Abstract
Multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) result in inflammatory white matter lesions in the CNS. However, information is sparse with regard to the effects of autoimmune demyelinating disease on gray matter regions. Therefore, we studied the late effects of chronic EAE in C57BL/6 mice on the spinal cord gray matter using immunohistochemistry. Here, EAE induced marked astrocytic, microglial, and macrophage activation in the ventral horn gray matter, without any motoneuron loss. Activated caspase-3 was also increased in the ventral horn gray matter. Furthermore, activated poly (ADP-ribose) polymerase (PARP), another apoptotic marker, co-localized with myelin basic protein (MBP) of oligodendrocyte processes, but not with the oligodendroglial cell body marker, adenomatous polyposis coli gene clone CC1 (APC-CC1), or with neurofilament marker (RT-97) or synaptophysin of axonal arbors. However, there was no associated increase in the number of terminal deoxynucleotidyl transferase (TdT) mediated-dUTP nick end labeling positive nuclei in the spinal cord gray matter of EAE mice. In addition, co-localization of MBP and the low-affinity neurotrophin receptor, p75, was demonstrated, further supporting the notion of apoptotic oligodendrocyte process degeneration in the gray matter of EAE mice.
Keywords: multiple sclerosis, inflammation, mouse, degeneration, demyelination
Multiple sclerosis (MS) is an immune-mediated disorder, which affects the CNS with inflammatory lesions and demyelination (Lucchinetti et al., 1998). In addition to the focal destruction of myelin in the white matter tracts of the brain and spinal cord, MS lesions may also exhibit transected axons (Trapp et al., 1998). The latter findings are of particular clinical importance, as neurological disability has been correlated with axonal loss in the spinal cord of chronic MS patients (Bjartmar et al., 2000). Interestingly, in experimental autoimmune encephalomyelitis (EAE), an animal model for MS, a similar axonal loss in spinal cord white matter is correlated with permanent neurological disability (Wujek et al., 2002). Therefore, from a pathological perspective, the axonal transections encountered in MS and EAE may result in partial or complete neurological disconnection syndromes, which are similar to those encountered following traumatic brain and spinal cord injuries.
In recent years, an emerging literature from the neuroimaging field has demonstrated pathologic changes remote from the inflammatory white matter lesions of MS and EAE. For instance, human imaging studies have demonstrated gray matter atrophy in patients with MS (Chard et al., 2002; Dalton et al., 2004). Gray matter atrophy has been similarly documented in the cerebellar cortical gray matter of mice with EAE (MacKenzie-Graham et al., 2006). However, pathological effects within gray matter structures remain unclear.
The goal of this study was to investigate long-term effects of EAE on spinal cord gray matter beyond the sites of inflammatory white matter lesions. During the chronic phase of EAE in C57BL/6 mice, we investigated late glial and inflammatory changes in the ventral horn gray matter as well as in the dorsal corticospinal tract. In addition, the ventral horn gray matter was studied for signs of neuronal and myelin degeneration. We report that EAE induces a marked astrocytic, microglial, and macrophage activation in the ventral horn gray matter in the absence of motoneuron death, but in the presence of apoptotic degeneration of oligodendrocyte processes. This degeneration of oligodendrocyte processes was associated with the expression of the low affinity neurotrophin receptor, p75.
Experimental Procedures
Animal procedures
All procedures were performed according to the standards established by the National Institutes of Health (NIH) Guide for the care and use of Laboratory Animals (NIH publications No. 80-23, revised 1996) and were approved by the Chancellor's Animal Research Committee at UCLA. All efforts were made to minimize the number of animals used and their suffering. Two-month-old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) were used for the study (n=14). To induce active EAE, rodent myelin oligodendrocyte glycoprotein (MOG) peptide, amino acids 35–55 (200 μg/mouse), mixed with mycobacterium tuberculosis (200 μg/mouse) in 0.2 ml complete Freund's adjuvant (CFA) was administered by s.c. flank injection on day 0 and day 7, and the pertussis toxin (500 ng/mouse in 200 μl PBS) was administered on day 0 and day 2 (Hjelmstrom et al., 1998). Controls consisted of gender, age and strain matched mice. The mice were observed daily, and neurological deficits were ranked and recorded on a five-point scale as previously described: 0, unaffected; 1, tail limpness; 2, failure to resist on attempt to roll over; 3, partial paralysis; 4, complete paralysis; 5, moribund (Suen et al., 1997).
Spinal cord tissue fixation and processing
All mice were deeply anesthetized (sodium pentobarbital, Abbott Laboratories, North Chicago, IL, USA) and perfused transcardially with phosphate-buffered 4% paraformaldehyde (pH 7.4) at 42 days post-immunization. The spinal cord was removed and post-fixed at room temperature with phosphate-buffered 4% paraformaldehyde (pH 7.4) for 1–2 h. Then, the spinal cord was immersed in 30% sucrose at 4 °C overnight prior to being embedded in O.C.T cryostat mounting compound (Sakura, Torrence, CA, USA). The L4 and L5 spinal cord segments were cryosectioned serially in the transverse plane (14 μm thickness). Subsequently, the spinal cord sections were mounted on glass slides for histological and immunohistochemical studies.
Histology
Spinal cord sections of the L4 and L5 segments were stained with 0.1% Cresyl Violet in 1% acetic acid for 15 min at room temperature. After a brief rinse with distilled water, the sections were dehydrated through increasing concentrations of ethanol and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA, USA).
Immunohistochemistry
For light stable immunohistochemistry, L4/L5 spinal cord sections on slides were first treated with 0.3% H2O2 for 10 min to quench endogenous peroxidase activity. Then, the sections were blocked with 5% BSA/0.3% Triton-X-100 in PBS (pH 7.4) at room temperature for 1 h followed by a brief rinse with PBS. Next, the sections were incubated with primary antibodies (Table 1), which were diluted in the blocking buffer overnight at 4 °C. After extensive washing in PBS, the tissue sections were incubated with biotinylated secondary antibodies of appropriate species (1:200 in PBS, Vector Laboratories, Burlingame, CA) at room temperature for 1 h, followed by incubation with avidin-biotin complex (1:200 in PBS, Vectastain ABC Elite kit; Vector Laboratories Inc., Burlingame, CA, USA) at room temperature for 1 h. Finally, immunoreactivity (IR) was visualized with diaminobenzidine (DAB; Sigma, St. Louis, MO, USA) and the sections were coverslipped in Permount. For fluorescent immunohistochemical staining, the tissue sections were blocked in 5% BSA/0.3% Triton-X-100 in PBS (pH 7.4) at room temperature for 1 h, followed by incubation with primary antibodies overnight at 4 °C. After extensive washing, the sections were incubated with secondary antibodies conjugated to Rhodamine Red™ (1:100 in PBS; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), or Alexa Fluor 488 (green) or 594 (red) of appropriate species (1:500 in PBS; Invitrogen, Carlsbad, CA, USA) at room temperature for 1 h. The fluorescence labeled sections were dried and coverslipped in Vectashield (Vector Laboratories Inc.). For colocalization studies, the sections were coverslipped in Vectashield with DAPI (Vector Laboratories Inc.).
Table 1.
Listing of primary antibodies used
| Antibody | Species and type | Dilution | Source | Reference |
|---|---|---|---|---|
| AIF1 | Goat polyclonal | 1:100 | Abcam #ab5076 | (Ramprasad et al., 1996) |
| APC-CC1 | Mouse monoclonal | 1:500 | Calbiochem #OP80 | (McTigue et al., 2001) |
| Caspase3 | Rabbit polyclonal | 1:100 | Promega #G748 | (Guseva et al., 2002) |
| CD68 | Rat monoclonal | 1:50 | Cell Science #HM1070 | (Autieri and Agrawal, 1998) |
| ChAT | Goat polyclonal | 1:200 | Chemicon #AB144P | (Shiromani et al., 1987) |
| GFAP | Rabbit polyclonal | 1:1000 | Chemicon #AB5804 | (Hammerle et al., 2003) |
| MBP | Rat monoclonal | 1:200 | Chemicon #MAB386 | (Glynn et al., 1987) |
| p75 | Rabbit polyclonal | 1:100 | Promega #G323A | (Chao and Hempstead, 1995) |
| PARP | Rabbit polyclonal | 1:100 | Cell signaling #9544 | (Oliver et al., 1998) |
| RT97 | Mouse monoclonal | 1:2000 | Hybridoma bank, NICHD | (Young and Black, 2004) |
| Synaptophysin | Mouse monoclonal | 1:2000 | Chemicon #MAB5258 | (Masliah et al., 2001) |
Terminal deoxynucleotidyl transferase (TdT) mediated-dUTP nick end labeling (TUNEL) staining
Apoptotic cells were detected with an in situ TUNEL kit (Chemicon, Temecula, CA, USA). L4/L5 spinal cord sections on slides were treated with pre-cooled ethanol:acetic acid (2:1) for 5 min at −20 °C for permeabilization. Then, the manufacturer's protocol was followed in labeling DNA fragments with digoxigenin-conjugated nucleotides and subsequently with anti-digoxigenin antibody that is conjugated to peroxidase. The apoptotic cells were visualized by DAB (Sigma). The tissue sections were counterstained with 0.5% (w:v) Methyl Green. The slides were mounted in Permount.
Light microscopy and quantitative analysis
Four non-overlapping light stable or fluorescent microscopic images of the L4-L5 ventral horn from all animals were captured (Objective lens 40×) with a Micropublisher five megapixel digital camera (Q Imaging, Burnaby, BC) attached to a Nikon E600 microscope (Nikon Inc., Melville, NY, USA) and analyzed using C-imaging software (Compix Inc., Sewickley, PA, USA). Two regions of interest (ROI) were selected for quantitative analysis. One ROI was within the ventral horn gray matter, which contains spinal motoneurons innervating hind limb muscles, and the other ROI was within the ventral portion of the dorsal funiculus (Fig. 2). The quantitative data were presented as mean labeled area as a percentage of the ROI. The motoneurons from eight hemi-sections per mouse were counted using the Abercrombie method (Coggeshall and Lekan, 1996). Sections labeled with fluorescent markers for colocalization studies were photographed using a confocal microscope (TCP-SP; Leica, Mannheim, Germany).
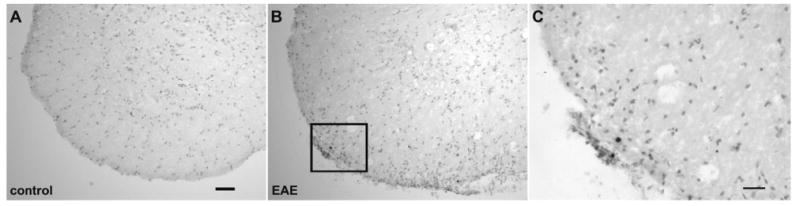
Fig. 2.
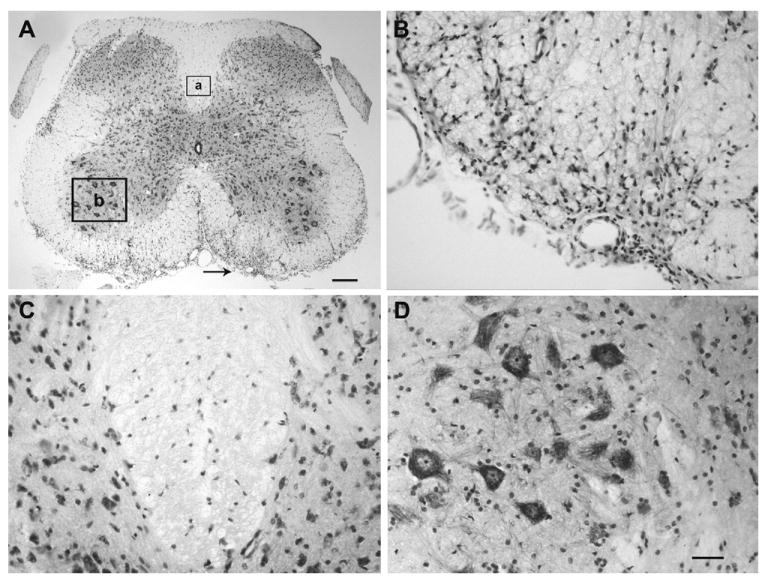
EAE induces inflammatory infiltrates in the spinal cord of EAE mice. (A) Cresyl Violet staining shows the inflammatory infiltrates in the white matter of the lumbar segment (L4/L5) of the EAE mouse. The arrow pointed region in (A) is shown at higher magnification in (B). The two boxes (box a and box b) in (A) indicate the areas chosen for quantitative analysis of activation of the inflammatory markers. These two boxed areas in (A) are shown at higher magnification in (C) for dorsal column and (D) for ventral horn after Cresyl Violet staining. Scale bar=250 μm (A); 50 μm (B–D).
Statistical analysis
All quantitative data were presented as mean±S.E.M. Statistical analysis was performed by using one-way analysis of variance (ANOVA) with Tukey's multiple comparison test (Sigma-stat 3.1, Systat Software, Inc., Point Richmond, CA, USA), and P<0.05 was regarded as reflecting a statistically significant difference between samples.
Results
Clinical and general pathological features of EAE mice
Mice of the EAE group demonstrated onset of clinical disease at 18 days after EAE induction (Fig. 1). Clinical signs peaked at 27 days after induction and remained stable until the termination of experiments at 42 days post-induction (clinical score=2.3±0.3, n=8) (Fig. 1). This relatively milder form of EAE was induced so that mice could be followed for weeks chronically without reaching a moribund state. In contrast, mice of the control series remained neurologically intact for the duration of the study period (n=6). The Cresyl Violet staining demonstrated patchy cellular infiltrates in the L4 and L5 spinal cord white matter of EAE mice (Fig. 2) but not in mice of the control series. These cell accumulations were often associated with vascular structures and were consistent with classic description of white matter inflammation in EAE. Yet, the dorsal columns, including our selected region of interest for quantitative morphological studies, were devoid of such cellular infiltrates at these segmental levels in all studied animals. Furthermore, the spinal cord gray matter demonstrated an absence of any local cellular infiltrates.
Fig. 1.
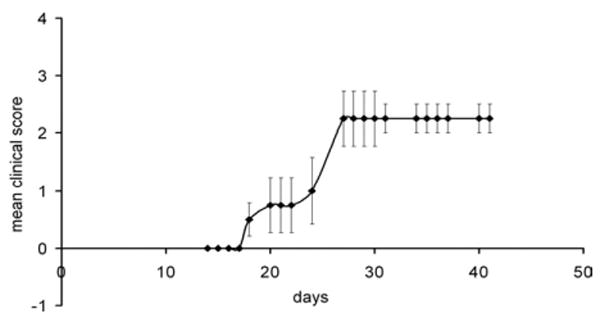
EAE induced neurological deficits in male C57BL/6 mice (n=8). The mice were observed daily for clinical signs and scored on a scale of 0–5 from 42 days after EAE induction.
Astroglial reactions
Immunohistochemistry for glial fibrillary acidic protein (GFAP) detected normal and reactive astroglia in the lumbar spinal cord of both control and EAE mice. In control mice, numerous astrocytic GFAP immunoreactive processes were detected throughout the gray and white matters (Fig. 3). In contrast, the gray matter of the L4/L5 spinal cord segments of EAE mice demonstrated astrocytes with swollen processes and increased GFAP IR. In control mice, 10.6±2.5% area of the ventral horn gray matter and 11.4±1.4% area of the dorsal column white matter expressed GFAP IR (n=4). In EAE mice, the ventral horn gray matter demonstrated a markedly increased GFAP IR (35.1±4.4%, n=4, P<0.05), whereas no significant increase was observed in GFAP IR in the dorsal column white matter (15.5±3.2%, n=4).
Fig. 3.
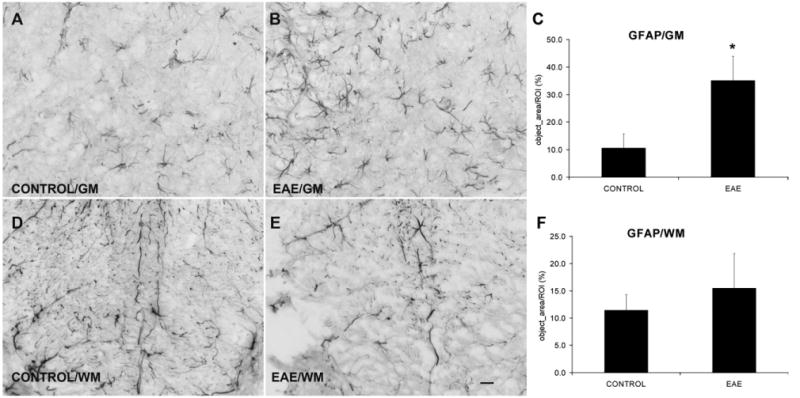
EAE increases astrocyte activation in the GM but not in the WM. Immunostaining of GFAP indicates the activation of astrocytes. (A, B) GFAP IR in the ventral horn gray matter from control and EAE mice, respectively. (D, E) GFAP IR in the WM from control and EAE mice, respectively. (C, F) Quantification of GFAP IR shows a significant astrocytic activation in the GM, but not in the WM after EAE induction (n=4) compared with controls (n=4). GM=gray matter; WM=white matter. Scale bar=25 μm (A, B, D, E).
Microglial activation
Allograft inflammatory factor (AIF1) IR demonstrated microglia with thin and ramified processes in both the gray and white matters of control mice. In EAE mice, however, several microglia were retracted and exhibited swollen processes particularly in gray matter as well as staining of prominent rounded structures, suggestive of cell bodies of either resting or activated macrophages (Fig. 4). In control mice, quantitative studies showed 5.3±1.1% area of the ventral horn gray matter and 4.9±0.8% area of the dorsal column white matter expressing AIF1 IR (n=4). In EAE mice, the ventral horn gray matter showed a markedly increased AIF1 IR (32.2±1.5%, n=4, P<0.05), whereas no significant difference in AIF1 IR was detected in the dorsal column white matter (6.3±0.5%, n=4).
Fig. 4.
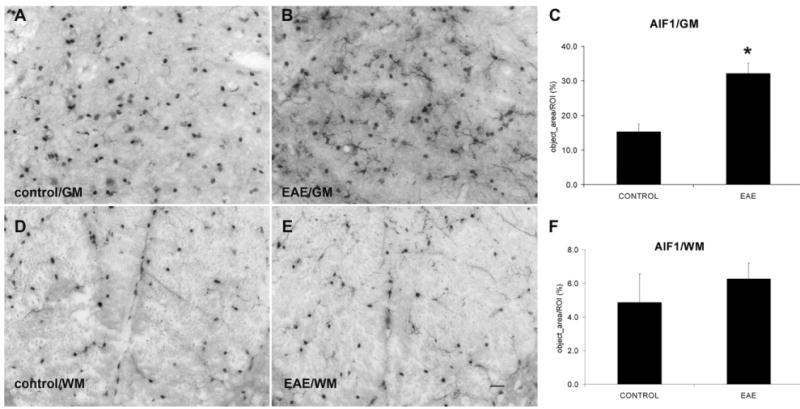
EAE increases microglial activation in the GM but not in the WM. (A, B) AIF1 IR in the ventral horn GM from control and EAE mice, respectively. (D, E) AIF1 IR in the WM from control and EAE mice, respectively. (C, F) Quantification of AIF1 IR shows a significant increase in microglial activation in the GM, but not in the WM after EAE induction (n=4) compared with controls (n=4). GM=gray matter; WM=white matter. Scale bar=25 μm (A, B, D, E).
Macrophage activation
IR for CD68 detected a few single activated macrophages in mice of the control series. In contrast, many macrophages were scattered in both the gray and white matter in EAE mice (Fig. 5). In control mice, quantitative studies demonstrated 0.3±0.1% area of the ventral horn gray matter and 0.1±0.0% area of the dorsal column white matter expressing CD68 (n=4). In EAE mice, the ventral horn gray matter and dorsal column white matter both showed a markedly increased CD68 IR (1.1±0.2% and 1.0±0.4%, respectively, n=4, P<0.05).
Fig. 5.
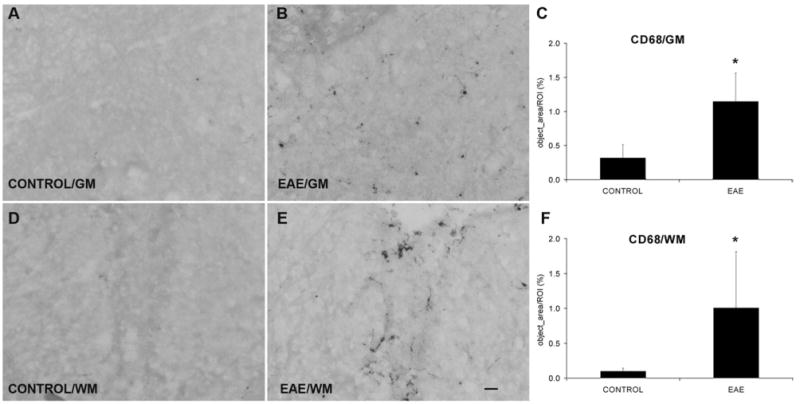
EAE increases macrophage activation both in the GM and in the WM. (A, B) CD68 immunostaining in the ventral horn GM from control and EAE mice, respectively. (D, E) CD68 IR in the WM from control and EAE mice, respectively. (C, F) Quantification of CD68 IR shows a significant increase in both the GM and the WM regions after EAE induction (n=4) compared with controls (n=4). GM=gray matter; WM=white matter. Scale bar=25 μm (A, B, D, E).
Absence of motoneuron loss in the ventral horn gray matter
In light of the glial, microglial, and macrophage activation in the ventral horn gray matter of EAE mice, we performed choline acetyl transferase (ChAT) immunohistochemistry and motoneuron counts to determine whether any neuronal degeneration took place in the L4/L5 ventral horn gray matter (Fig. 6). Only ChAT immunoreactive motoneurons exhibiting a nucleus were included in the analyses. No difference in nuclear size was present between the ChAT immunoreactive motoneurons of the control and experimental series (see Experimental Procedures). In the control series, 5.9±0.6 ChAT immunoreactive motoneurons were detected per hemi-section (n=4). In EAE mice, 6.0±0.5 ChAT immunoreactive motoneurons were detected per hemisection (n=4). There was no statistical difference between the number of ChAT immunoreactive motoneurons in the control and experimental series.
Fig. 6.
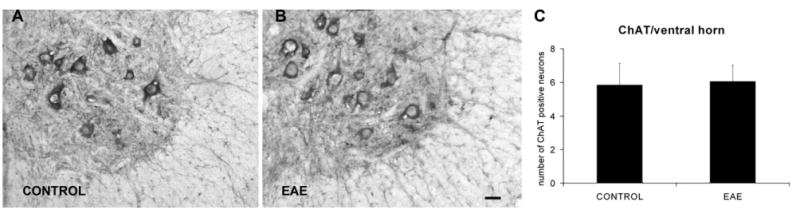
EAE does not induce motoneuron loss. Motoneurons were identified using ChAT immunohistochemistry. (A, B) Motoneurons in the L4 ventral horn region of control and EAE mice, respectively. (C) There is no significant difference in motoneuron counts between control (n=4) and EAE mice (n=4). Scale bar=10 μm (A, B).
Activation of apoptosis in ventral horn neuropil
Although our neural counts showed no signs of any motoneuron loss in the ventral horn of EAE mice, we pursued additional morphological studies in attempts to identify other signs of neural degeneration to explain our unexpected findings of marked glial and inflammatory reactions in the spinal cord gray matter in these mice. For this purpose, TUNEL staining and immunohistochemistry for the activated form of caspase-3, a marker for apoptosis, were performed.
In mice of the control series, TUNEL staining of nuclei was very rarely encountered (Fig. 7). In contrast, in the EAE series, TUNEL positive nuclei were frequently encountered in the white matter, typically in small clusters at the surface of the spinal cord and in close proximity to vascular structures, reminiscent of the location of cellular infiltrates associated with the inflammatory disease (Fig. 7). However, the ventral horn motor nuclei were nearly devoid of TUNEL positive cells in mice of both the control and EAE series and there was no significant difference between the two groups. Specifically, 0.00±0.00 cells in the ventral horn per section were TUNEL positive in the control series (n=4) and 0.20±0.28 cells in the ventral horn per section were positive in the EAE series (n=4) (Fig. 7).
Fig. 7.
TUNEL positive nuclei maybe frequently encountered in the white matter but not in the gray matter in EAE mice. (A, B) TUNEL staining in the L4 ventral horn of control and EAE mice, respectively. In the control series (A), it is rare to encounter any TUNEL positive nuclei, whereas in EAE series (B), the TUNEL positive staining is frequently encountered in the white matter but not in the gray matter. (C) The boxed region in (B) is showed at higher magnification. Scale bar=50 μm (A, B); 25 μm (C).
In mice of both the control and EAE series, activated caspase-3 was not detected in the somata of neurons or glial cells. However, in the EAE series, numerous small and irregularly shaped particles were immunoreactive to activated caspase-3. These were encountered throughout the gray matter, typically in small clusters and short fragments in the gray matter (Fig. 8). Often, the caspase-3 immunoreactive structures demonstrated a string-of-pearl appearance with small beaded structures being interconnected by very thin thread-like segments. In contrast, the spinal cord gray matter of mice of the control series demonstrated only sparse such IR for activated caspase-3 (Fig. 8). Quantitative analysis demonstrated a significant increase in IR for activated caspase-3 within the ventral horn of EAE mice (n=4) compared with the corresponding area in the mice of the control series (n=4; P<0.05) (Fig. 8).
Fig. 8.
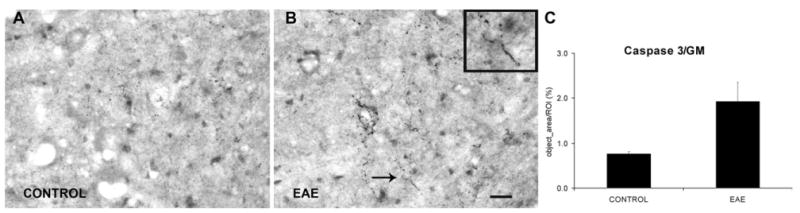
The apoptotic pathway is activated in EAE mice. (A, B) Activated caspase-3 IR in the neuropil of the L4 ventral horn GM of control and EAE mice respectively. The region indicated by arrow in (B) is shown at higher magnification in the inset of (B). (C) Increased expression of activated caspase-3 was detected at the L4 ventral horn region in EAE mice (n=4) compared with control (n=4). GM=gray matter. Scale bar=25 μm (A, B).
Degeneration of oligodendrocyte processes in the spinal cord gray matter
In attempts to determine the nature of the structures exhibiting IR for the activated caspase-3, double labeling immuno-fluorescence studies combined with confocal microscopy were performed. Poly (ADP)-ribose polymerase (PARP) was another apoptosis marker used in this study. The activation of PARP was achieved by cleavage of 116 kDa PARP into 24 kDa and 89 kDa fragments by activated caspases. The PARP antibody used in this study only recognized the 89 kDa fragments generated by activated caspase-3 mediated cleavage. In this study, IR for synaptophysin and neurofilament marker (RT-97) served to demonstrate axonal arbors, whereas IR for myelin basic protein (MBP) served to detect myelin.
Detailed double immunofluorescence studies showed absence of co-localization of PARP and RT-97 in the spinal cord gray matter of mice of the control and EAE series (Fig. 9). Similarly, PARP and synaptophysin also did not co-localize in the control or EAE series (Fig. 9). Thus, there was no support for active axonal degeneration by apoptosis in the spinal cord gray matter. However, in mice of the EAE series, PARP demonstrated frequent co-localization with MBP with absence of colocalization with adenomatous polyposis coli gene clone CC1 (APC-CC1) in oligodendrocyte cell bodies (Fig. 10). This suggests active degeneration of oligodendrocyte processes (Fig. 10). In contrast, no co-localization of PARP and MBP was detected in mice of the control series. In addition, as p75, the low-affinity neurotrophic factor receptor, has previously been associated with degeneration of myelin forming oligodendrocyte processes by apoptosis after spinal cord injury (Beattie et al., 2002), we performed co-localization studies for MBP and p75. Interestingly, we detected frequent colocalization of MBP and p75 in the spinal cord gray matter of mice of the EAE series (Fig. 10), providing additional support for active degeneration of myelin in these animals. In contrast, mice of the control series showed an absence of p75 and MBP co-localization in the spinal cord.
Fig. 9.
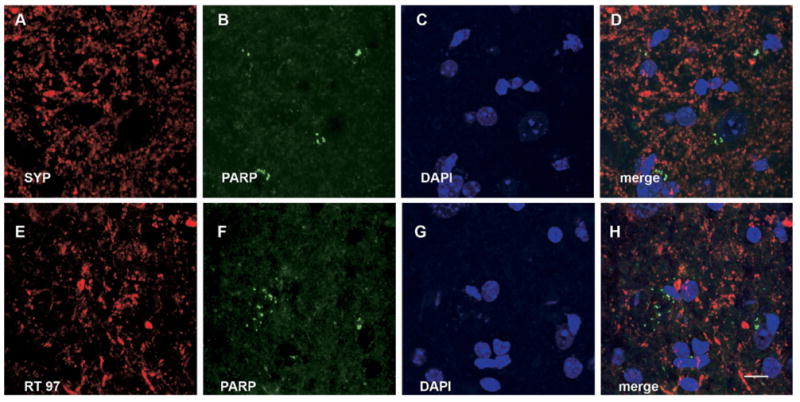
The apoptotic cell death markers PARP is not associated with synaptophysin or with neurofilament (RT 97) in the ventral horn gray matter of EAE mice. (A, E) Synaptophysin and RT97 IR, respectively; (B, F) PARP IR; (C, G) DAPI staining. (D) Merge of (A), (B) and (C). (H) Merge of (E), (F) and (G). (D, H) There is no association between PARP and SYP or between PARP and RT97. Scale bar=10 μm.
Fig. 10.
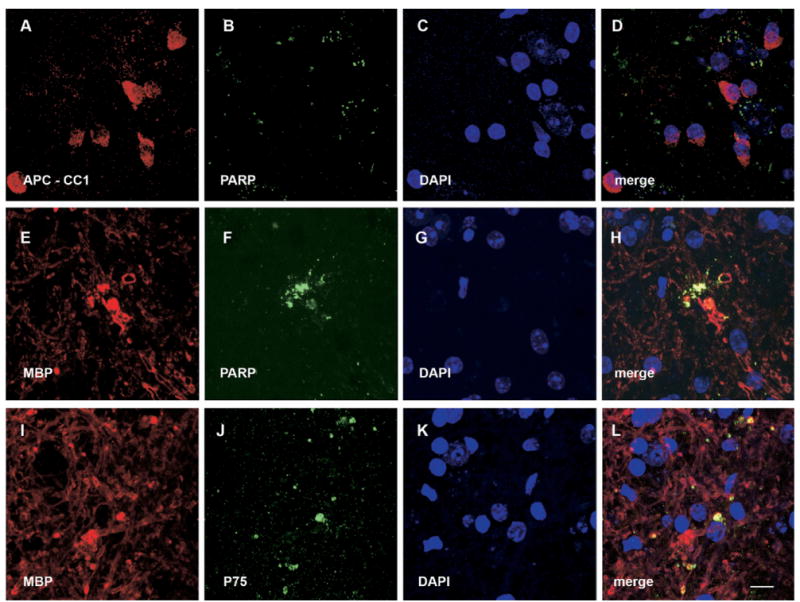
The apoptotic cell death markers PARP and p75 are associated with MBP, but not detectable in oligodendrocyte cell bodies (APC-CC1) in the ventral horn gray matter of EAE mice. (A) APC-CC1. (E, I) MBP IR. (B, F) PARP IR. (J) p75 IR. (C, G, K) DAPI staining. (D) Merge of (A), (B) and (C), and it indicates that there is no association between PARP and APC-CC1. (H) Merge of (E), (F) and (G), and it shows that MBP is colocalized with PARP. (L) Merge of (I), (J) and (K), and it shows that MBP is colocalized with p75. Scale bar=10 μm.
Discussion
Here, we examined glial and inflammatory reactions beyond classic inflammatory lesions of the white matter tracts in EAE mice. We show an increased IR for astrocytes, microglia, and macrophages in the ventral horn of the lumbar spinal cord in the absence of any detectable motoneuron loss. However, a marked activation of caspase-3, a marker for apoptosis, was encountered in the gray matter neuropil. Also, PARP, a product of the enzymatic activity of activated caspase-3, co-localized with myelin fragments but not with axonal markers in the ventral horn white matter. TUNEL positive nuclei were nearly absent in the spinal cord gray matter in the mice of both the control and experimental series. Furthermore, p75, which may be associated with apoptosis, also co-localized with the myelin debris. Taken together, our data show that the induction of EAE results in a marked increase in glial activation and suggest degeneration of oligodendrocyte processes by apoptosis in spinal cord gray matter.
Glial and inflammatory reactions in the spinal cord gray matter
Astrocytes and microglia are activated in the CNS after various forms of injury (Popovich et al., 1997; Fawcett and Asher, 1999). In both EAE and clinical MS, inflammatory lesions occur in the white matter tracts of the CNS, resulting in demyelination and a transection injury of axons (Trapp et al., 1998, 1999). Nodules composed of microglia and macrophages can be observed in close proximity to the plaque margins of such inflammatory lesions affecting the white matter tracts (Prineas et al., 2001). Interestingly, morphological studies of intracortical lesions in MS have demonstrated signs of axonal or neuronal injury, microglial activation, but an absence of lymphocytic infiltrates (Bo et al., 2003; Vercellino et al., 2005). While few studies have addressed gray matter abnormalities in EAE, the activities of astrocytes and microglia have been described (Liedtke et al., 1998; Aharoni et al., 2005). Ultrastructurally, microglia may be seen to phagocytose dark degenerating terminals in the spinal cord gray matter in EAE (Gehrmann et al., 1993). In the present study, we report the activation of astrocytes, microglia, and macrophages in the ventral horn of the spinal cord gray matter in response to EAE. It is interesting to note that the activation of these cells occurs without the formation of distinct inflammatory infiltrates or nodules. It is unclear whether this inflammation within the spinal cord gray matter exerts a primary role in the destruction of myelin, or whether it may reflect a delayed response to the EAE-induced wallerian degeneration of axons and secondary destruction of myelin.
Effects of EAE on motoneuron survival
It was of particular interest here to address the fate of spinal motoneurons following the induction of EAE, as previous studies have demonstrated that motoneuron death may be associated with the activation of macrophages in the ventral horn of spinal cord gray matter (Mattsson et al., 1999). With regard to EAE-induced effects on the fate of spinal motoneurons, there is some controversy in the literature, which has suggested both presence and absence of motoneuron loss. For instance, immunization of Lewis rats with MBP resulted in a quantitative loss of ventral horn neurons with motoneurons also being subject to a direct lymphocyte attack (Smith et al., 2000). In contrast, when EAE was induced in mice by the MOG peptide and motoneurons were identified by a yellow fluorescent protein transgene driven by thy1 promoter elements, there were no signs of motoneuron loss (Bannerman et al., 2005). Similarly, there was no support for degeneration and death of spinal motoneurons in the present study, where motoneurons were identified immunohistochemically by the expression of ChAT. It is plausible that differences in experimental models and methods of analysis may contribute to the above differences between studies.
Wallerian degeneration of axons after lesions to white matter tracts
Traumatic injuries and inflammatory lesions of the white matter tracts of the brain and spinal cord may result in wallerian degeneration of the distal axonal segment, which has been separated from its parent cell body. The distal portion of the axon and its terminal boutons undergo a series of characteristic morphological changes, including dense and filamentous degeneration, which may be detected ultrastructurally within the first few days after the lesion (Ralston, 1990; Peters and Webster, 1991). More recent studies have demonstrated that wallerian degeneration of the distal axonal segment may be associated with apoptosis after injuries in the CNS. Specifically, a transection of the medial forebrain bundle in the neonatal rat induces within hours after the injury a retrograde effect with apoptosis of the dopaminergic neurons in the substantia nigra but also activation of caspases and detection of caspase cleavage products in their degenerating axons distal to the lesion (El-Khodor and Burke, 2002). Similarly, a traumatic brain injury in adult rats induces early activation of caspase-3 and cleavage of β-amyloid precursor protein in the terminal processes of lesioned axons, detectable by immunohistochemistry within hours after the injury (Buki et al., 2000; Stone et al., 2002). These expressions of markers for apoptosis subside over days and may be notably reduced by 10 days after the axonal lesion (Stone et al., 2002). Thus, both the ultrastructural and immunohistochemical studies suggest that wallerian degeneration takes place early, primarily within the first week, after the axonal lesion in the CNS. As it has been previously reported that axonal loss has already taken place at a late time point in chronic EAE mice (DeBoy et al., 2007; Tiwari-Woodruff et al., 2007), it is not surprising that our axonal markers, RT-97 and synaptophysin, did not co-localize with markers for apoptosis in our EAE mice examined at the similar late time point.
Secondary degeneration of myelin in the spinal cord gray matter
Although we did not encounter any examples of active axonal degeneration by apoptosis at these relatively later time points examined during EAE, we did detect support for apoptosis in association with oligodendrocyte processes but not oligodendrocyte cell bodies in EAE mice. Specifically, we demonstrated co-localization of cleaved PARP, a product of proteolytic cleavage by activated caspase-3, and MBP in the spinal cord gray matter of EAE mice. However, apoptosis of oligodendrocyte cell bodies in the spinal cord gray matter has been suggested in earlier studies of EAE in the Lewis rat (Pender et al., 1991). Interestingly delayed associations between markers of apoptosis and oligodendroglia have previously been encountered after traumatic spinal cord lesions in both rodents and non-human primates and correlate with lesion extension along fiber tracts undergoing wallerian degeneration (Crowe et al., 1997; Shuman et al., 1997; Warden et al., 2001). Such injury-induced death of oligodendrocytes requires the presence of p75, a low-affinity neurotrophin receptor (Beattie et al., 2002). In addition, all the apoptosis markers used in this study are late markers of apoptosis, including activated caspase-3, PARP, and TUNEL staining. Following CNS injury, these committed steps of apoptosis may be preceded by the cleavage of multiple proteins and activation of enzymatic reactions along different pathways, including the extrinsic and mitochondrial (intrinsic) pathways (Yakovlev and Faden, 2001; Stirling et al., 2005). The detailed features of the early steps of caspase-dependent apoptosis pathways in the spinal cord gray matter in EAE remain to be investigated.
One interesting finding in the present study was that IR for apoptotic markers p75 was demonstrated in association with degenerating myelin but not in the cell bodies of oligodendrocytes. Thus, our data suggest that oligodendroglial cells may undergo apoptotic elimination of a subset of their myelin forming processes without death of the oligodendrocyte itself. A similar process may take place in neurons during the normal development of the nervous system, where select axon collateral branches are eliminated without loss of the parent neuron (Raff et al., 2002). Furthermore, axotomized spinal motoneurons may undergo partial elimination of whole intramedullary motor-axon collateral trees without retrograde neuronal death following a peripheral nerve transection (Havton and Kellerth, 1990). We speculate that the secondary and delayed apoptosis of oligodendrocyte processes reported here may contribute to chronic demyelination and dysfunction of the spinal cord in the settings of post-inflammatory myelopathies. In addition, our neuropathologic findings in the MS model further support the notion of using neuroimaging focused on the gray matter to discern neurodegenerative aspects of clinical disease.
Acknowledgments
This study was supported by grants from the NIH/NCRR (1 U54 RR021813) and National Multiple Sclerosis Society (CA 1028 and RG 3593). The RT97 monoclonal antibody developed by Dr. John Wood was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Abbreviations
- AIF1
allograft inflammatory factor
- APC-CC1
adenomatous polyposis coli gene clone CC1
- CFA
complete Freund's adjuvant
- ChAT
choline acetyl transferase
- DAB
diaminobenzidine
- EAE
experimental autoimmune encephalomyelitis
- GFAP
glial fibrillary acidic protein
- IR
immunoreactivity
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NIH
National Institutes of Health
- PARP
poly (ADP-ribose) polymerase
- ROI
region of interest
- RT-97
neurofilament marker
- TUNEL
terminal deoxynucleotidyl transferase (TdT) mediated-dUTP nick end labeling
References
- Aharoni R, Arnon R, Eilam R. Neurogenesis and neuroprotection induced by peripheral immunomodulatory treatment of experimental autoimmune encephalomyelitis. J Neurosci. 2005;25:8217–8228. doi: 10.1523/JNEUROSCI.1859-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autieri MV, Agrawal N. IRT-1, a novel interferon-gamma-responsive transcript encoding a growth-suppressing basic leucine zipper protein. J Biol Chem. 1998;273:14731–14737. doi: 10.1074/jbc.273.24.14731. [DOI] [PubMed] [Google Scholar]
- Bannerman PG, Hahn A, Ramirez S, Morley M, Bonnemann C, Yu S, Zhang GX, Rostami A, Pleasure D. Motor neuron pathology in experimental autoimmune encephalomyelitis: studies in THY1-YFP transgenic mice. Brain. 2005;128:1877–1886. doi: 10.1093/brain/awh550. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- Buki A, Okonkwo DO, Wang KK, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. p75 And Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, Plant GT, Thompson AJ, Miller DH. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127:1101–1107. doi: 10.1093/brain/awh126. Epub 2004 Mar 1103. [DOI] [PubMed] [Google Scholar]
- DeBoy CA, Zhang J, Dike S, Shats I, Jones M, Reich DS, Mori S, Nguyen T, Rothstein B, Miller RH, Griffin JT, Kerr DA, Calabresi PA. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007;130:2199–2210. doi: 10.1093/brain/awm122. Epub 2007 Jun 2198. [DOI] [PubMed] [Google Scholar]
- El-Khodor BF, Burke RE. Medial forebrain bundle axotomy during development induces apoptosis in dopamine neurons of the substantia nigra and activation of caspases in their degenerating axons. J Comp Neurol. 2002;452:65–79. doi: 10.1002/cne.10367. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Gold R, Linington C, Lannes-Vieira J, Wekerle H, Kreutzberg GW. Microglial involvement in experimental autoimmune inflammation of the central and peripheral nervous system. Glia. 1993;7:50–59. doi: 10.1002/glia.440070110. [DOI] [PubMed] [Google Scholar]
- Glynn P, Chantry A, Groome N, Cuzner ML. Basic protein dissociating from myelin membranes at physiological ionic strength and pH is cleaved into three major fragments. J Neurochem. 1987;48:752–759. doi: 10.1111/j.1471-4159.1987.tb05581.x. [DOI] [PubMed] [Google Scholar]
- Guseva NV, Taghiyev AF, Rokhlin OW, Cohen MB. Contribution of death receptor and mitochondrial pathways to Fas-mediated apoptosis in the prostatic carcinoma cell line PC3. Prostate. 2002;51:231–240. doi: 10.1002/pros.10095. [DOI] [PubMed] [Google Scholar]
- Hammerle B, Elizalde C, Galceran J, Becker W, Tejedor FJ, Carnicero A, Ceron J, Martinez S, Ferrus A. The MNB/DYRK1A protein kinase: neurobiological functions and Down syndrome implications. J Neural Transm Suppl. 2003;17:129–137. doi: 10.1007/978-3-7091-6721-2_11. [DOI] [PubMed] [Google Scholar]
- Havton L, Kellerth JO. Elimination of intramedullary axon collaterals of cat spinal alpha-motoneurons following peripheral nerve injury. Exp Brain Res. 1990;79:65–74. doi: 10.1007/BF00228873. [DOI] [PubMed] [Google Scholar]
- Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–4483. [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Chiu FC, Kucherlapati R, Raine CS. Experimental allergic encephalomylitis in mice lacking glial fibrillary acidic protein is characterized by a more severe clinical course and an infiltrative central nervous system lesion. Am J Pathol. 1998;152:251–259. [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti CF, Brueck W, Rodriguez M, Lassmann H. Multiple sclerosis: lessons from neuropathology. Semin Neurol. 1998;18:337–349. doi: 10.1055/s-2008-1040885. [DOI] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Tinsley MR, Shah KP, Aguilar C, Strickland LV, Boline J, Martin M, Morales L, Shattuck DW, Jacobs RE, Voskuhl RR, Toga AW. Cerebellar cortical atrophy in experimental autoimmune encephalomyelitis. Neuroimage. 2006;32:1016–1023. doi: 10.1016/j.neuroimage.2006.05.006. Epub 2006 Jun 1027. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. Epub 12001 Sep 12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson P, Meijer B, Svensson M. Extensive neuronal cell death following intracranial transection of the facial nerve in the adult rat. Brain Res Bull. 1999;49:333–341. doi: 10.1016/s0361-9230(98)00178-6. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- Pender MP, Nguyen KB, McCombe PA, Kerr JF. Apoptosis in the nervous system in experimental allergic encephalomyelitis. J Neurol Sci. 1991;104:81–87. doi: 10.1016/0022-510x(91)90219-w. [DOI] [PubMed] [Google Scholar]
- Peters APS, Webster HdeF. The fine structure of the nervous system. 3rd edition. Oxford England: Oxford University Press; 1991. [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Ralston HJ., 3rd Analysis of neuronal networks: a review of techniques for labeling axonal projections. J Electron Microsc Tech. 1990;15:322–331. doi: 10.1002/jemt.1060150403. [DOI] [PubMed] [Google Scholar]
- Ramprasad MP, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1996;93:14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromani PJ, Armstrong DM, Bruce G, Hersh LB, Groves PM, Gillin JC. Relation of pontine choline acetyltransferase immunoreactive neurons with cells which increase discharge during REM sleep. Brain Res Bull. 1987;18:447–455. doi: 10.1016/0361-9230(87)90019-0. [DOI] [PubMed] [Google Scholar]
- Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- Stone JR, Okonkwo DO, Singleton RH, Mutlu LK, Helm GA, Povlishock JT. Caspase-3-mediated cleavage of amyloid precursor protein and formation of amyloid beta peptide in traumatic axonal injury. J Neurotrauma. 2002;19:601–614. doi: 10.1089/089771502753754073. [DOI] [PubMed] [Google Scholar]
- Suen WE, Bergman CM, Hjelmstrom P, Ruddle NH. A critical role for lymphotoxin in experimental allergic encephalomyelitis. J Exp Med. 1997;186:1233–1240. doi: 10.1084/jem.186.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. Epub 12007 Sep 14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P. Grey matter pathology in multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:1101–1107. doi: 10.1097/01.jnen.0000190067.20935.42. [DOI] [PubMed] [Google Scholar]
- Warden P, Bamber NI, Li H, Esposito A, Ahmad KA, Hsu CY, Xu XM. Delayed glial cell death following wallerian degeneration in white matter tracts after spinal cord dorsal column cordotomy in adult rats. Exp Neurol. 2001;168:213–224. doi: 10.1006/exnr.2000.7622. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J Neuropathol Exp Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Faden AI. Caspase-dependent apoptotic pathways in CNS injury. Mol Neurobiol. 2001;24:131–144. doi: 10.1385/MN:24:1-3:131. [DOI] [PubMed] [Google Scholar]
- Young HE, Black AC., Jr Adult stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:75–102. doi: 10.1002/ar.a.10134. [DOI] [PubMed] [Google Scholar]


