyme beginning in the early stages of lung morphogenesis. Despite murine knockout studies showing the pertinence of these Hox genes to embryogenesis including thoracic and in some cases pulmonary development, there is minimal to no information addressing the expression and regulation of their corresponding Hox proteins past the earliest stages of lung morphogenesis (on or before Gd15) and no clear correlations between present or potential lung phenotypes that may be ascribed to altered spatial and cellular expression of these Hox proteins (Ramirez-Solis 1993; Rancourt 1995; Aubin 1997; Manley 2001; McIntyre 2007). Other studies in lung and other organ systems show that Hox gene expression patterns change during developmental stages in organogenesis and in the adult reflecting changes in the regulatory effects of a particular Hox protein transcription factor (reviewed in Pavlopoulos and Akam 2007) (Bogue 1996; Volpe 1997; Golpon 2001; Hombria and Lovegrove 2003). This makes complete developmental evaluations essential to understanding the regulatory roles of Hox proteins in developing lung and in lung-specific disease states (Krumlauf 1994; Mark 1997; Hogan 1999). Further, despite studies by us and others that show abnormalities in retinoid, thyroid and steroid receptor signaling in PH with CDH and known regulation of Hox proteins by these regulatory mechanisms, there are no studies addressing the expression and regulation of these Hox proteins in pulmonary hyopolasia (Manson 1986; Brandsma 1994; Chinoy 2001; Archavachotikul 2002; Greer 2003; Volpe 2003a; Babiuk 2004; Noble 2007).
The objective of this study was first to determine the spatial and temporal expression domains and relative balance of Hoxa5, Hoxb4 and Hoxb6 in normal murine lung development, and second to determine how these are altered by nitrofen-induced pulmonary hypoplasia (NT-PH). We hypothesized that Hoxa5, Hoxb4 and Hoxb6 protein expression patterns (1) would support distinct roles for these Hox proteins in normal lung development, and (2) would be modified in a pattern consistent with hypoplastic pulmonary development. We show that during normal lung development precise relationships exist between the temporal, cellular and spatial expression domains for Hoxa5, Hoxb4 and Hoxb6 that are significantly altered in murine nitrofen-induced pulmonary hypoplasia. These findings indicate that Hoxa5, Hoxb4 and Hoxb6 likely control key regulatory mechanisms during normal lung morphogenesis and that these regulatory mechanisms are perturbed in pulmonary hypoplasia.
Methods
Animal care and the investigational protocol followed Institutional Animal Care and Usage Committee International (IACUCI), National Institute of Health guidelines for Care and Use of Laboratory Animals, and the American Veterinary Medical Association Panel on Euthanasia guidelines. Timed pregnant CD-1 mice (Charles River Laboratories, Wilmington, MA) were either gavaged with 25 mg nitrofen in 0.5 ml olive oil on gestational day 8 (Gd8, Gd 0 defined as morning of vaginal plug) or treated as sham controls (no nitrofen = normal controls) as previously described (one animal each for normal control and nitrofen at each gestational age for each of five experiments) (Cilley 1997). This dose of nitrofen is not toxic to pregnant dams, but is selectively teratogenic to developing embryos. Using this methodology, at least 90% of nitrofen-exposed fetuses exhibit pulmonary hypoplasia with CDH. Nitrofen-treated and normal control dams were euthanized by halothane anesthesia and fetal lungs removed under sterile conditions. Neonatal mice (Neo, postnatal day 1) were euthanized by halothane anesthesia at less than 30 hours of age. Neonatal NT-PH pups were alive prior to sacrifice even though frequently experiencing respiratory distress and being less active than normal healthy pups. Our previous experiments have shown that nitrofen-exposed pups with pulmonary hypoplasia and CDH do not survive beyond 30 hours (Cilley 1997). Fetal and neonatal lungs from nitrofen-exposed fetuses with pulmonary hypoplasia + CDH (NT-PH group) were compared to normal lungs from fetuses and neonates of sham-treated control dams (Normal Control group).
Reagents
Individual goat polyclonal primary antibodies that cross-react with mouse and human Hoxa5, Hoxb4 and Hoxb6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used for both Western blot analysis and immunohistochemistry evaluations. These antibodies identify and were produced against unique regions within Hoxa5, Hoxb4 and Hoxb6 proteins (Hoxa5, SC-13199; Hoxb4, SC-15001; Hoxb6, SC-17171). Mouse monoclonal GAPDH antibody was purchased from Research Diagnostics (Flanders, NJ) and horseradish peroxidase (HRP)-linked Donkey anti-goat and anti-mouse secondary antibodies from Jackson Immunoresearch Laboratories (West Grove, PA). Immunostaining reagents were from Vector (Burlingame, CA). All other reagents were from Sigma (St. Louis, MO) unless otherwise specified.
Western blot analyses
Individual protein aliquots from five individual experiments were prepared for Western blots as previously described (Cilley 1997; Volpe 2007). Briefly, protein aliquots from normal control and NT-PH fetuses at Gd13.5 were prepared by pooling 5 lungs from the same litter for each sample for each experiment. The total number of experiments and thus samples obtained at Gd13.5 (N=5) used for analysis was comprised of samples from different litters to address the possible variability between the different litters exposed to nitrofen and also to address natural variability in the normal control litters. One lung each from normal control and NT-PH fetuses at Gd16, Gd19 and neonates was processed for proposed experiments from each of the five experiments (N=5). At Gd16 and later gestations, the individual lungs are of significantly large size compared to those at Gd13.5. Therefore, to control for possible variability between litters, individual lungs taken from different litters for each of five experiments were used for each analysis at Gd16 and later gestational ages. Individual Western blots for Hoxa5, Hoxb4 and Hoxb6 were processed from the same protein aliquots from these five experiments. After Western blot chemiluminescence detection for Hoxa5, Hoxb4 and Hoxb6 protein expression, each blot was subsequently probed with mouse monoclonal anti-mouse GAPDH antibody. Densitometry analysis was performed on the closely clustered group of isoforms specifically detected for each Hox protein under study. Densitometeric analysis of GAPDH blots was then used as an internal control for normalizing protein levels of Hoxa5, Hoxb4 and Hoxb6. As changes in the levels of protein isoforms detected for Hoxa5 and Hoxb6 were apparent across gestation in both normal and NT-PH lungs, further densitometric analyses were performed to assess protein levels of the smaller protein isoforms for Hoxa5 (32 kDa) and Hoxb6 (32–37 kDa) as compared to total densitometric values for the whole cluster of bands at detected for Hoxa5 and Hoxb6. In preliminary experiments, the specificity of antibodies used in Western blot reactions was confirmed by absence of specific bands in absence of primary antibody as well as by identifying a unique set of bands for each Hox protein under study as compared to other goat polyclonal primary antibodies.
Immunostaining
Two sets of lungs from two separate experiments were harvested from Gd13.5, Gd16, Gd19 and neonates of normal controls and NT-PH fetuses and prepared for Hoxa5, Hoxb4 and Hoxb6 immunostaining as we previously described with modifications appropriate for the proteins under study (Volpe 2000). The antibodies against Hoxa5, Hoxb4 and Hoxb6 used in immunostaining were the same antibodies used in Western blot analysis, which were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Sequential coronal lung cryosections (6 microns) from normal controls and NT-PH lungs were processed simultaneously by overnight incubation (4°C) with either Hoxa5, Hoxb4 or Hoxb6 primary antibodies (1/400), followed by room temperature incubation with horse-antigoat secondary antibody (1/200), avidin-biotin complex conjugated to alkaline phosphatase, blue alkaline phosphatase chromagen + levamisole (endogenous alkaline phosphatase blocker) and conterstained with Fast Red (Vector, Burlingame, CA). Lung sections from normal control and NT-PH lungs were incubated for the same period of time in the alkaline phosphatase chromagen solution. A total of thirty sections per lung for each Hox protein were visualized. To confirm specificity of immunostaining reactions, each experiment included adjacent sections with omission of respective primary antibody.
Data Analysis
Computer densitometry (Alpha Innotech, San Leandro, CA) results of individual Hoxa5, Hoxb4 and Hoxb6 western blots of protein aliquots from five separate experiments were performed as described above and statistically analyzed for each Hox gene based on gestational age and treatment using ANOVA, with correction for multiple comparisons, or t-Test with Welch correction where appropriate (Instat, Graphpad Software, San Diego, CA). The level of significance was defined at p<0.05. For Hoxa5, Hoxb4 and Hoxb6 immunostaining, coronal lung sections of fetal and neonatal lungs obtained from two experiments were visually assessed and compared by light microscopy.
Results
Hoxa5, Hoxb4, and Hoxb6 total protein levels do not vary during normal lung development but are developmentally modified in NT-PH
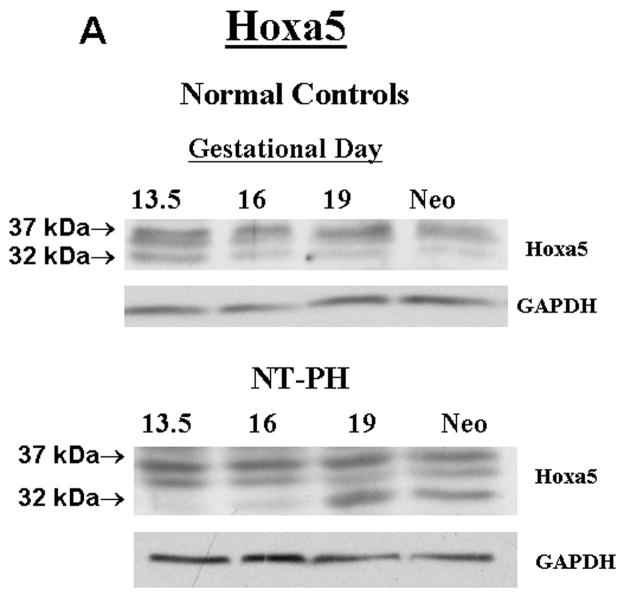
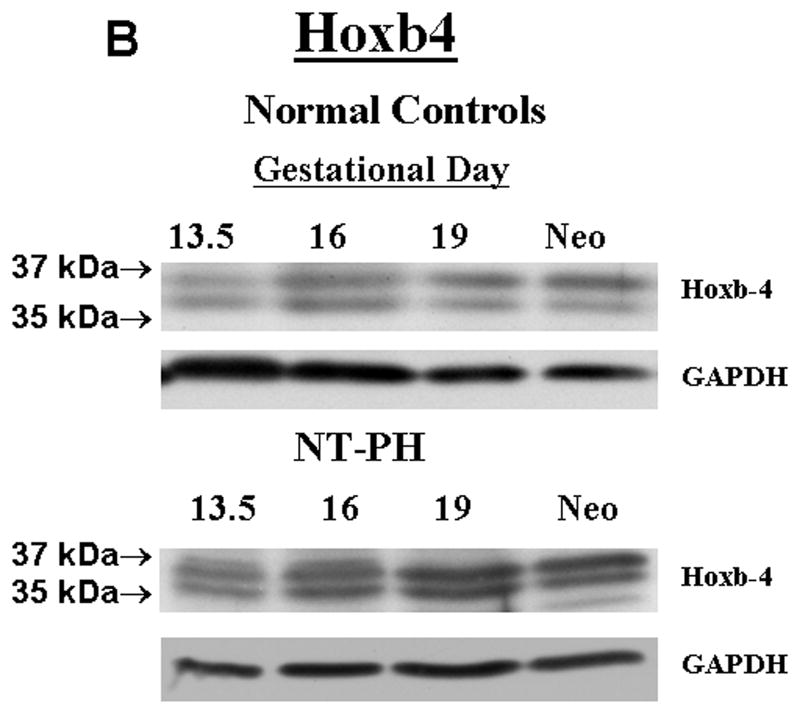
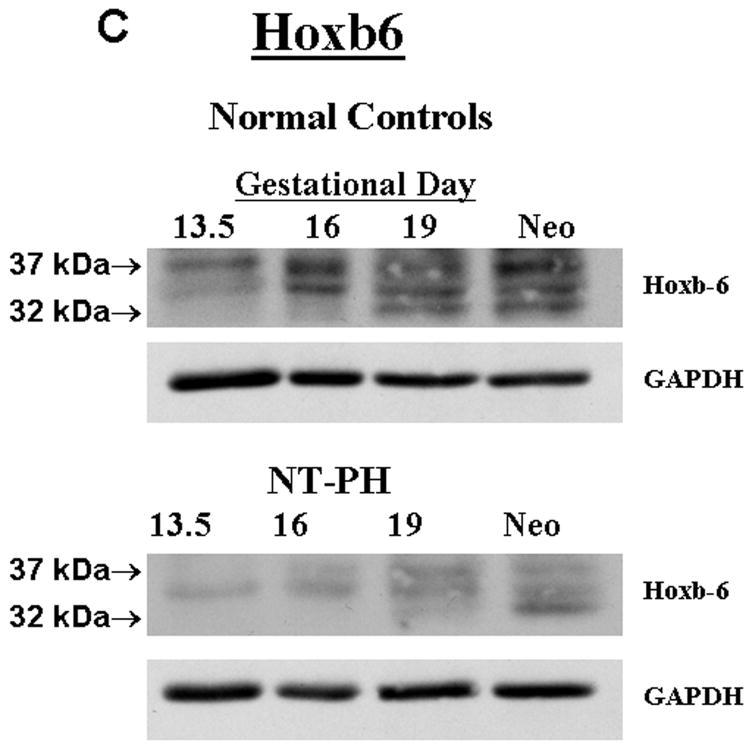
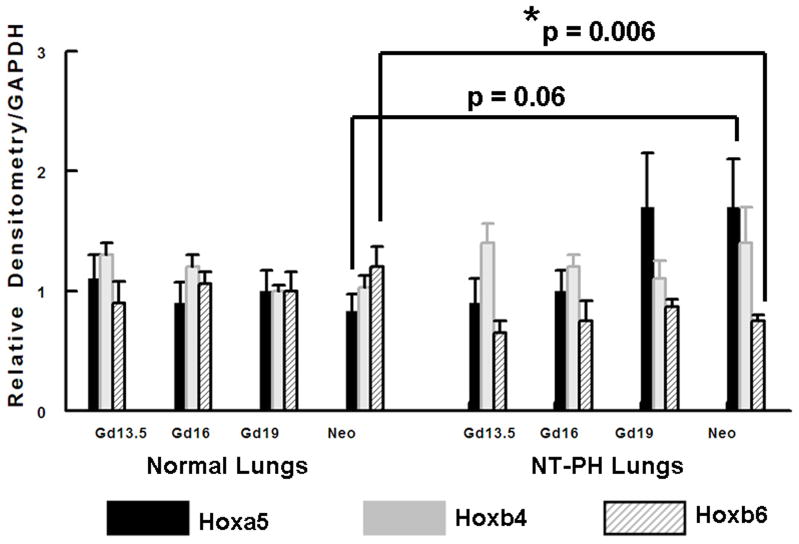
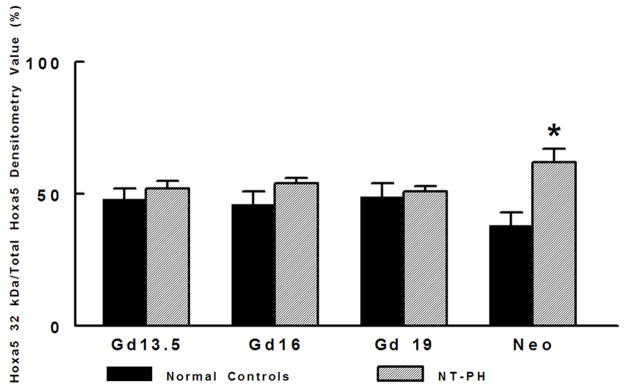
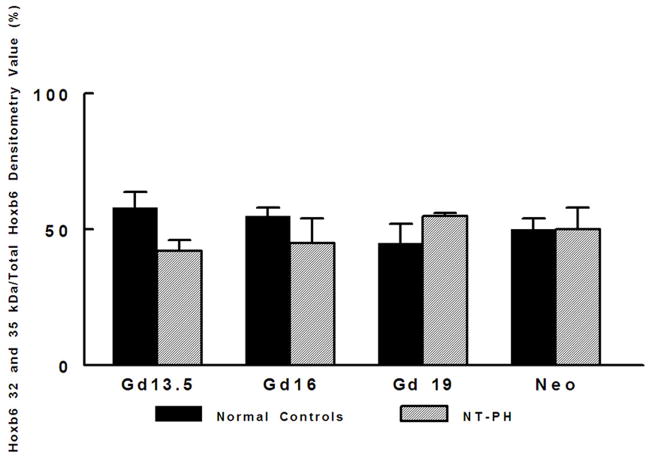
Representative Western blots are shown in Figure 1A (Hoxa5), Figure 1B (Hoxb4) and Figure 1C (Hoxb6). Similar to what has been shown for other Hox proteins, several closely clustered isoforms were detected for Hoxa5 (3 isoforms), Hoxb4 (2 isoforms) and Hoxb6 (3 isoforms) at 32–37 kDa. For each of these proteins, densitometry analysis was first performed on these closely clustered bands as a group that were detected with specific antibodies to Hoxa5, Hoxb4 or Hoxb6 (Krumlauf 1994; Hombria and Lovegrove 2003). Similar to our previous evaluations looking at Hoxb5 protein levels in mouse lung development, this was done to eliminate potential contribution of each of the closely clustered isoforms to its neighboring band in the cluster (Volpe 1997). These results summarizing data from five experiments are shown in Figure 1D. We found that although protein levels of each Hox protein studied were relatively unchanged across gestation, normal lung has higher Hoxa5 and Hoxb4 than Hoxb6 prior to Gd16 during airway branching morphogenesis (Fig. 1D, Left side of graph). However, as lung development progresses into the terminal sac period in the neonate, this relationship became reversed with normal lung having higher Hoxb6 relative to Hoxa5 and Hoxb4. This change in relative proportions of these Hox proteins did not occur in NT-PH lungs, in which patterns of expression characteristic of earlier lung development were still present and further exaggerated (Fig. 1D, right side of graph). As compared to normal lungs at equivalent gestational ages, Hoxa5 protein levels were increased by at least 40% in Gd19 and neonatal NT-PH lungs (Fig. 1A and 1D) with a trend towards statistical significance. Hoxb4 (Fig. 1B and 1D) also increased somewhat in NT-PH neonatal lungs by 40% although this did not show statistical significance. For the protein isoforms detected for Hoxb6, NT-PH lungs had lower Hoxb6 protein levels at each gestational age. This reached statistical significance in neonatal lungs, in which Hoxb6 protein levels were decreased by 45% in NT-PH lungs compared to normal controls (Fig. 1C and 1D). As changes in the smallest detected isoforms visualized for Hoxa5 and Hoxb6 appeared to occur in both normal lung development and in NT-PH lungs, we also compared the densitometric values of the smallest isoforms for Hoxa5 and Hoxb6 to the total densitometric values obtained for the cluster of bands detected for each of these Hox proteins. Evaluation of the lower molecular weight protein isoforms for Hoxa5 and Hoxb6 are shown in Figure 1E and 1F respectively. When evaluating the 32 kDa Hoxa5 band compared to total densitometric values for the whole cluster of bands at 32–37 kDa, we found that the contribution of the 32 kDa isoform was significantly increased in neonatal NT-PH lungs compared to normal neonatal lungs (Fig. 1E) (62% in NT-PH neonatal lungs verses 38% in normal neonatal lungs). However, prior to the neonatal period the proportional amount of this smaller isoform compared to the total Hoxa5 cluster was similar between normal and NT-PH lungs. By evaluating the relative contribution of the 32 and 35 kDa Hoxb6 protein isoforms compared to total densitometric values for the whole Hoxb6 cluster (Fig. 1F), we found that the levels of the smallest protein isoforms detected for Hoxb6 (32 and 35 kDa) changed from being barely detectable to appearing more prominent as gestation progressed. However, the contributions of these smaller two Hoxb6 isoforms to the densitometric value of the whole cluster were somewhat decreased with advancing gestational age in normal lungs (58% at Gd13.5 lungs to 50% in neonates) but increased somewhat in NT-PH lungs (42% at Gd13.5 to 50% in NT-PH neonatal lungs), however the overall signal detection in NT-PH was lower than that seen in the normal lungs at all stages of developing lung. This did not reach statistical significance. Hox protein-specific Western blot signals for each of the Hox proteins under study were not identified in absence of each primary antibody (data not shown).
Figure 1. Hoxa5, Hoxb4 and Hoxb6 western blots and densitometric protein levels in normal and NT-PH lungs.
As shown by representative western blots, Hoxa5 (A), Hoxb4 (B), and Hoxb6 (C) were each detected as a cluster of three protein bands at approximate molecular weights of 32–37 kDa. Summary densitometry analysis on the cluster of protein isoforms detected for each Hox protein (from five separate blots for each protein from five experiments) is shown in (D). Densitometry of the lower molecular weight protein isoforms are shown for Hoxa5 (E) and Hoxb6 (F). Prior to Gd19, normal lung (D, left side of graph) had higher Hoxa5 and Hoxb4 than Hoxb6 followed by higher Hoxb6 compared to Hoxa5 and Hoxb4 after Gd19. This change in relative protein levels of Hoxa5, Hoxb4 and Hoxb6 did not occur in NT-PH lungs (D, Right side of graph) with Gd19 and Neo protein levels of these three Hox proteins resembling an exaggerated pattern to that seen in earlier stages of lung development. Compared to normal lungs, Gd19 and neonatal NT-PH lungs showed a trend towards increased Hoxa5 protein by at least 40% (p=0.06, N=5, Mean ± SEM) and in neonatal lungs, a similar increase of Hoxb4 by 40%. Neonatal NT-PH lungs had a significant decrease in Hoxb6 by 45% (*p = 0.006, N = 5, Mean ± SEM, Neo normal control verses Neo NT-PH). When evaluating changes in the smaller protein isoforms identified for Hoxa5 and Hoxb6, the 32 kDa isoform for Hoxa5 (E) contributed to a significant increase in the total protein levels of Hoxa5 in neonatal NT-PH lungs compared to normal controls (*p = 0.004, N = 5, Mean ± SEM, Neo NT-PH lungs verses Neo normal controls) whereas the relative proportion of densitometric signal for the smaller bands for Hoxb6 (F) was only slightly increased with advancing gestation in NT-PH lungs.
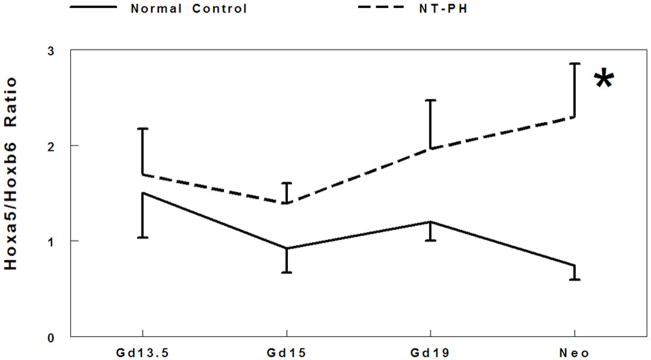
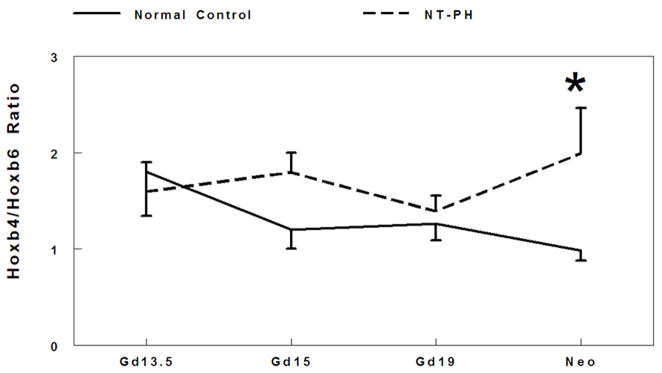
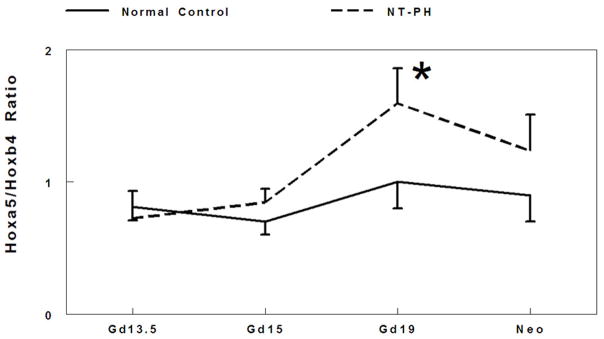
There is extensive cross regulation and concentration dependent activity demonstrated by Hox proteins. The balance and threshold of Hox protein expression levels are crucial to coordinate transcriptional control (Rancourt 1995; Mollard and Dziadek 1997; Hooiveld 1999; Pavlopoulos and Akam 2007). Therefore, we examined the potential relationship between the balance of Hox protein levels by evaluating ratios of the cluster of isoforms detected for Hoxa5 to Hoxb6 (Fig. 2A), Hoxb4 to Hoxb6 (Fig. 2B), and Hoxa5 to Hoxb4 (Fig. 2C) in normal developing mouse lungs and in NT-PH lungs. In normal control lungs, the ratio of Hoxa5 to Hoxb6 protein levels gradually decreased throughout gestation. However, in NT-PH lungs the ratio of Hoxa5 to Hoxb6 became greater with advancing gestational age, reaching an increase of 240% in neonatal NT-PH lungs compared to normal control lungs (Fig. 2A). The ratio of Hoxb4 to Hoxb6 protein levels also showed a gradual decrease across gestation in normal lungs. This showed little overall change in NT-PH lungs on or before Gd19. However, in neonatal NT-PH lungs the ratio of Hoxb4 to Hoxb6 was significantly elevated by 102% compared to normal controls (Fig. 2B). The ratio of Hoxa5 to Hoxb4 was relatively unchanged across early to mid-gestation in normal controls but significantly elevated by 60% in Gd19 NT-PH lungs compared to normal controls (Fig. 2C), but this finding did not persist in neonatal NT-PH lungs.
Figure 2. Ratio of Hoxa5 to Hoxb6, Hoxb4 to Hoxb6, and Hoxa5 to Hoxb4 protein levels.
Densitometry analysis showed that Hoxa5 to Hoxb6 ratio (A) gradually decreased throughout gestation in normal controls. However in NT-PH lungs, Hoxa5 to Hoxb6 ratio increased throughout gestation and was significantly increased in neonatal NT-PH lungs (*P<0.05, N=5, Mean ± SEM, Neo normal controls verses Neo NT-PH). On or before Gd19, Hoxb4/Hoxb6 ratio (B) was not different in normal verses NT-PH lungs but became significantly greater in neonatal NT-PH lungs (*P<0.05, N=5, Mean ± SEM, Neo normal controls verses Neo NT-PH). Normal lung Hoxa5 to Hoxb4 ratio (C) was unchanged across gestation but significantly elevated in Gd19 NT-PH lungs compared to normal controls (*P<0.05, N=5, Mean ± SEM, Gd19 Normal Controls verses Gd19 NT-PH).
Developing normal mouse lung has unique spatial and cellular localization patterns of Hoxa5, Hoxb4 and Hoxb6 proteins that are developmentally modified in NT-PH
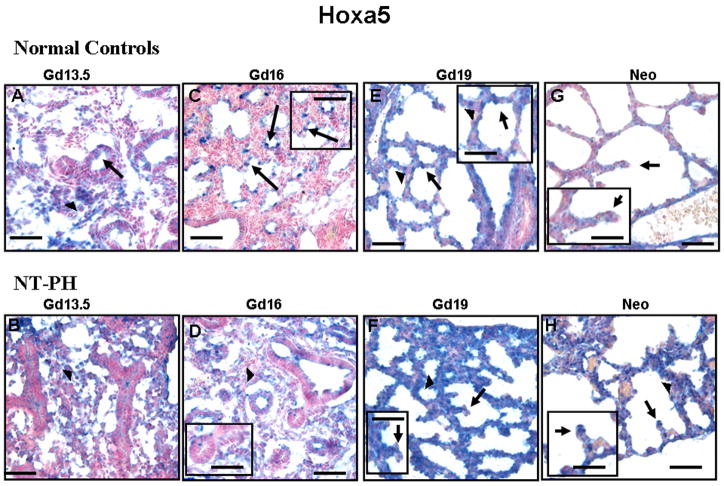
Normal mouse lung at the specific developmental stages studied showed different spatial and cellular expression of Hoxa5, Hoxb4 and Hoxb6. Typically, such differences for regulatory factors such as Hox proteins indicate different regulatory roles. These patterns of expression were altered in NT-PH. In Gd13.5 normal lungs, Hoxa5 protein was mainly localized to nuclei in clusters of mesenchymal cells (Fig. 3A). Gd13.5 NT-PH lungs had thicker mesenchyme that had more diffuse immunostaining for Hoxa5 protein while the epithelial cells were mostly negative (Fig. 3B). In Gd16 normal lungs, Hoxa5 protein localization changed, becoming mostly restricted to epithelial cells and immediately adjacent mesenchymal cells of branching airway tips (Fig. 3C and corresponding higher power insert). However, in Gd16 NT-PH lungs (Fig. 3D), these changes in the cellular localization of Hoxa5 protein did not occur and expression remained similar to that seen at Gd13.5. Correspondingly, progression of lung airway branching was delayed with mesenchyme remaining thicker and airway branches having narrow lumens lined by columnar epithelial cells reminiscent of an earlier stage of lung development. At Gd 19 in normal control lungs, Hoxa5 protein was localized to nuclei of discrete epithelial cells of developing saccules (Fig. 3E). Adjacent mesenchyme had occasional Hoxa5 positive cells. The thicker mesenchyme in NT-PH Gd19 lungs (Fig. 3F) remained strongly positive for Hoxa5. Although epithelial cells were also positive, NT-PH Gd19 lungs lacked the discrete saccular epithelial cell expression seen in normal lungs. In normal control neonatal lungs (Fig. 3G), Hoxa5 epithelial expression was less intense (Fig. 3G, see higher power insert). However, neonatal NT-PH lungs had stronger mesenchymal and epithelial expression than normal control neonatal lungs especially at tips of developing septae (Fig. 3H, see higher power insert).
Figure 3. Hoxa5 protein immunolocalization in normal developing mouse lung and in NT-PH lungs. Bar = 50μ in Panels A–H and 20μ in corresponding inserts.
In Gd13.5 normal controls (A) and NT-PH (B) lungs, Hoxa5 protein was mostly localized to mesenchyme (arrowheads) with minimal expression in epithelial cells of columnar-lined airways (arrows). In Gd16 normal lungs (C), Hoxa5 protein localization changed being mostly restricted to epithelial cells of branching airway tips (arrows) (see higher magnification insert). This change was not seen in Gd16 NT-PH lungs where Hoxa5 remained mostly diffusely expressed in mesenchyme (arrowheads). Hoxa5 protein remained mostly localized to epithelial cells (arrows) in Gd 19 normal controls (E). Gd19 NT-PH lungs (F) also had persistent expression in thicker mesenchyme (arrowheads) whereas epithelial cells (arrows) especially at septal tips remained negative. In neonatal normal controls (G), Hoxa5 epithelial cell expression was less intense as compared to strong Hoxa5 mesenchymal (arrowheads) and epithelial expression (arrows) especially at septal tips in hypoplastic lungs (H).
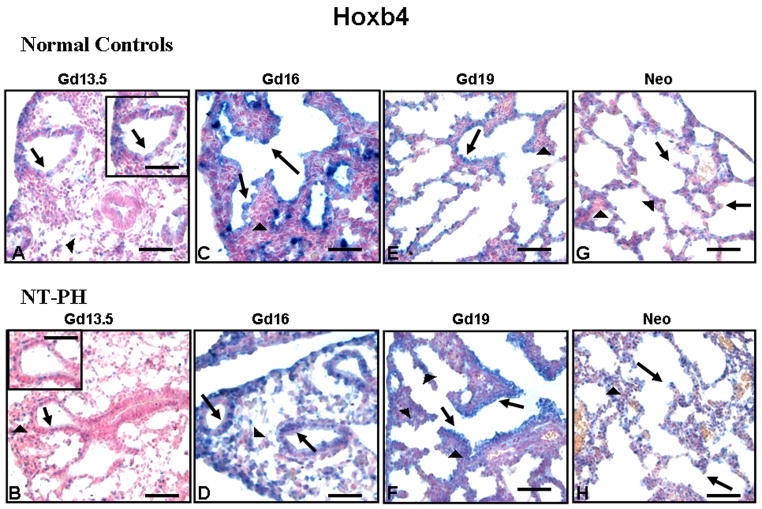
Epithelial and mesenchymal cell-specific localization of Hoxb4 was very different than that of Hoxa5. Hoxb4 was seen mainly in epithelial and less in mesenchymal cells from the earliest gestation studied. In Gd13.5 normal control (Fig. 4A) and NT-PH (Fig. 4B) lungs, Hoxb4 positive nuclei were seen in scattered mesenchymal and epithelial cells (also see higher power insert). Epithelial cell expression of Hoxb4 became more intense in both Gd16 normal control (Fig. 4C) and NT-PH lungs (Fig. 4D), but Hoxb4 mesenchymal cell expression was stronger in Gd16 NT-PH lungs than in normal control lungs. This demonstrates a change in cell-specific expression and level of intensity of mesenchymal Hoxb4 expression that was beyond the limits of detection by Western blot of total lung protein levels. Compared to Gd19 normal control lungs (Fig. 4E), Hoxb4 expression in mesenchyme around distal airways and saccules of Gd19 NT-PH lungs (Fig. 4F) remained more diffusely intense. Bronchiolar epithelial expression of Hoxb4 also remained stronger in NT-PH lungs compared to Gd19 normal control lungs. Both normal control (Fig. 4G) and NT-PH neonatal lungs (Fig. 4H) had occasional Hoxb4 positive epithelial cells. However, mesenchyme and epithelial cells in neonatal NT-PH lungs continued to have increased Hoxb4 positive cells compared to controls. Hoxa5 and Hoxb4 expression were both seen in cartilage rings in both normal control and NT-PH lungs (data not shown).
Figure 4. Hoxb4 protein immunolocalization in normal developing mouse lung and in NT PH lungs, Bar = 50μ in Panels A–H and 20μ in corresponding inserts.
Hoxb4 protein was localized to both mesenchymal (arrowheads) and epithelial cells (arrows) in Gd13.5 normal controls (A) and NT-PH lungs (B) but epithelial cell expression was more intense. Gd16 normal controls (C) and NT-PH lungs (D) had strong epithelial cell expression, but Hoxb4 mesenchymal cell (arrowheads) expression was more intense in NT-PH lungs than in the normal controls. Gd19 normal controls (E) and NT-PH lungs (F) had similar Hoxb4 mesenchymal (arrowheads) and epithelial (arrows) cell localization, but NT-PH lungs had increased intensity of Hoxb4 in epithelial cells (arrows), especially in bronchiolar airways as well as somewhat increased mesenchymal expression (arrowheads). Compared to normal neonatal lungs (G), epithelial cell (arrow) Hoxb4 staining remained stronger in neonatal NT-PH lungs (H). Thicker mesenchyme (arrowhead) in NT-PH lungs continued to have greater intensity of Hoxb4 positive cells than seen in the normal lungs.
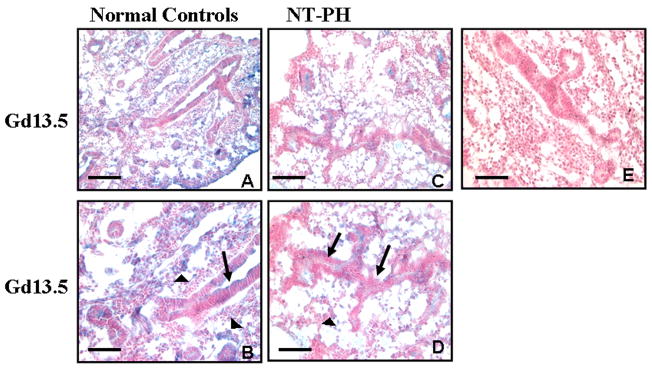
Unlike Hoxa5 and Hoxb4, Hoxb6 protein was mostly localized to mesenchymal cells around distal bronchioles at Gd13.5 (Fig. 5A and 5B). Columnar epithelial cells of proximal, more central airways remained negative. As Hoxb6 is located more 5′ in the Hox cluster DNA sequence, this result is consistent with the paradigm that Hox genes positioned more 5′ in the gene cluster are expressed more caudally within organs (Krumlauf 1994; Hogan 1999). Consistent with this caudal expression pattern, and different than Hoxa5 and Hoxb4, Hoxb6 was not expressed in cartilage rings which are more proximal and central rather than distal structures in lung (data not shown). Although Hoxb6 cellular localization was not different in Gd13.5 NT-PH lungs (Fig. 5C and 5D), mesenchymal expression was decreased compared to controls. In normal controls at Gd16 (Fig. 5F), Hoxb6 remained mostly localized to mesenchymal cells around airways with cuboidal epithelium but occasional cuboidal epithelial cells were also positive for Hoxb6. In contrast, in NT-PH lungs (Fig. 5I) whose morphology remained similar to early pseudoglandular period lung, mesenchymal and epithelial expression of Hoxb6 was less intense compared to normal control lungs. Hoxb6 epithelial and mesenchymal cell expression was present in Gd19 normal control lungs (Fig. 5G). Scattered intensely positive epithelial cells were seen in developing saccules. This expression pattern was similar but much less intense in Gd19 NT-PH lungs (Fig. 5J). In normal control neonatal lungs (Fig. 5H), Hoxb6 mesenchymal cell expression around saccules was more diffuse, whereas, in NT-PH lungs (Fig. 5K), saccules were surrounded by small clusters of Hoxb6 positive mesenchymal cells. Hoxb6 epithelial expression remained minimal. Negative controls, including omission of primary antibodies for Hoxa5, Hoxb4 or Hoxb6, were performed with each immunostaining experiment and showed no blue alkaline phosphatase staining in absence of primary antibody (Fig. 5E). Overall, we were able to identify key changes in the immunolocalization of these Hox proteins in both normal lung and NT-PH lungs prior to changes that could be detected by Western blots and showed that in general, the intensities of immnunohistochemcial localization of the respective proteins supported the observations of the densitometric analyses of the Western blots: Hoxa5 and Hoxb4 signals were more intense in NT-PH than normal lungs, whereas Hoxb6 signals in NT-PH lungs were weaker than those seen in normal developing lungs.
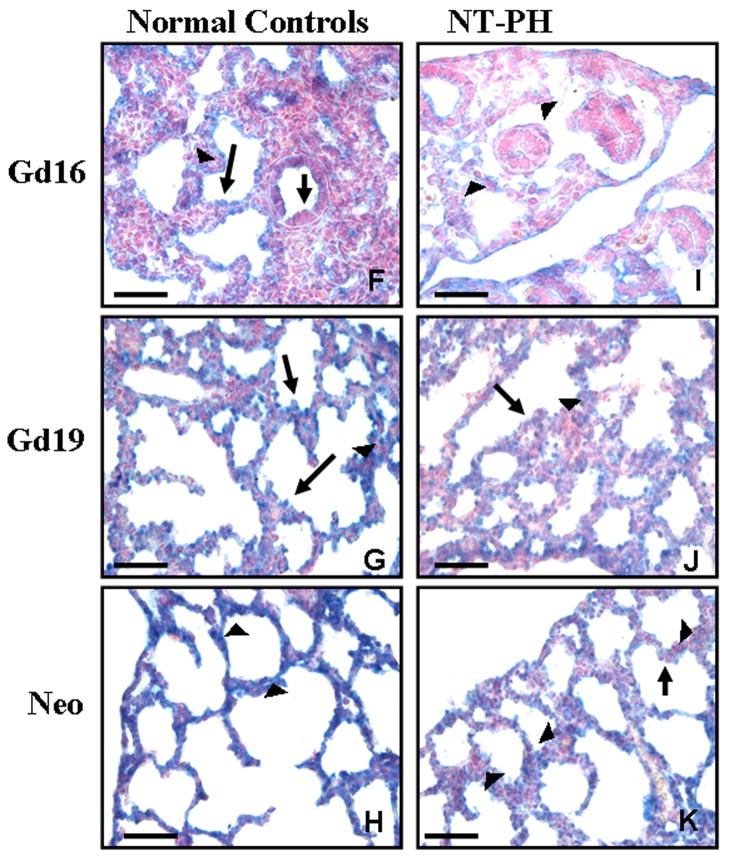
Figure 5. Hoxb6 protein immunolocalization in normal developing mouse lung and in NT-PH lungs, Bar = 100μ in A and C and 50μ in B, D, E and F through K.
Hoxb6 protein was mostly localized to mesenchyme (arrowheads) at Gd13.5 in normal control lungs (A, B) but decreased in NT-PH lungs (C, D). Epithelial cells of proximal more central airways (arrows in B and D) were mostly negative. Gd16 normal lungs (F) had Hoxb6 positive mesenchymal cells (arrowheads) around distal airways with cuboidal epithelial cells (long arrow in F). Although some cuboidal epithelial cells (long arrow in F) were positive for Hoxb6 at Gd16, columnar epithelial cells of proximal airways (short arrow in F) remained mostly negative. Mesenchymal expression (arrowheads in I) remained decreased around less well-developed airways in Gd16 NT-PH lungs (I). In normal lungs at Gd19 (G), Hoxb6 epithelial (arrow) and mesenchymal cell (arrowhead) expression was present and more intense compared to Gd19 NT-PH lungs (J). In normal neonatal lungs (H), mesenchymal Hoxb6 protein expression (arrowheads) was more diffuse around developing saccules. In neonatal NT-PH lungs (K) saccules were less developed with thicker surrounding mesenchyme (arrowhead) that had clusters of Hoxb6 positive mesenchymal cells and epithelial (arrow) expression remained minimal.
Discussion
We and others have previously reported that in nitrofen-induced pulmonary hypoplasia, retinoid signaling through RARs is altered (Manson 1986; Brandsma 1994; Chinoy 2001; Chinoy 2002a; Babiuk 2004; Noble 2007). Nitrofen partially acts by decreasing the levels of retinoic acid dehydrogenase 2 (RALDH2), a key enzyme expressed in developing lung that regulates local production of active retinoic acid (Malpel 2000; Greer 2003; Babiuk 2004). Consistent with the hypothesis that abnormal retinoid signaling is part of the mechanism that contributes to human and experimental models of pulmonary hypoplasia with CDH, RAR knockout mice show evidence of hypoplastic lungs and abnormal regulation of alveolar septation (Mendelsohn 1994; Greer 2003; Chinoy 2003a). Retinoids are key regulators of Hox gene expression. The interactions of Hox genes and retinoid signaling are made more intriguing as cross regulation exists between Hox genes and the RAR beta receptor (Serpente 2005). The focus of our current study was to determine the spatial and temporal expression patterns and relative balance of Hoxa5, Hoxb4 and Hoxb6 in normal murine lung development and whether the pattern of expression of these Hox genes would be altered in nitrofen-induced pulmonary hypoplasia. Based on these observations and the current literature, we can suggest that in our mouse model of nitrofen-induced pulmonary hypoplasia that the ability of nitrofen to alter retinoid signaling modifies the normal developmental patterns of Hoxa5, Hoxb4 and Hoxb6 expression. Current literature supports that it may be the reduced availability or reduced optimal levels of retinoids and not necessarily abnormal retinoid receptor expression that is responsible for hypoplastic lung development. The ability to measure levels of active and available RA in fetal lungs during nitrofen-exposure was not possible during our current studies, but our data suggest that part of this mechanism occurs through altered Hox gene expression, as Hox genes are downstream of RA signaling. The knowledge that retinoid replacement does not completely eliminate pulmonary hypoplasia and CDH in animal models fits with the possibility that other mechanisms including abnormal thyroid and glucocorticoid hormone receptor signaling are also an important part of the mechanism leading to pulmonary hypoplasia with CDH (Manson 1986; Brandsma 1994; Chinoy 2001; Babiuk 2004). Current literature shows conflicting reports on steroid-thyroid-retinoid receptor levels and localization in pulmonary hypoplasia with CDH in mouse and rat models versus humans (Chinoy 2001; Greer 2003; Rajatapiti 2006; Noble 2007). However, the levels and localization of RARs, TRs and glucocorticoid hormone receptors (GR) may not be as critical as abnormal receptor activity due to blockade of thyroid and retinoid receptors by nitrofen (Manson 1986; Brandsma 1994). Given this information, it is important to note that retinoids are only one group of molecules and retinoid signaling only one pathway regulating Hox genes in developing embryos and in adults. Although less well understood, Hox genes are also regulated by thyroid and steroid hormones with DNA response elements for these other endocrine regulators being identified within Hox gene promoter regions (Awgulewitsch 1990; Chinoy 1998; Archavachotikul 2002; Volpe 2003a; Daftary and Taylor 2006). Non availability or reduced availability of receptors due to blockade by nitrofen may result in altered retinoid and thyroid responses as RXRs heterodimerize with TRs and RARs further adding to possible mechanisms that could have altered Hox gene expression in our model (Manson 1986; Brandsma 1994; Chinoy 2001; Noble 2007). Altered receptor signaling through these other mechanisms could explain the altered levels of Hoxa5 expression seen in our study as compared to the study by Wang et al. (Wang 2006) looking at RALDH2 knockout mice with/without retinoid supplementation. Hoxa5 levels and expression patterns in pulmonary hypoplasia may be affected not only by retinoid levels but also by altered retinoid, thyroid and glucocorticoid receptor (RARs, TR and GR) signaling. This is supported by our previous studies in which we showed altered TR and GR expression at gestational ages consistent with the time period where we now report abnormalities in Hox protein expression (Chinoy 2001).
We found a precise developmental relationship between Hoxa5, Hoxb4 and Hoxb6 protein levels in the different stages of normal murine lung development. This relationship was modified in the term (Gd19) and neonatal NT-PH lungs to a pattern similar but exaggerated from that seen at earlier developmental stages. In NT-PH lungs, the changed levels of Hoxb6 in the terminal sac period (Gd19 and Neo) reversed the relationship of Hoxb6 to Hoxa5 and Hoxb4 during this period of lung development. This decreased level of Hoxb6 protein expression was preceded by a trend towards increased levels of Hoxa5 and Hoxb4. Although the increased total protein levels of Hoxa5 and Hoxb4 were not statistically significant, potential biologic significance is worth considering, as these changes led to significant alterations in the relationship of protein levels of Hoxa5, Hoxb4 and Hoxb6. Given the results of our study, the changed levels of the smaller protein isoform for Hoxa5 could have contributed to these alterations, subsequently affecting downstream signaling. Subtle changes in Hox protein levels are the mechanism by which Hox gene abnormalities alter transcriptional regulation and subsequent organ and tissue morphogenesis and function (Barrow and Capecchi 1996; Mollard and Dziadek 1997; Hooiveld 1999; Foucher 2002; Pavlopoulos and Akam 2007). Further, Hox gene cross-regulation within and between Hox clusters also occurs. For example, we have previously shown that Hoxb5 expression is maintained in an expression pattern similar to earlier lung development in NT-PH lungs (Chinoy 2003b). Hoxb5 has been shown in other studies to up-regulate Hoxb4, which could contribute to altered Hox protein expression seen in our current study (Hooiveld 1999). The ability of Hoxa5 and/or Hoxb4 to regulate their own or each others expression and the subsequent regulation of Hoxb6 in normal lung and in pulmonary hypoplasia remains to be determined, but is plausible considering the results of our study. We have not evaluated the expression of these Hox proteins prior to Gd13.5. It is possible that altered Hox protein expression is present closer in timing to the insult by nitrofen. Some of our observations may represent a consequence of or a compensation for a developmental hit to lung morphogenesis. The developmental hit by nitrofen likely causes a cascade of events throughout lung development. Part of this mechanism may be through altered sequential activation of Hox genes in lung that is likely to occur through auto and cross regulation as it does in other organs. For example, in the NT-PH model it is not known if compensatory effects lead to maintaining the level of Hoxa5 expression. Does this alter timing of sequential Hox gene expression?
To our knowledge, we are the first to describe in this level of detail that several specific protein isoforms exist for Hoxa5, Hoxb4 and Hoxb6 in developing lung and that their expression changed both with development as well as in NT-PH lungs compared to normal lungs. The presence of a cluster of protein isoforms detected for each Hox protein in our study is consistent with identification of multiple mRNA transcripts detected for many Hox genes including Hoxa5, Hoxb4 and Hoxb6 by other researchers. Transcriptional (multiple promoters and transcriptional start sites) and post-transcriptional regulatory mechanisms (mRNA splicing) have been identified for Hox genes. However, post-translational modification also occurs for Hox genes which could contribute to our findings as well, particularly for the change in expression of specific protein isoforms with different gestational ages (Fibi 1988; Odenwald 1989; Coletta 1991; Wall 1992; Gutman 1994; Mollard and Dziadek 1997; Larochelle 1999; Kim and Nielsen 2000). How these protein isoforms differ in different organs, whether binding or protein stability is affected, or if regulatory mechanisms controlled by the different isoforms change at different gestational ages or in pulmonary hypoplasia remains to be studied. Other studies, however, suggest that different protein isoforms for specific Hox proteins including Hoxb6 may determine specific and changed roles for these transcriptional regulators in the progression of organ development and cellular differentiation (Komuves 2000).
Our work is in agreement with work in mouse (mRNA and protein) and human (mRNA) developing lung showing relatively unchanged Hoxa5 levels across gestation and in the adult (Kim and Nielsen 2000; Golpon 2001). Similarly, our data on Hoxb6 protein levels agrees with data from mouse and chick developing lung showing that Hoxb6 mRNA is expressed at least at consistent levels and in distal lung structures across gestation and in newborn lung (Bogue 1996; Mollard and Dziadek 1997; Sakiyama 2000). The differences in reported mRNA levels for these Hox genes in rat compared to what we have shown for protein expression could be explained by changes in mRNA translation or protein stability or species-specific regulation (Bogue 1994; Cardoso 1996). DNA methylation does occur for Hox genes including Hoxa and Hoxb genes that could contribute to decreased mRNA production (Hershko 2003; Rinn 2007).
An understanding of the ability of Hoxa5, Hoxb4 and Hoxb6 to modify downstream developmental networks in lung also depends on the knowledge of the localized cellular and spatial expression domains of each of these Hox proteins at different developmental stages and how these patterns are modified in lung diseases including pulmonary hypoplasia (Graba 1997; Hombria and Lovegrove 2003; Volpe 2007). By evaluating spatial and cellular expression of these Hox proteins across key developmental stages we identified distinct temporal and cellular expression domains in and between normal and hypoplastic lungs that could not be identified by simply looking at total lung protein levels or only one gestational time point. This strengthens the applicability of our findings for understanding the mechanisms regulated by each protein during normal and disrupted lung morphogenesis. The potential significance of these findings for airway branching, terminal sac/alveolar formation, and lung cell fate and maturation are discussed below.
Previous studies in which Hoxa5 mRNA expression was only evaluated in early lung development (on or before Gd13.5) showed that Hoxa5 is expressed in lung mesenchyme (Aubin 1997). Our results at Gd13.5 are consistent with these findings. However, in addition to this our studies revealed a transition of Hoxa5 to epithelial expression, in line with other studies in human lung that show Hoxa5 mRNA in epithelial cells of developing bronchioles and alveolar ducts in fetal pseudoglandular and canalicular stage lungs and in adult lung septal tips and Type II cells (Golpon 2001). Interestingly, lungs from surviving homozygous Hoxa5 knockout mice have emphysematous airways with decreased septal number, reminiscent of adult humans with emphysema in whom Hoxa5 mRNA expression is decreased in alveolar septal tips. Our current data together with these studies suggest a more direct involvement of Hoxa5 in epithelial cell differentiation than previously believed, including an important function in alveolar septal formation (Golpon 2001; Kinkead 2004). We further found that the transition from mesenchyme to epithelial expression of Hoxa5 protein was delayed by approximately 1–2 days in NT-PH lungs, consistent with delayed airway development. The increased intensity of Hoxa5 expression in septal tips and alveolar epithelial cells of neonatal hypoplastic lung could be a compensatory response to correct this altered progression of lung maturation and may contribute more directly to surfactant protein expression and production than previously suspected (Aubin 1997; Ijsselstijn 1998; Golpon 2001; Kinkead 2004; Davey 2005). We cannot rule out the possibility that the different protein isoforms for Hoxa5 noted in both normal and NT-PH lungs and the changing levels of the smallest protein isoform for Hoxa5 that was increased in neonatal NT-PH lungs contributes to the altered cellular expression patterns and to mechanisms regulated by Hoxa5 in normal and NT-PH lungs.
Our study and studies by Golpon (2001) and Aubin (Aubin 1997) differs from the study by Packer (Packer 2000) that showed a more proximal localization of Hoxa5 mRNA expression up to Gd14.5 in developing mouse lung. The differences in proximal to distal extension of mRNA versus protein may be due to differences in the anterior to posterior boundary of protein versus mRNA which has been reported for Hox genes in CNS development (Wall 1992; Krumlauf 1994). These differences could also result from differences in mRNA production, stability or rate of translation to protein as well as variable levels of experimental detection in whole mount in situ studies compared to tissue sections, which may have better permeability to reagents.
Wang (Wang 2006) showed that lack of RA signaling through RALDH2 in RALDH2−/− mice leads to decreased Hoxa5 mRNA which is only partially reversed by retinoic acid supplementation. The differences in Hoxa5 expression in this study versus our nitrofen model suggests that other mechanisms are active in altering Hoxa5 expression besides retinoic acid availability such as altered signaling through RARs and TRs. Our results may indicate that preferential binding of nitrofen to RARs and TRs may still trigger downstream signaling cascades, however the outcomes could be different if this trigger is initiated by the teratogen nitrofen rather than RA or thyroid hormone (Manson 1986; Chinoy 2001). This difference in Hoxa5 localization may have lead to altered airway branching and airway maturation through downstream changes in FGF signaling (Causak 1998; Aubin 2002; Wang 2006; Volpe 2007).
We found that Hoxb4 is also expressed in some epithelial cells but possibly different cell types from those identified by Hoxa5. The identity of the epithelial cells expressing Hoxa5 and Hoxb4 is not known at this time but the localization pattern of expression suggests that Hoxb4 may identify more proximal epithelial cells and Hoxa5 more distal epithelial cells. The more intense epithelial expression of Hoxb4 especially in bronchiolar epithelium in hypoplastic lungs after Gd16 could contribute to alterations in proximal to distal maturation of airway epithelium. The stronger mesenchymal expression of Hoxb4 in hypoplastic lungs after Gd13.5 is partially explained by the thicker mesenchyme present in NT-PH lungs. We previously reported decreased expression of c-myc in hypoplastic lungs. The increased levels of Hoxb4 we observed may contribute to the hypoplasia through regulation of c-myc (Chinoy 2001; Pan and Simpson 2001). In different cell types, Hoxb4 can increase cell proliferation or activate apoptotic pathways (Morgan 2004). Therefore, Hoxb4 effects may also differ in pulmonary mesenchymal and epithelial cells, helping balance cell proliferation and apoptosis during airway remodeling (Levesque 2000).
Hoxb6 protein was spatially confined to more distal lung regions than Hoxa5 or Hoxb4. This is similar to previous studies looking at localization of Hoxb6 mRNA expression in developing lung (Bogue 1996; Cardoso 1996; Sakiyama 2000). Further, at the transition from the pseudoglandular to canalicular stage at Gd16, it is important to note that the balance of Hoxb6 and Hoxb4 expression in mesenchymal cells was altered in hypoplastic lungs. This transitional period in lung development represents a critical time in which distal airway buds progress from a program of branching morphogenesis of bronchioles to beginning development of alveolar ducts and terminal sacs (Ten Have-Opbroek 1991). Thus, in NT-PH lungs, lack of Hoxb6 expression in more distal lung mesenchyme may further contribute to altered proximal to distal airway branching and distal saccular development and maturation. As Hoxb6 protein expression is indicative of more caudal lung structural development, these changes with NT-PH further indicate that Hoxb6 participates in regulation of more distal lung development. Decreased Hoxb6 protein expression seen in the NT-PH lungs may have also modified proliferation of mesenchymal cells and potentially certain mesenchymal cell phenotypes. Hoxb genes are the main Hox genes expressed in human endothelial cells, where they play an important role in vasculogenesis (Belotti 1998). Modified Hoxb6 mesenchymal expression in hypoplastic lungs could contribute to the altered vascular development seen in this condition (Chinoy 2002b; Greer 2003; Gallot 2005). The antibody used in our immunostaining study detected three protein isoforms for Hoxb6 by Western blot. The levels of these protein isoforms appeared to change with gestational age. We cannot determine if altered localized intensity of Hoxb6 immunostaining is partially due to the changing isoforms. In skin development altered Hoxb6 protein isoform structure contributes to protein localization and possibly determines different regulatory roles at different stages of skin development and differentiation (Komuves 2000).
Although we noted some of the changes in protein levels and cellular expression of these Hox proteins in neonatal lungs, these changes cannot be attributed only to changes in oxygenation in neonatal mice at birth as several of the noted Hox protein changes occurred before birth and predominantly in NT-PH lungs. NT-PH neonatal lungs would be expected to be less well oxygenated than normal control neonatal lungs due to respiratory distress of neonatal NT-PH mice. However, it is important to consider that some of the differences we noted between normal neonatal lungs and neonatal NT-PH lungs could result from better oxygenation of the normal controls altering the genetic regulation of Hox protein expression, important changes that might not occur in neonates with pulmonary hypoplasia due to poor oxygenation.
We conclude that our current study adds key, previously unknown information on the protein expression domains of Hoxa5, Hoxb4 and Hoxb6 at different lung development stages and in pulmonary hypoplasia. The unique cellular expression patterns of these three Hox proteins and the contrasts between normal and NT-PH lungs support their individual roles in lung cell fate and provide mechanistic insight into possible regulatory events controlled by Hox proteins in normal lung morphogenesis and in congenital lung defects. We speculate that in normal lung development regulated levels and cellular expression patterns of Hoxa5, Hoxb4 and Hoxb6 proteins participate in the control of proximal to distal mesenchymal and epithelial cell fate, contributing to a “Hox code” for the developing lung. We propose that with nitrofen-induced pulmonary hypoplasia, the “environmental hit” acting on regulatory pathways in lung development alters this Hox code resulting in modified proximal to distal airway development and cellular maturation. Such changes may contribute to the morbidity and mortality associated with pulmonary hypoplasia in human infants (Krumlauf 1994; Graba 1997; Hombria and Lovegrove 2003; Greer 2003; Gallot 2005).
In summary, it is clear from many studies, only a subset of which have been done in lung, that Hox proteins help regulate many different signaling pathways to assist cells in determining their subsequent cell-cell interactions, including cell adhesion, cell migration and cell fate (Foucher 2002; Theokli 2003; Moens and Selleri 2006; Volpe 2007). As Hox gene-regulated mechanisms play key roles in embryonic development, it is essential to consider how their expression and function are altered in lung development and lung disease. Our study can assist in understanding the interactions between Hox proteins and these other developmental pathways in lung morphogenesis, which may eventually lead to innovative new regimens for prevention and treatment of pulmonary hypoplasia.
Acknowledgments
Grant Information and Support: NICHD (HD-044784), NHLBI (HL-37930), and the Peabody Foundation
The authors would like to thank Shane Miller for technical assistance, Dr. Sujatha Ramadurai and Dr. Sandy Murray for helpful comments and Erdene Haltiwanger for secretarial support. NICHD (HD-044784) and NIHLBI (HL-37930) research support is greatly appreciated.
Footnotes
Presented at the 2006 Pediatric Academic Societies’ Annual Meeting, April 29 to May 2, 2006, San Francisco, California.
Literature Cited
- Archavachotikul K, Ciccone TJ, Chinoy MR, Nielsen HC, Volpe MV. Thyroid hormone affects embryonic mouse lung branching morphogenesis and cellular differentiation. Am J Physiol Lung Cell Mol Physiol. 2002;282:L359–L369. doi: 10.1152/ajplung.00400.2000. [DOI] [PubMed] [Google Scholar]
- Aubin J, De’ry U, Lemieuz M, Chailler P, Jeannotte L. Stomach regional specification requires Hoxa-5 driven mesenchymal-epithelial signaling. Dev. 2002;129:4075–4087. doi: 10.1242/dev.129.17.4075. [DOI] [PubMed] [Google Scholar]
- Aubin J, Lemieuz M, Tremblay M, Berard J, Jennotte L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol. 1997;192:432–445. doi: 10.1006/dbio.1997.8746. [DOI] [PubMed] [Google Scholar]
- Awgulewitsch A, Bieberich C, Bogarad L, Shashikant C, Ruddle FH. Structural analysis of the Hox3.1 transcription unit and the Hox3.2-Hox3.1 intergenic region. Proc Natl Acad Sci USA. 1990;87:6428–6432. doi: 10.1073/pnas.87.16.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk RP, Thebaud B, Greer JJ. Reductions in the incidence of nitrofen-induced diaphragmatic hernia by vitamin A and retinoic acid. Am J Physiol Lung Cell Mol Physiol. 2004;286:970–973. doi: 10.1152/ajplung.00403.2003. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Capecchi MR. Targeted disruption of the Hoxb-2 locus in mice interferes with expresssion of Hoxb-1 and Hoxb-4. Dev. 1996;122:3817–3828. doi: 10.1242/dev.122.12.3817. [DOI] [PubMed] [Google Scholar]
- Belotti D, Clausse N, Flagiello D, Alami Y, Daukandt M, Deroanne C, Malfoy B, Boncinelli E, Faiella A, Castronovo V. Expression and modulation of homeobox genes from cluster B in endothelial cells. Lab Invest. 1998;78:1291–1299. [PubMed] [Google Scholar]
- Bogue CW, Gross I, Vasavada H, Dynia DW, Wilson CM, Jacobs HC. Identification of Hox genes in newborn lung and effects of gestational age and retinoic acid on their expression. Am J Physiol. 1994;266:L448–L454. doi: 10.1152/ajplung.1994.266.4.L448. [DOI] [PubMed] [Google Scholar]
- Bogue CW, Lou LJ, Vasavada H, Wilson CM, Jacobs HC. Expression of Hoxb genes in the developing mouse foregut and lung. Am J Respir Cell Mol Biol. 1996;15:163–171. doi: 10.1165/ajrcmb.15.2.8703472. [DOI] [PubMed] [Google Scholar]
- Brandsma AE, Tibboel D, Vulto IM, de Vijlder JJM, Have-Opbroek AAW, Wiersinga WM. Inhibition of T3-receptor binding by nitrofen. Biochim Biophys Acta. 1994;1201:266–270. doi: 10.1016/0304-4165(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Mitsialis S, Brody JS, Williams MC. Retinoic acid alters the expression of pattern related genes in the developing lung. Dev Dyn. 1996;207:47–59. doi: 10.1002/(SICI)1097-0177(199609)207:1<47::AID-AJA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Causak RA, Zgleszewski SE, Zhang L, Cilley RE, Krummel TM, Chinoy MR. Differential gene expression at gestational days 14 and 16 in normal and nitrofen-induced hypoplastic murine fetal lungs with coexistent diaphragmatic hernia. Pediatr Pulm. 1998;26:301–311. doi: 10.1002/(sici)1099-0496(199811)26:5<301::aid-ppul1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Chinoy MR. Pulmonary hypoplasia and congenital diaphragmatic hernia: advances in the pathogenetics and regulation of lung development. J Surg Res. 2002a;106:209–223. doi: 10.1006/jsre.2002.6390. [DOI] [PubMed] [Google Scholar]
- Chinoy MR, Chi X, Cilley RE. Down-regulation of regulatory proteins for differentiation and proliferation in murine fetal hypoplastic lungs: altered mesenchymal-epithelial interactions. Pediatr Pulmnol. 2001;30:1–13. doi: 10.1002/ppul.1099. [DOI] [PubMed] [Google Scholar]
- Chinoy MR. Lung growth and development. Frontiers in Bioscience. 2003a;8:392–415. doi: 10.2741/974. [DOI] [PubMed] [Google Scholar]
- Chinoy MR, Graybill MM, Miller SA, Lang CM, Kauffman GL. Angiopoietin-1 and VEGF in vascular development and angiogenesis in hypoplastic lungs. Am J Physiol Lung Cell Mol Physiol. 2002b;283:L60–L66. doi: 10.1152/ajplung.00317.2001. [DOI] [PubMed] [Google Scholar]
- Chinoy MR, Nielsen HC, Volpe MV. Mesenchymal nuclear transcription factors in nitrofen-induced hypoplastic lung. J Surg Res. 2003b;108:203–211. doi: 10.1006/jsre.2002.6550. [DOI] [PubMed] [Google Scholar]
- Chinoy MR, Volpe MV, Cilley RE, Zgleszewski SE, Vosatka RJ, Martin A, Nielsen HC, Krummel TM. Growth factors and dexamethasone regulate Hoxb-5 protein in cultured murine fetal lungs. Am J Physiol. 1998;274:L610–L620. doi: 10.1152/ajplung.1998.274.4.L610. [DOI] [PubMed] [Google Scholar]
- Cilley RE, Zgleszewski SE, Krummel TM, Chinoy MR. Nitrofen dose-dependent gestational day-specific murine lung hypoplasia and left-sided diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. 1997;272:L362–L371. doi: 10.1152/ajplung.1997.272.2.L362. [DOI] [PubMed] [Google Scholar]
- Coletta PL, Shimeld SM, Chaudhuri C, Muller U, Clarke JP, Sharpe PT. Characterisation of the murine Hox3.3 gene and its promoter. Mech Dev. 1991;35:129–142. doi: 10.1016/0925-4773(91)90063-c. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Taylor HS. Endocrine Regulation of Hox genes. Endocrine Reviews. 2006;27:331–355. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- Davey MG, Biard J-M, Robinson L, Schwarz U, Danzer E, Adzick S, Flake AW, Hedrick HL. Surfactant protein expression is increased in the ipsilateral but not contralateral lungs of fetal sheep with left-sided diaphragmatic hernia. Pediatr Pulm. 2005;39:359–367. doi: 10.1002/ppul.20175. [DOI] [PubMed] [Google Scholar]
- Deschamps J, Van Den Akker E, Forlani S, De Graaff W, Oosterven T, Roelen B, Roelfsema J. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int J Dev Biol. 1999;43:635–650. [PubMed] [Google Scholar]
- Fauza DO, Wilson JM. Congenital diaphragmatic hernia and associated anomalies: their incidence, identification, and impact on prognosis. J Pediatr Surg. 1994;29:1113–1117. doi: 10.1016/0022-3468(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Fibi M, Zink B, Kessel M, Colberg-Poley AM, Labeit S, Lehrach H, Gruss P. Coding sequence and expression of the homeobox gene Hox1.3. Dev. 1988;102:349–359. doi: 10.1242/dev.102.2.349. [DOI] [PubMed] [Google Scholar]
- Foucher I, Volovitch M, Frain M, Kim JJ, Souberbielle J-C, Gan L, Unterman TG, Prochiantz A, Trembleau A. Hoxa-5 over expression correlates with IGFBP1 up regulation and postnatal dwarfism: evidence for an interaction between Hoxa-5 and forkhead box transcription factors. Dev. 2002;129:4065–4074. doi: 10.1242/dev.129.17.4065. [DOI] [PubMed] [Google Scholar]
- Gallot D, Marceau G, Coste K, Hadden H, Robert-Gnansia E, Laurichesse H, Labbe’ A, Dastugue B, Lemery D, Sapin V. Congenital diaphragmatic hernia: A retinoid-signaling pathway disruption during lung development. Birth Defects Research Part A: Clin Mol Teratol. 2005;73:523–531. doi: 10.1002/bdra.20151. [DOI] [PubMed] [Google Scholar]
- Golpon HA, Gerace MW, Moore MD, Miller HL, Tuder RM, Voelkel NF. Hox genes in human lung: altered expression in primary pulmonary hypertension and emphysema. Am J Pathol. 2001;158:955–966. doi: 10.1016/S0002-9440(10)64042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graba Y, Aragnol D, Pradel J. Drosophila hox complex downstream targets and the function of homeotic genes. Bioessays. 1997;19:379–388. doi: 10.1002/bies.950190505. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Babiuk RP, Barnard T. Etiology of congenital diaphragmatic hernia: the retinoid hypothesis. Pediatr Res. 2003;53:726–730. doi: 10.1203/01.PDR.0000062660.12769.E6. [DOI] [PubMed] [Google Scholar]
- Gutman A, Gilthorpe J, Bigby PWJ. Multiple positive and negative regulatory elements in the promoter of the mouse homeobox gene Hoxb-4. Mol Cell Biol. 1994 Dec;:8143–8154. doi: 10.1128/mcb.14.12.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko AY, Kafri T, Fainsod A, Razin A. Methylation of Hoxa-5 and Hoxb-5 and its relevance to expression during mouse development. Gene. 2003;302:65–72. doi: 10.1016/s0378111902010910. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Hombria JCG, Lovegrove B. Beyond homeoosis - Hox function in morphogenesis and organogenesis. Differentiation. 2003;71:461–476. doi: 10.1046/j.1432-0436.2003.7108004.x. [DOI] [PubMed] [Google Scholar]
- Hooiveld MHW, Morgan R, InDerRieden P, Houtzager E, Pannese M, Boncinelli E, Durston AJ. Novel interactions between Hox genes. Int J Dev Biol. 1999;43:665. [PubMed] [Google Scholar]
- Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics;2004. Pediatrics. 2006;117:168–182. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- Ijsselstijn H, Zimmerman LJ, Bunt JE, deJongste JC, Tibboel D. Prospective evaluation of surfactant composition in bronchoalveolar lavage fluid of infants with congenital diaphragmatic hernia and age-matched controls. Crit Care Med. 1998;26:573–580. doi: 10.1097/00003246-199803000-00035. [DOI] [PubMed] [Google Scholar]
- Kappen C. Hox genes in the lung. Am J Respir Cell Mol Biol. 1996;15:156–162. doi: 10.1165/ajrcmb.15.2.8703471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer R, Liu J, Deimling J, Tibboel D, Post M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156:1299–1306. doi: 10.1016/S0002-9440(10)65000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Nielsen HC. Hoxa-5 in mouse developing lung: cell-specific expression and retinoic acid regulation. Am J Physiol Lung Cell Mol Physiol. 2000;279:863–871. doi: 10.1152/ajplung.2000.279.5.L863. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Leblanc M, Gulemetova R, Lalancette-Hebert M, Lemieux M, Mandeville I, Jeannotte L. Respiratory adaptations to lung morphological defects in adult mice lacking Hoxa-5 gene function. Pediatr Res. 2004;56:553–562. doi: 10.1203/01.PDR.0000139427.26083.3D. [DOI] [PubMed] [Google Scholar]
- Komuves LG, Shen W, Kwong A, Stelnicki E, Rozenfeld S, Oda Y, Blink A, Krishnan K, Lau B, Mauro T. Changes in Hoxb6 homeodomain protein structure and localization during human epidermal development and differentiation. Dev Dyn. 2000;218:636–647. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1014>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Larochelle C, Tremblay M, Bernier D, Aubin J, Jeannotte L. Multiple cis-acting regulatory regions are required for restricted spatio-temporal Hoxa5 gene expression. Dev Dyn. 1999;214:127–140. doi: 10.1002/(SICI)1097-0177(199902)214:2<127::AID-AJA3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Le M, Zhongyong L, Cilley RE, Donahue HJ, Chinoy MR. Connexin 43 gene expression in mice with cardiopulmonary defects. Frontiers in Bioscience. 2006;11:3014–3025. doi: 10.2741/2029. [DOI] [PubMed] [Google Scholar]
- Levesque BM, Vosatka RJ, Volpe MV. Dihydrotestosterone stimulates branching morphogeneisis, cell proliferation and programmed cell death in mouse embryonic lung explants. Pediatr Res. 2000;47:481–491. doi: 10.1203/00006450-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Dev. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- Manley NR, Barrow JR, Zhang T, Capecchi MR. Hoxb-2 and Hoxb-4 act together to specify ventral body wall formation. Dev Biol. 2001;237:130–144. doi: 10.1006/dbio.2001.0365. [DOI] [PubMed] [Google Scholar]
- Manson JM. Mechanisms of Nitrofen Teratogenesis. Environmental Health Perspec. 1986;70:137–147. doi: 10.1289/ehp.8670137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Rijli FM, Chambon P. Homeobox genes in embryogenesis and pathogenesis. Pediatr Res. 1997;42(4):421–429. doi: 10.1203/00006450-199710000-00001. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Rakshit S, Yallowitz AR, Loken L, Jeannotte L, Capecchi MR, Wellik DM. Hox patterning of the vertebrate rib cage. Dev. 2007;134:2981–2989. doi: 10.1242/dev.007567. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, Lemeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development. II. Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Mollard R, Dziadek M. Homeobox genes from clusters A and B demonstrate characteristics of temporal colinearity and differential restrictions in spatial expression domains in the branching mouse lung. Int J Dev Biol. 1997;41:655–666. [PubMed] [Google Scholar]
- Morgan R, Pettengell R, Sohal J. The double life of Hoxb-4. FEBS Letters. 2004;578:1–4. doi: 10.1016/j.febslet.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Noble BR, Babiuk RP, Clugston RD, Underhill TM, Sun H, Kawaguchi R, Walfish PG, Blomhoff R, Gundersen TE, Greer JJ. Mechanisms of action of the congenital diaphragmatic hernia-inducing teratogen nitrofen. Am J Lung Cell Mol Physiol. 2007;293:L1079–L1087. doi: 10.1152/ajplung.00286.2007. [DOI] [PubMed] [Google Scholar]
- Odenwald WF, Garbern J, Arnheiter H, Tournier-Lasserve E, Lazzarine RA. The Hox1.3 homeo box protein is a sequence-specific DNA binding phosphoprotein. Genes and Development. 1989;3:158–172. doi: 10.1101/gad.3.2.158. [DOI] [PubMed] [Google Scholar]
- Packer AI, Mailutha KG, Ambrozewicz LA, Wolgemuth DJ. Regulation of the Hoxa4 and Hoxa5 genes in the embryonic mouse lung by retinoic acid and TGFbeta1: implications for lung development and patterning. Dev Dyn. 2000;217(1):62–74. doi: 10.1002/(SICI)1097-0177(200001)217:1<62::AID-DVDY6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Pan G, Simpson RU. Antisense knockout of Hoxb-4 blocks 1,25-dihydroxyvitamin D3 inhibition of c-myc expression. J Endocrin. 2001;169:153–159. doi: 10.1677/joe.0.1690153. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos A, Akam M. Hox go omics; insights from drosophila into Hox gene targets. Genome Biol. 2007;8:208. doi: 10.1186/gb-2007-8-3-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajatapiti P, Keijzer R, Blommaart PE, Lamers WH, Visser TJ, Tibboel D, Rottier R. Spatial and Temporal Expression of Glucocorticoid, Retinoid, and Thyroid hormone receptors is not altered in lungs of congenital diaphragmatic hernia. Pediatr Res. 2006;60:693–698. doi: 10.1203/01.pdr.0000246245.05530.02. [DOI] [PubMed] [Google Scholar]
- Ramirez-Solis R, Zheng H, Whiting J, Krumlauf R, Bradley A. Hoxb-4 (Hox2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993;73:279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Rancourt DE, Tsuzuki T, Capecchi MR. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes and Development. 1995;9:108–112. doi: 10.1101/gad.9.1.108. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugman SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human Hox loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama J, Yokouchi Y, Kuroiwa A. Coordinated expression of Hoxb genes and signaling molecules during development of the chick respiratory tract. Dev Biol. 2000;227:12–27. doi: 10.1006/dbio.2000.9880. [DOI] [PubMed] [Google Scholar]
- Serpente P, Tumpel S, Ghyselinck NB, Niederreither K, Wielemann LM, Dolle’ P, Chambon P, Krumlauf R, Gould AP. Direct crossregulation between retinoic acid receptor beta and hox genes during hindbrain segmentation. Dev. 2005;132:503–513. doi: 10.1242/dev.01593. [DOI] [PubMed] [Google Scholar]
- Ten Have-Opbroek AAW. Lung development in the mouse embryo. Exp Lung Res. 1991;17:111–130. doi: 10.3109/01902149109064406. [DOI] [PubMed] [Google Scholar]
- Theokli C, El-Kadi AS, Morgan R. TALE class homeodomain gene Irx5 is an immediate downstream target for hoxb4 transcription regulation. Dev Dyn. 2003;227:48–55. doi: 10.1002/dvdy.10287. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Martin A, Vosatka RJ, Mazzoni CL, Nielsen HC. Hoxb-5 expression in the developing mouse lung suggests a role in branching morphogenesis and epithelial cell fate. Histochem Cell Biol. 1997;108:495–504. doi: 10.1007/s004180050190. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Nielsen HC, Archavachotikul K, Ciccone TJ, Chinoy MR. Thyroid hormone affects airway morphogenesis and epithelial cell fate during the late pseudoglandular period of mouse lung development. Molecular Genetics and Metabolism. 2003a;80:242–254. doi: 10.1016/j.ymgme.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Pham L, Lessin M, Ralston SJ, Bhan I, Cutz E, Nielsen HC. Expression of Hoxb-5 during human lung development and in congenital lung malformations. Birth Defects Research Part A: Clin Mol Teratol. 2003b;67:550–556. doi: 10.1002/bdra.10086. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Ramadurai SM, Pham LD, Nielsen HC. Hoxb-5 down regulation alters tenascin-C, FGF10 and Hoxb gene expression patterns in pseudoglandular period fetal mouse lung. Frontiers in Bioscience. 2007;12:860–873. doi: 10.2741/2108. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Vosatka RJ, Nielsen HC. Hoxb-5 control of early airway formation during branching morphogenesis in the developing mouse lung. Biochim Biophys Acta. 2000;1475:337–345. doi: 10.1016/s0304-4165(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Wall NA, Jones MJ, Hogan BLM, Wright CVE. Expression and modification of Hox 2.1 protein in mouse embryos. Mech Dev. 1992;37:111–120. doi: 10.1016/0925-4773(92)90073-s. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dolle’ P, Cardoso WV, Niederreither K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol. 2006;297:433–445. doi: 10.1016/j.ydbio.2006.05.019. [DOI] [PubMed] [Google Scholar]