Summary
Background
The glycoprotein (GP) Ib-IX complex is critically involved in platelet adhesion to von Willebrand factor and in the initial step of platelet activation. How this complex is assembled is not clear. We previously showed that the transmembrane domains of the GPIbα and GPIbβ subunits interact and participate in complex assembly.
Objectives and Methods
Here, we have investigated the role of the transmembrane and cytoplasmic domains of GPIX in assembly of the GPIb-IX complex, by analyzing the mutational effects on complex expression and assembly in transiently transfected Chinese hamster ovary cells.
Results
Replacing the cytoplasmic domain of GPIX with a poly-alanine sequence had little effect on surface expression and structural integrity of the GPIb-IX complex. In contrast, replacing the GPIX transmembrane domain (residues 132–153) with a poly-leucine-alanine sequence markedly disrupted complex formation of GPIX with GPIbα, interfered with GPIb formation, and decreased surface expression of the host complex. We further analyzed the contributions of a number of GPIX transmembrane residues to complex formation by mutagenesis and found significant roles for Asp135 and several Leu residues.
Conclusions
The transmembrane domain, rather than the cytoplasmic domain, of GPIX plays an important role in expression and assembly of the GPIb-IX complex by interacting with its counterparts of GPIb. These transmembrane domains may form a parallel four-helical bundle structure in the complex.
Introduction
The glycoprotein (GP) Ib-IX-V complex is one of the major adhesion receptors expressed on the surface of circulating platelets. Through interaction with various ligands and counter-receptors, it is critically involved in platelet genesis, adhesion, activation, and clearance [1–3]. The GPIb-IX-V complex consists of four membrane-spanning polypeptides, GPIbα, GPIbβ, GPIX, and GPV [4–7]. GPIbα is linked to two GPIbβ subunits through membrane-proximal disulfide bonds [8]. The αβ2 complex, also known as GPIb, is noncovalently associated with GPIX [9]. GPV is more loosely associated with two GPIb-IX complexes [10, 11].
Deficiency or dysfunction of this complex results in a severe bleeding disorder known as the Bernard-Soulier Syndrome (BSS) [12]. BSS-causing mutations identified from patients have been mapped to the genes encoding GPIbα, GPIbβ, and GPIX (http://www.bernardsoulier.org and references therein). Consistently, all three subunits are required for efficient expression of the GPIb-IX complex on the plasma membrane of transfected cells [13]. In the absence of GPIbβ and GPIX, most of GPIbα is degraded in the lysosome [14]. The unassembled GPIbβ and GPIX accumulate in the cytoplasm, presumably in non-native conformations, with only a minority reaching the cell surface [15]. These results suggested that correct assembly of the GPIb-IX complex is a prerequisite for its trafficking to the plasma membrane. Although the underlying molecular basis is not clear, this feature — assembly before trafficking — can be utilized to probe inter-subunit interactions in the complex through analysis of mutational effects on complex surface expression. GPIbβ was thereby found to play a central role in complex assembly by interacting with both GPIbα and GPIX [16]. Recently, the transmembrane (TM) domains were identified as a major site of interaction between GPIbα and GPIbβ [17]. The TM-TM interactions are thought to bring the membrane-proximal Cys residues into proximity to facilitate formation of the disulfides between the GPIbα and 2 GPIbβ subunits.
The interaction between GPIX and GPIb is strong and specific [9], although the nature of the interaction is not clear. The significant effect of mutations in the N-terminal region of GPIbβ on GPIX expression implicates direct interaction between the extracellular domains of the two subunits [18]. Such interaction is also supported by the observation that monoclonal antibodies against GPIX only bind their epitope when in the presence of GPIbβ [15]. However, the interaction of GPIX with GPIb may not be limited to its extracellular domain. A GPIX mutant lacking the entire TM and cytoplasmic domains abolishes expression of the GPIb-IX-V complex in platelets and causes BSS [19], suggesting participation of the TM and/or cytoplasmic domains of GPIX in assembly and efficient surface expression of the GPIb-IX complex.
In this paper we report studies of the role of the TM and cytoplasmic domains of GPIX in assembly and expression of the GPIb-IX complex using a transfected cell model. Our results indicate that the TM domain is much more important than the cytoplasmic domain. Furthermore, the GPIX TM domain interacts with the GPIb TM domain(s) and modulates formation of the disulfide bonds between GPIbα and GPIbβ. Based on these results, we propose a parallel 4-helical bundle model for the TM domains of the GPIb-IX complex.
Materials and methods
Materials
The expression vector pDX was used in earlier studies on expression of the GPIb-IX complex in Chinese hamster ovary (CHO) cells [13, 16]. The HA-tagged GPIbβ cDNA has been described [8]. The CHO K1 cell line was obtained from ATCC. WM23, an anti-GPIbα monoclonal antibody, was provided by Dr. Michael Berndt.
Mutagenesis of GP IX
The pDX vectors containing domain-deleted IXE and IXET cDNAs were obtained with nonsense mutations at residues Al 25 and Ala155, respectively, using the QuikChange kit (Stratagene). Construction of the IXETpA cDNA involved two rounds of mutations (firstly residues Thr156–Glu158, then residues Leu160 Asp161, to alanines). To construct GPIX variants with mutation(s) in the TM domain, the TM-encoding DNA fragment containing the desired mutation was generated by PCR amplification, digested with restriction enzymes and ligated into the pDX-GPIX vector with unique XmaI and XbaI sites flanking the TM domain [17]. All the mutant cDNA sequences were confirmed by DNA sequencing (SeqWright, Houston, TX).
Expression of GPIb-IX complex in transiently transfected CHO cells
Transient transfection of CHO K1 cells with pDX vectors containing GPIbα, GPIbβ or GPIX cDNAs was carried out as described [17], and the cells were grown for additional 2 days before being analyzed for protein expression. Surface expression of GPIbα and GPIX was measured by flow cytometry using the antibodies AK2 and FMC25 (Millipore), respectively [17]. The quantified mean fluorescence value of each cell population (10,000 cells) was normalized with the value of the wild-type CHOαβIX cells being 100% and that of CHOpDX cells 0%. Overall expression levels were measured by Western blot of the resolved cell lysate in SDS gels with specific antibodies as described [17].
Immunoprecipitation
Lysates from approximately 5 × 105 transfected cells were first pretreated with 20 μL 50% Protein G-agarose beads (Sigma) at 4 °C for 1 h to clear nonspecifically associated proteins, then immunoprecipitated with 1 μg of the indicated antibody and 20 μL 50% Protein G-agarose beads at 4°C overnight. After being washed with PBS containing 0.1% Triton X-100 for three times, the proteins bound to the beads were eluted in the Tris-glycine SDS sample buffer, separated in a 4–12% Bis-Tris SDS gel under reducing conditions and immunoblotted with appropriate antibodies. The blotted bands were quantified by densitometry and normalized against those from CHOαβIX cells.
Results
The GPIX cytoplasmic domain is not critical to efficient surface expression and proper assembly of the GPIb-IX complex
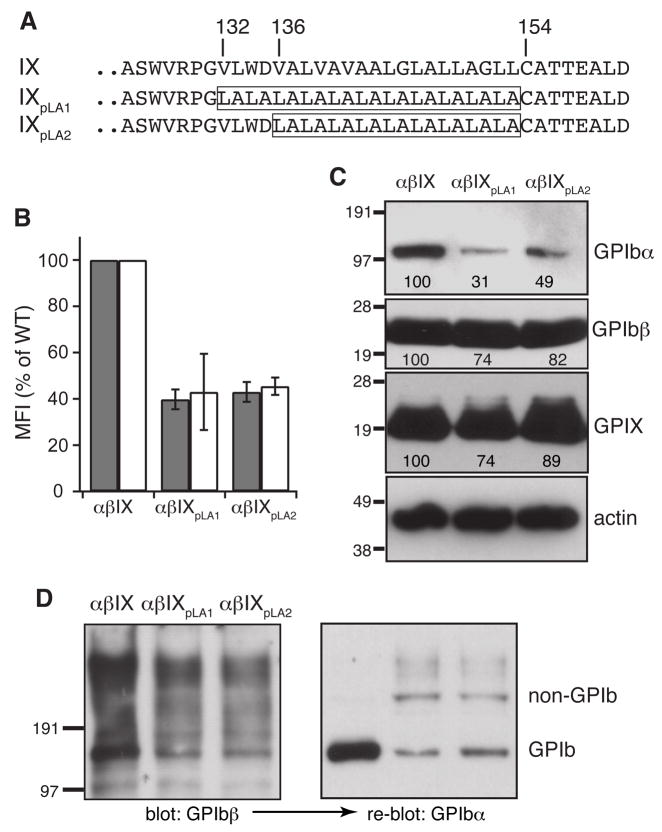
Association of GPIX mutants missing the TM and cytoplasmic domains with BSS highlights the importance of the regions in efficient expression of the complex [19]. Whether both domains are important, and why, is not clear. Three mutant GPIX cDNAs, IXET, IXE and IXETpA, were constructed (Fig. 1A). The IXET construct lacked the cytoplasmic domain, whereas IXE lacked the TM and cytoplasmic domains. In IXETpA, the cytoplasmic domain was replaced by a poly-alanine sequence. Each mutant GPIX gene was cotransfected transiently with wild-type GPIbα and GPIbβ cDNAs into CHO cells. To ensure proper comparison of complex expression levels among various transfected cells, key parameters of the experiment, such as the amount of vectors and transfection efficiency, were kept constant as described [17]. Moreover, CHO cells transfected with all three wild-type cDNAs (CHOαβIX cells), with only GPIbα and GPIbβ cDNAs (CHOαβ cells), and with pDX vectors (CHOpDX cells) were included in the same experiment for direct comparison. Therefore, the difference in surface expression of GPIbα and GPIX can be attributed solely to the mutation(s) in GPIX.
Fig. 1. Effect of domain deletion in GPIX on expression and assembly of the GPIb-IX complex in transiently transfected CHO cells.
(A) Illustration of mutant GPIX constructs. (B) Surface expression levels of GPIbα (gray bar) and GPIX (white bar) in mutant cells. The vector containing wild type or mutant GPIX cDNA was co-transfected transiently with wild type GPIbα and GPIbβ cDNAs into CHO cells. After 2 days, the cells were harvested, stained by antibodies against GPIbα (AK2) or GPIX (FMC25), and analyzed by flow cytometry. The cells were identified by the subunits transfected. “α” stands for GPIbα, “β” GPIbβ, and “IX” GPIX. The subscript denotes the mutation within the subunit. The mean fluorescence intensity (MFI) were normalized such that the level in wild-type CHOαβIX cells is 100% and that in sham vector transfected CHOpDX cells 0%. The data are calculated from 3 independent experiments and presented as the mean ± s.d.. (C) Overall expression of GPIbα and GPIX in transfected CHO cells. Cell lysates were resolved in reducing SDS gels and transferred to the PVDF membrane. The membrane was probed separately with antibodies against GPIbα (WM23), GPIbβ (Gi27), GPIX (polyclonal), and actin. Band intensities for each complex subunit were quantitated and expressed as a percentage of the wild type level. The numbers below the bands are the average of 3 independent experiments, and the deviation is usually 10%. (D) Effect of domain deletion in GPIX on formation of the GPIbα-GPIbβ disulfide bonds (i.e. GPIb formation). Cell lysates were resolved in non-reducing SDS gels and transferred to PVDF membrane. The membrane was probed with Gi27 first, stripped and re-probed with WM23. The blots are representatives of 2 independent experiments. The membrane was also probed directly with WM23 in 3 additional independent experiments (not shown). The GPIb and non-GPIb bands are indicated on the right.
In CHOαβIX cells, GPIbα, GPIbβ and GPIX were all expressed at high levels. Deletion of the GPIX cytoplasmic domain in CHOαβIXET cells did not cause a significant change in surface expression of GPIbα, while GPIX expression was decreased modestly (Fig. 1B). Replacing it with a poly-alanine sequence in CHOαβIXETpA cells caused no change in surface expression of GPIbα and GPIX. The overall expression levels of GPIbα and GPIX in these cells, estimated from Western blots of the cell lysates, showed a similar pattern (Fig. 1C). In contrast, deletion of the TM and cytoplasmic domains of GPIX in CHOαβIXE cells resulted in total absence of GPIXE on the cell surface (Fig. 1B). Immunoprecipitation of the culture medium with anti-GPIX antibody yielded no evidence for GPIXE secretion from the cells (data not shown). Surface expression of GPIbα was also decreased significantly compared to its expression in CHOαβIX cells. Therefore, whereas lack of only the cytoplasmic domain of GPIX did not significantly decreased complex expression, lack of both the TM and cytoplasmic domains did.
Formation of disulfide bonds between GPIbα and 2 GPIbβ subunits (i.e., GPIb formation), visualized in the non-reducing SDS gel, is a definitive indicator of proper complex assembly. GPIb formation was disrupted when the TM domain of either GPIbα or GPIbβ was replaced with an unrelated sequence [17], suggesting it depends on the proper interaction between the TM domains of GPIbα and GPIbβ. In CHOαβIXET cells, the majority of GPIbα was in the GPIb complex, with a small portion in undefined protein complexes with apparent molecular masses of over 200 kDa (Fig. 1D). The defect in GPIb formation may be due to unintended complications from domain deletion such as defects in protein insertion into the membrane, because wild-type-like GPIb formation was observed in CHOαβIXETpA cells. Thus, the GPIX cytoplasmic domain is not critical for correct assembly of the GPIb-IX complex. Since deletion of both TM and cytoplasmic domains of GPIX in CHOαβIXE cells totally abolished GPIb formation, the TM domain must be important to complex assembly.
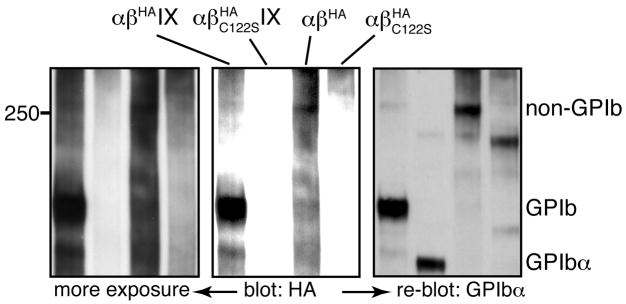
The non-GPIb complex contains GPIbα and GPIbβ
When GPIb formation is disrupted in CHOαβIXE or CHOαβ cells, GPIbα was instead present in high molecular weight protein complexes, which was dominated by one complex we termed “non-GPIb complex” (Fig. 1D). The smearing background in blots against GPIbβ made it difficult to ascertain directly the presence of GPIbβ in the non-GPIb complex. Nonetheless, mutating residue Cys122 of GPIbβ to Ser abolished formation of the non-GPIb complex in CHOαβ cells (Fig. 2), indicating that GPIbβ was in the non-GPIb complex through a disulfide bond involving Cys122. The presence of both GPIbα and GPIbβ in the non-GPIb complex, plus the difference in molecular masses between GPIb and non-GPIb complexes, led us to conclude that the non-GPIb complex is the disulfide-linked GPIbβ-GPIbα-GPIbα-GPIbβ complex. Although formation of a disulfide bond between GPIbα and GPIbβ does not require GPIX, the presence of GPIX, and particularly its TM domain, is necessary for correct formation of the two disulfide bonds in the GPIb complex.
Fig. 2. The non-GPIb complex contains GPIbα and GPIbβ.
Cell lysates were resolved in a non-reducing 5% Tris-glycine SDS gel and probed with anti-HA antibody for HA-tagged GPIbβ (two exposures shown) and re-probed with WM23 for GPIbα. The blots are representatives of 3 independent experiments. The anti-HA antibody was used instead of Gi27 for slightly better GPIbβ blotting. Addition of the HA epitope tag to the C-terminus of GPIbβ did not affect GPIbα blotting (not shown).
Replacement of the GPIX TM domain
The GPIX TM domain is defined in the GenBank database as residues Val136–Leu153, which is shorter than its GPIbα and GPIbβ counterparts. In contrast, the widely used TM-prediction program TMHMM [20] predicted the TM domain as Gly131–Leu153, which matched the length of the GPIbα and GPIbβ TM domains. Another program, HMMTOP 2.0 [21, 22], predicted the TM domain as Val132–Thr156. GPIX is myristoylated at residue Cys154 [23], which should mark the C-terminal end of the TM domain. The predicted boundaries mostly differed on whether residue Asp135 was a part of the TM domain (Fig. 3A).
Fig. 3. Mutational effect on complex expression and assembly by replacement of the GPIX TM domain with pLA sequences.
(A) TM sequences in wild type and domain replacement GPIX constructs. The pLA sequence in each mutant is boxed. (B) Surface expression levels of GPIbα (gray) and GPIX (white) in domain replacement cells. Measurement and quantitation followed the description in Fig 1B. (C) Overall expression of GPIbα and GPIX. Cell lysates were resolved in reducing SDS gels. The relative band intensity is the average of 3 independent experiments. (D) GPIb formation in domain replacement cells. Cell lysates were resolved in non-reducing SDS gels and probed as indicated. The blots are representatives of 2 independent experiments.
We had reported that replacing residues Val136–Leu153 with a poly-leucine (pL) sequence did not notably affect complex expression and GPIb formation, but replacing them with a poly-leucine-alanine (pLA) sequence did [17]. Given the discrepancy in TM domain boundary, we first tested whether replacing residues Val132–Leu153 — that is, the longer GPIX TM domain — with a pLA sequence would have the same effect (Fig. 3A). In CHOαβIXpLA1 cells, replacing the longer TM domain with the pLA sequence resulted in a significantly lower surface expression level of the GPIb-IX complex than the wild type level. The extent of decrease was similar to that observed in CHOαβIXpLA2 cells, in which the shorter TM domain was replaced with the pLA sequence (Fig. 3B,C). Furthermore, while GPIb formation was perturbed in both CHOαβIXpLA1 and CHOαβIXpLA2 cells, the former appeared to produce a more pronounced effect (Fig. 3D). These results suggested that residues Val132–Asp135 may be an integral part of the GPIX TM domain to play a critical role in proper assembly of the GPIb-IX complex.
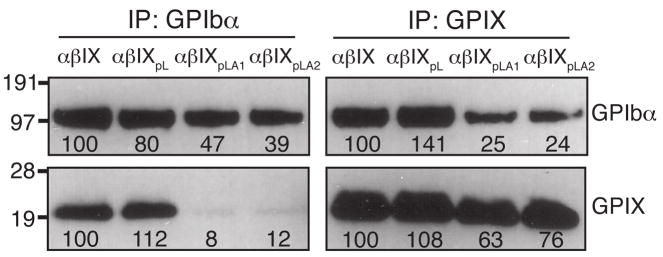
The simplest explanation for the ability of the GPIX TM domain to modulate the interaction and concurrently formation of the disulfide bonds between GPIbα and GPIbβ is that the GPIX TM domain interacts with either or both of its Ibα and Ibβ counterparts directly. In CHOαβ cells, which expressed no GPIX and therefore was free of any GPIX-involved interactions, GPIb was not formed. Instead, GPIbα was mostly present in the non-GPIb complex, which was also present in CHOαβIXpLA1 and CHOαβIXpLA2 cells (Fig. 1D, 3D). In addition, much less GPIX (or GPIbα) was co-immunoprecipitated by the anti-GPIbα (or anti-GPIX) antibody from the lysates of CHOαβIXpLA1 or CHOαβIXpLA2 cells than from wild-type CHOαβIX cells (Fig. 4), clearly indicating that the interaction between GPIXpLA1 or GPIXpLA2 and GPIb was not as strong as that between the wild-type subunits. Consistent with our earlier observation that replacement of the GPIX TM domain with the pL sequence did not cause any defects in GPIb formation and complex membrane expression [17], little defect in complexation of GPIbα with GPIXpL was observed in CHOαβIXpL cells. Overall, these results showed that replacement of the GPIX TM domain with an unrelated sequence such as pLA interfered with the interaction among the TM domains, which led to improper assembly and decreased surface expression of the receptor complex.
Fig. 4. Replacement of the GPIX TM domain with pLA sequences, but not the pL sequence, weakened the interaction between GPIb and GPIX.
Cell lysates were co-immunoprecipitated with antibodies AK2 or FMC25, resolved in a reducing SDS gel, and immunoblotted with WM23 or anti-IX polyclonal antibody. Intensities of the GPIbα and GPIX bands were quantitated by densitometry and expressed as a percentage of the wild type level. The numbers below the bands are the average of 3 independent experiments.
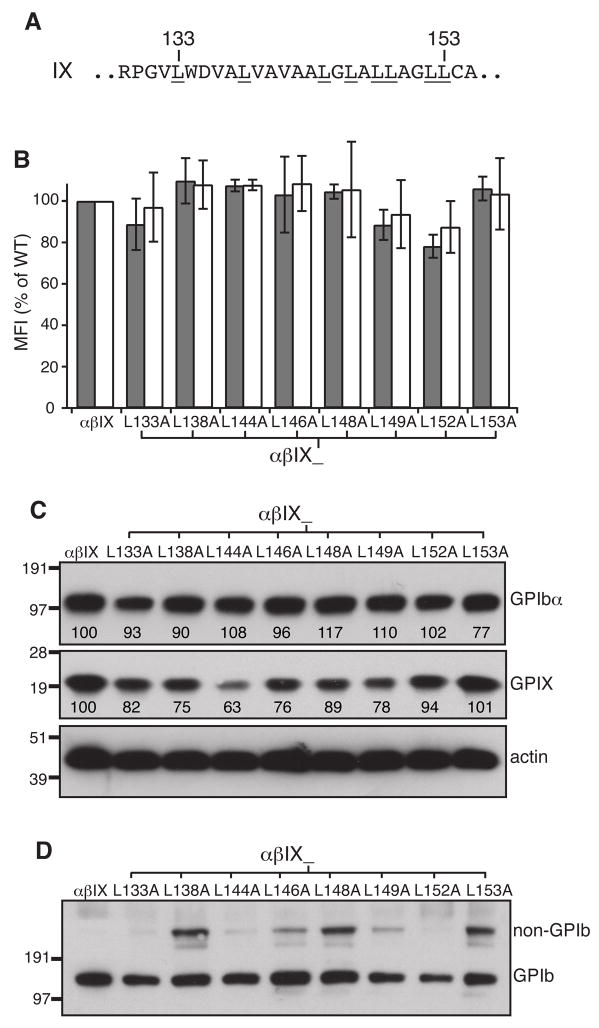
Scanning for critical Leu residues in the GPIX TM domain
Since the pL sequence, but not the pLA sequence, in the GPIX TM domain could largely maintain the integrity of the GPIb-IX complex as well as its surface expression level [17], certain Leu residues in the wild-type TM domain may account for the difference between the pL and pLA sequences. We thus screened for them by mutating all the Leu residues in the GPIX TM domain, one at a time, to Ala and measuring the effects of these mutations on complex expression and GPIb formation. Although most of the Leu→Ala mutations decreased the overall expression levels of GPIX in transfected cells, surface expression of GPIX in these cells were not affected significantly compared to wild type levels (Fig. 5B,C). Neither the overall nor surface expression of GPIbα were markedly affected by any of the mutations. However, 5 mutants, L138A, L146A, L148A, L149A and L153A, interfered with GPIb formation to various degrees (Fig. 5D), suggesting that these 5 Leu residues may play a role in complex assembly.
Fig. 5. Certain Leu residues in the GPIX TM domain hampered GPIb formation but not surface expression of the GPIb-IX complex in transfected CHO cells.
(A) Sequence of the GPIX TM domain. All Leu residues are underlined with 2 residue numbers marked on top. (B) Surface expression levels of GPIbα (gray) and GPIX (white) in Leu mutant cells. Measurement and quantitation followed the description in Fig 1B. (C) Overall expression of GPIbα and GPIX. Cell lysates were resolved in reducing SDS gels and immunoblotted with antibodies as indicated. The relative band intensity is the average of 3 independent experiments. (D) GPIb formation in Leu mutant cells. Cell lysates were resolved in non-reducing SDS gels and immunoblotted with WM23. The blot shown represents 4 independent experiments.
Asp135 in the GPIX TM domain contributes to GPIb formation
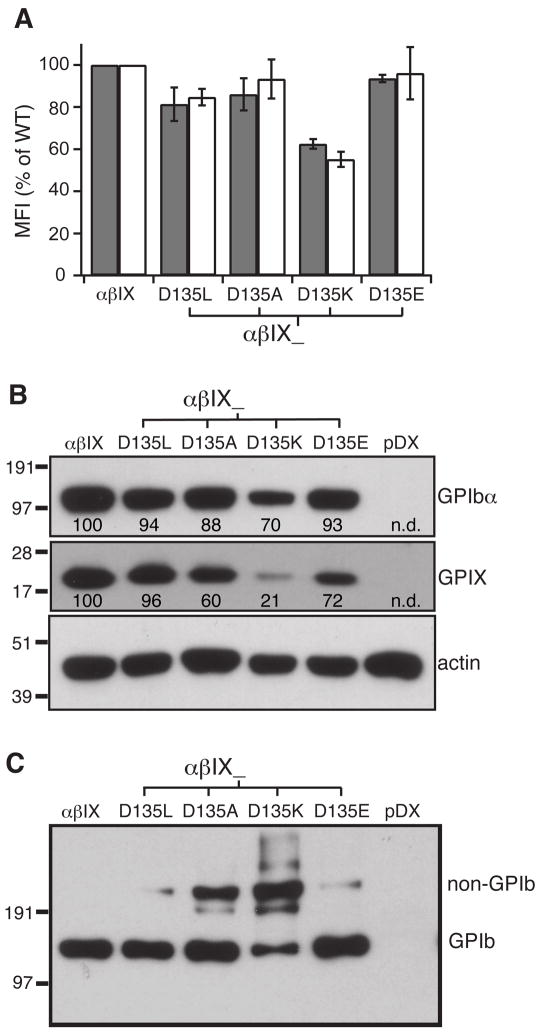
Polar residues present in the TM domain often play an important role in interactions between TM helices [24]. Since it is possible that the GPIX TM domain includes a polar residue — Asp135, we next tested whether Asp135 served a definable role in disulfide formation between GPIbα and GPIbβ by mutating it to apolar (Leu, Ala) as well as other polar (Lys, Glu) residues. Mutations D135L, D135A, and K135E did not significantly decrease surface expression of the GPIb-IX complex; in contrast D135K did (Fig. 6A,B). The mutational effects on GPIb formation in general were more pronounced. Mutating Asp135 to Ala or Lys hampered GPIb formation the most, whereas mutating it to Leu or Glu produced a smaller but reproducible effect (Fig. 6C). Therefore, it appeared that both size of the side chain and the type of polar group were important for residue 135. Furthermore, it was noteworthy that Asp135 is on the same side of the TM helix as the 5 Leu residues identified above.
Fig. 6. Asp135 in the GPIX TM domain is important to complex assembly and expression.
(A) Surface expression levels of GPIbα (gray) and GPIX (white), as well as (B) overall expression of GPIbα and GPIX, in Asp135 mutant cells were measured as described earlier. The relative intensity marked under each band is the average of 3 independent experiments. (C) GPIb formation in Asp135 mutant cells. Cell lysates were resolved in non-reducing SDS gels and immunoblotted with WM23. The blot shown represents 3 independent experiments.
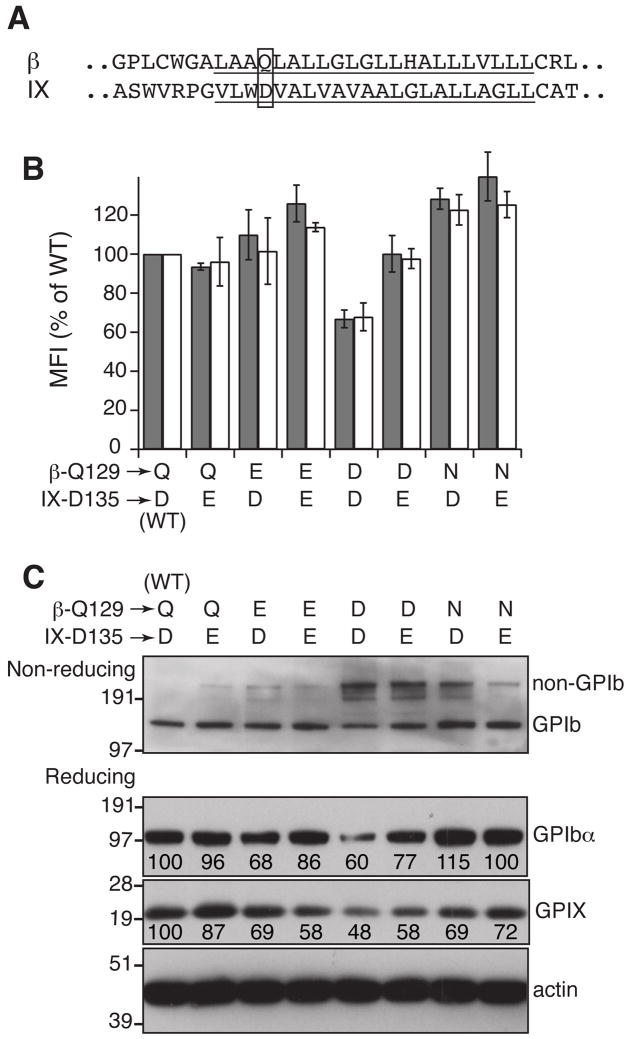
In a TM helical complex, polar residues often interact with nearby polar residues through hydrogen bonds [25, 26]. Unpaired polar residues in a TM helix, particularly acidic or basic residues, can induce degradation of the host protein [27, 28]. Alignment of the GPIbβ and GPIX TM domains revealed that Gln129 of GPIbβ (β-Q129) was in a similar position as IX-D135 (Fig. 7A). β-Q129 has been implicated in the inter-subunit interactions because mutating it to Leu markedly decreased surface expression of the GPIb-IX complex [17]. We postulated that β-Q129 and IX-D135 may interact with one another, partly through hydrogen bonds. Since the putative hydrogen bonds among these residues depend on the precise positions of the polar groups in the side chains, even a small perturbation of their positions should compromise the distance constraints of the hydrogen bonds and thus interfere with the interactions. When β-Q129 or IX-D135 was mutated to Glu, no significant changes in complex surface expression but only a small-scale disruption of GPIb formation was observed, indicating that Glu could be tolerated at both positions (Fig. 7). In comparison, changing β-Q129 to either Asn or Asp, the residues with a shorter side chain but the same or similar ionizable groups, notably hampered GPIb formation. More importantly, the extent of GPIb formation in transfected CHO cells with the β-Q129N (or β-Q129D) mutation was markedly less than that in cells containing the combined IX-D135E/β-Q129N (or β-Q129D) mutations (Fig. 7C). In other words, increasing the side chain length of residue 135 of GPIX could ameliorate, at least partially, the negative effect caused by decreasing the side chain length of β-Q129, clearly implicating a direct interaction between β-Q129 and IX-D135.
Fig. 7. Probing the interaction between Asp135 of GPIX and Gln129 of GPIbβ.
(A) The TM sequences (underlined) of GPIbβ and GPIX. Residues Gln129 of GPIbβ and Asp135 of GPIX are boxed. (B,C) Effects of mutating Gln129 and Asp135 to certain polar residues on surface expression levels of GPIbα (gray bar) and GPIX (white bar), GPIb formation as well as overall expression of the complex. The data shown were either the average or a representative of 3–4 independent experiments.
Ala140 is not in the interface of the GPIb-IX complex
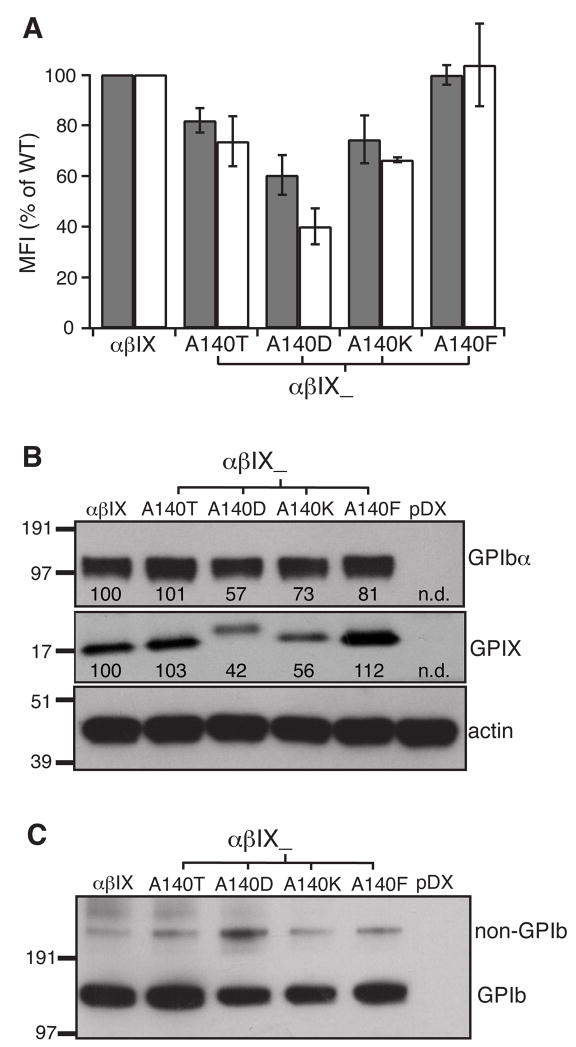
The A140T mutation in the GPIX TM domain was first described as a polymorphism because no adverse effects on platelet number, morphology and GPIb-IX expression were found in homozygotes [29, 30]. However, it was the only mutation in the GPIb-IX complex of a BSS patient [31]. This mutation was also found to associate with low platelet count in a large Asian Indian kindred [32]. In transiently transfected CHO cells, A140T was reported to abolish surface expression, but not overall expression, of GPIbα and GPIX [31]. Since its effect on complex assembly had not been characterized, we tested whether this mutation affected GPIb formation. Ala140 was also mutated to Asp, Lys or Phe for comparison. The A140T mutation, as well as A140D and A140K, reproducibly reduced surface expression of GPIbα and GPIX in transfected CHO cells (Fig. 8A,B). However, the extent of decrease caused by A140T was much less than those reported in [31], suggesting that A140T alone is not sufficient to cause BSS. Additional factors may be at play in recognizing the A140T mutation and exacerbating its effect in those BSS patients with the A140T mutation.
Fig. 8. Ala140 in the GPIX TM domain is not critical to assembly and surface expression of the GPIb-IX complex.
Ala140 was mutated to Thr, Asp, Lys, or Phe, and the mutational effects on the surface (A) and overall (B) expression level of GPIbα (gray bar) and GPIX (white bar), as well as (C) the effects on GPIb formation were characterized. For some reason, GPIXA140D migrated at a pace different from the other variants. The data shown were either the average or a representative of 4 independent experiments.
Except for the small but reproducible effect caused by A140D, the other Ala140 mutations showed minimal disruption of GPIb formation (Fig. 8C). If Ala140 participated in any direct and meaningful interaction with the GPIbβ or GPIbα TM domains, mutating it to other residues would have produced a more severe defect in GPIb formation. In particular, changing Ala140 to Phe, which has a much larger side chain, failed to produce any effects on complex assembly and expression, indicating that Ala140 is not located on a helical face engaging in the TM-TM interactions within the GPIb-IX complex.
Discussion
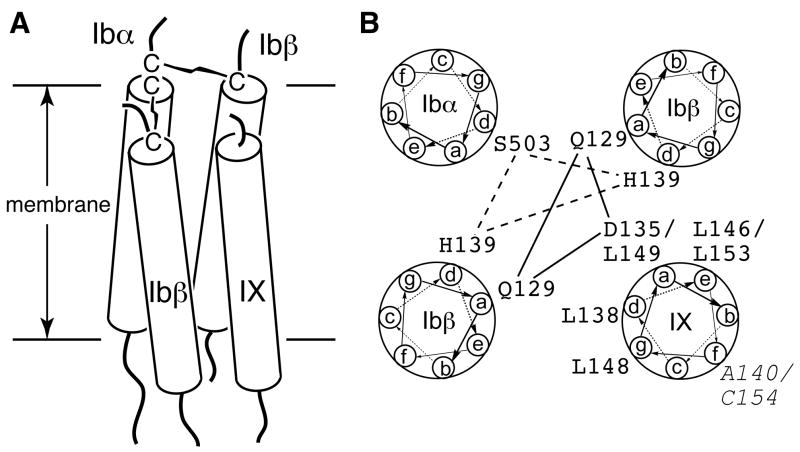
The structural integrity of the GPIb-IX-V complex is essential to its function as well as its synthesis and presentation on the platelet surface. In this paper, we have provided direct evidence, for the first time, that supports an interaction between the TM domains of GPIb and GPIX. Replacing the GPIX TM domain with an unrelated pLA sequence hindered the ability of GPIX to complex with GPIbα and interfered with formation of the two disulfide bonds between GPIbα and GPIbβ in the GPIb complex. The TM domains of GPIbα, GPIbβ and GPIX, all of which are type I transmembrane proteins, are expected to take on α-helical conformation. Given the evidence supporting the interaction between the GPIbα and GPIbβ TM domains [8, 17], as well as that between the GPIbβ and GPIX TM domains presented here, we propose a parallel 4-helical bundle as the structural model for the TM domains in the GPIb-IX complex (Fig. 9A). In this model, two GPIbβ TM helices are placed in diagonal and between the GPIbα and GPIX helices, consistent with the notion that GPIbβ is central to complex assembly [16]. Such arrangement should satisfy the distance constraints placed on the GPIbα and GPIbβ TM helices by the membrane-proximal inter-subunit disulfide bonds.
Fig. 9. A four-helical bundle model for the TM domains of the GPIb-IX complex.
(A) The side view of the TM bundle. Each TM helix is shown as a rod traversing the membrane bilayer. (B) The helical wheel diagram of the TM bundle. The possible interactions among the polar residues are marked by lines. The residues in GPIX that play an role in modulating GPIb formation, as well as A140 and Cys154 (Italic), are marked.
Through analysis of site-specific mutational effects on complex expression and assembly, Asp135 and several Leu residues in the GPIX TM domain have been identified as critical to complex assembly. It is significant that these residues are located on the same side of the TM helix, suggesting this side as the interaction interface with the GPIbβ TM domains (Fig. 9B). Furthermore, the mutation IX-D135E could partly reverse the disruptive impact on GPIb formation caused by β-Q129N, suggesting a direct contact between these polar residues in the TM bundle. Replacing the GPIX TM domain (residues 136–153) with pLA sequence, but not with pL sequence, disrupts the TM-TM interaction, suggesting the presence of a leucine-zipper-like sequence motif, rather than the GxxxG motif [24, 33], in the GPIX TM domain. Overall, it appears that a combination of polar and leucine-zipper interactions mediate the TM-TM association in the GPIb-IX complex.
The orientation of the GPIX TM helix shown in the helical wheel diagram of the GPIb-IX TM bundle places the aforementioned critical residues in the TM interaction interface (Fig. 9B). It also places residue Ala140 away from the other TM helices in the bundle, consistent with our finding that residue Ala140 does not participate directly in complex assembly. It is noteworthy that Ala140 is on the same helical face as Cys154, which is myristoylated in human platelets [23] and should be located on the outside of the TM bundle.
Many site-specific mutations analyzed in this study induced detectable defects in GPIb formation but had little impact on the surface expression level of GPIbα. This is because disruption of GPIb formation gives rise to a non-GPIb protein complex that appears to have the disulfide-linked GPIbβ-GPIbα-GPIbα-GPIbβ structure. The non-GPIb complex can be expressed in the plasma membrane of the transfected CHO cells, albeit with less efficiency than GPIb. Thus, a mild perturbation of GPIb formation may not cause a significant change in surface expression of GPIbα. It is not clear, however, whether a similar non-GPIb protein complex is present in platelets with assembly defects of the GPIb-IX complex.
Acknowledgments
We thank Dr. Michael Berndt for providing the WM23 antibody and members of the Li lab for helpful discussions. This work was supported by NIH (HL082808), American Heart Association (0565078Y), and the Welch Foundation (AU-1581). R.L. is partly supported by the Howard Temin Award (CA096706). X.M. is a recipient of the Harry S. and Isabel C. Cameron Foundation Fellowship.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have on conflict of interest.
References
- 1.Berndt MC, Shen Y, Dopheide SM, Gardiner EE, Andrews RK. The vascular biology of the glycoprotein Ib-IX-V complex. Thromb Haemost. 2001;86:178–88. [PubMed] [Google Scholar]
- 2.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–7. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel TP, Hartwig JH. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 4.Lopez JA, Chung DW, Fujikawa K, Hagen FS, Papayannopoulou T, Roth GJ. Cloning of the alpha chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc Natl Acad Sci USA. 1987;84:5615–9. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez JA, Chung DW, Fujikawa K, Hagen FS, Davie EW, Roth GJ. The alpha and beta chains of human platelet glycoprotein Ib are both transmembrane proteins containing a leucine-rich amino acid sequence. Proc Natl Acad Sci USA. 1988;85:2135–9. doi: 10.1073/pnas.85.7.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey MJ, Williams SA, Roth GJ. Human platelet glycoprotein IX: an adhesive prototype of leucine-rich glycoproteins with flank-center-flank structures. Proc Natl Acad Sci USA. 1989;86:6773–7. doi: 10.1073/pnas.86.17.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickey MJ, Hagen FS, Yagi M, Roth GJ. Human platelet glycoprotein V: characterization of the polypeptide and the related Ib-V-IX receptor system of adhesive, leucine-rich glycoproteins. Proc Natl Acad Sci USA. 1993;90:8327–31. doi: 10.1073/pnas.90.18.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo S-Z, Mo X, Afshar-Kharghan V, Srinivasan S, Lopez JA, Li R. Glycoprotein Ibα forms disulfide bonds with 2 glycoprotein Ibβ subunits in the resting platelet. Blood. 2007;109:603–9. doi: 10.1182/blood-2006-05-024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du X, Beutler L, Ruan C, Castaldi PA, Berndt MC. Glycoprotein Ib and glycoprotein IX are fully complexed in the intact platelet membrane. Blood. 1987;69:1524–7. [PubMed] [Google Scholar]
- 10.Modderman PW, Admiraal LG, Sonnenberg A, von dem Borne AE. Glycoproteins V and Ib-IX form a noncovalent complex in the platelet membrane. J Biol Chem. 1992;267:364–9. [PubMed] [Google Scholar]
- 11.Li CQ, Dong JF, Lanza F, Sanan DA, Sae-Tung G, Lopez JA. Expression of platelet glycoprotein (GP) V in heterologous cells and evidence for its association with GP Ibα in forming a GP Ib-IX-V complex on the cell surface. J Biol Chem. 1995;270:16302–7. doi: 10.1074/jbc.270.27.16302. [DOI] [PubMed] [Google Scholar]
- 12.Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91:4397–418. [PubMed] [Google Scholar]
- 13.Lopez JA, Leung B, Reynolds CC, Li CQ, Fox JEB. Efficient plasma membrane expression of a functional platelet glycoprotein Ib-IX complex requires the presence of its three subunits. J Biol Chem. 1992;267:12851–9. [PubMed] [Google Scholar]
- 14.Dong JF, Gao S, Lopez JA. Synthesis, assembly, and intracellular transport of the platelet glycoprotein Ib-IX-V complex. J Biol Chem. 1998;273:31449–54. doi: 10.1074/jbc.273.47.31449. [DOI] [PubMed] [Google Scholar]
- 15.Lopez JA, Li CQ, Weisman S, Chambers M. The glycoprotein Ib-IX complex-specific monoclonal antibody SZ1 binds to a conformation-sensitive epitope on glycoprotein IX: implications for the target antigen of quinine/quinidine-dependent autoantibodies. Blood. 1995;85:1254–8. [PubMed] [Google Scholar]
- 16.Lopez JA, Weisman S, Sanan DA, Sih T, Chambers M, Li CQ. Glycoprotein (GP) Ibβ is the critical subunit linking GP Ibα and GP IX in the GP Ib-IX complex. Analysis of partial complexes. J Biol Chem. 1994;269:23716–21. [PubMed] [Google Scholar]
- 17.Mo X, Lu N, Padilla A, Lopez JA, Li R. The transmembrane domain of glycoprotein Ibβ is critical to efficient expression of glycoprotein Ib-IX complex in the plasma membrane. J Biol Chem. 2006;281:23050–9. doi: 10.1074/jbc.M600924200. [DOI] [PubMed] [Google Scholar]
- 18.Kenny D, Morateck PA, Montgomery RR. The cysteine knot of platelet glycoprotein Ibβ (GPIbβ) is critical for the interaction of GPIbβ with GPIX. Blood. 2002;99:4428–33. doi: 10.1182/blood.v99.12.4428. [DOI] [PubMed] [Google Scholar]
- 19.Noda M, Fujimura K, Takafuta T, Shimomura T, Fujimoto T, Yamamoto N, Tanoue K, Arai M, Suehiro A, Kakishita E, et al. Heterogeneous expression of glycoprotein Ib, IX and V in platelets from two patients with Bernard-Soulier syndrome caused by different genetic abnormalities. Thromb Haemost. 1995;74:1411–5. [PubMed] [Google Scholar]
- 20.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 21.Tusnady GE, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol. 1998;283:489–506. doi: 10.1006/jmbi.1998.2107. [DOI] [PubMed] [Google Scholar]
- 22.Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics (Oxford, England) 2001;17:849–50. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- 23.Schick PK, Walker J. The acylation of megakaryocyte proteins: glycoprotein IX is primarily myristoylated while glycoprotein Ib is palmitoylated. Blood. 1996;87:1377–84. [PubMed] [Google Scholar]
- 24.Curran AR, Engelman DM. Sequence motifs, polar interactions and conformational changes in helical membrane proteins. Curr Opin Struct Biol. 2003;13:412–7. doi: 10.1016/s0959-440x(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 25.DeGrado WF, Gratkowski H, Lear JD. How do helix-helix interactions help determine the folds of membrane proteins? Perspectives from the study of homo-oligomeric helical bundles. Protein Sci. 2003;12:647–65. doi: 10.1110/ps.0236503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Call ME, Schnell JR, Xu C, Lutz RA, Chou JJ, Wucherpfennig KW. The structure of theζζ transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell. 2006;127:355–68. doi: 10.1016/j.cell.2006.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonifacino JS, Cosson P, Klausner RD. Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell. 1990;63:503–13. doi: 10.1016/0092-8674(90)90447-m. [DOI] [PubMed] [Google Scholar]
- 28.Bonifacino JS, Cosson P, Shah N, Klausner RD. Role of potentially charged transmembrane residues in targeting proteins for retention and degradation within the endoplasmic reticulum. EMBO J. 1991;10:2783–93. doi: 10.1002/j.1460-2075.1991.tb07827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi T, Suzuki K, Akiba J, Yahagi A, Tajima K, Satoh S, Sasaki H. G-->A transition at nucleotide 2110 in the human platelet glycoprotein (GP) IX gene resulting in Ala139(ACC)-->Thr(GCC) substitution. Jpn J Hum Genet. 1997;42:369–71. doi: 10.1007/BF02766961. [DOI] [PubMed] [Google Scholar]
- 30.Kunishima S, Tomiyama Y, Honda S, Fukunishi M, Hara J, Inoue C, Kamiya T, Saito H. Homozygous Pro74-->Arg mutation in the platelet glycoprotein Ibβ gene associated with Bernard-Soulier syndrome. Thromb Haemost. 2000;84:112–7. [PubMed] [Google Scholar]
- 31.Wang Z, Zhao X, Duan W, Fu J, Lu M, Wang G, Bai X, Ruan C. A novel mutation in the transmembrane region of glycoprotein IX associated with Bernard-Soulier syndrome. Thromb Haemost. 2004;92:606–13. doi: 10.1160/TH04-04-0240. [DOI] [PubMed] [Google Scholar]
- 32.Garner C, Best S, Menzel S, Rooks H, Spector TD, Thein SL. Two candidate genes for low platelet count identified in an Asian Indian kindred by genome-wide linkage analysis: glycoprotein IX and thrombopoietin. Eur J Hum Genet. 2006;14:101–8. doi: 10.1038/sj.ejhg.5201499. [DOI] [PubMed] [Google Scholar]
- 33.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol. 2000;296:911–9. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]