Abstract
Objectives
Obsessive-Compulsive Disorder (OCD) is a common co-morbid condition in schizophrenia, associated with poor prognosis. However, the prevalence of obsessive compulsive symptomatology (OCS) and its relationship to outcome has not been evaluated in adolescents at ultra high-risk for psychosis (UHR).
Methods
Sixty-four UHR and 26 non-prodromal comparison (NPC) youth were ascertained using the Structured Interview for Prodromal Syndromes (SIPS). Participants completed diagnostic interviews and the Padua Inventory (Sanavio, 1988), a self-report measure of OCS.
Results
UHR youth reported significantly higher rates of OCS on the Padua Inventory compared to NPC youth. Clinical diagnosis of OCD (20% of sample) was associated with lower risk of conversion to psychosis over the follow-up period, but was unrelated to clinical severity or psychosocial functioning. However, dimensional ratings of OCS were significantly associated with positive symptom severity, self-reported depression, and a trend toward increased suicidal ideation within the UHR sample.
Conclusions
OCS rates in UHR youth are well above estimated prevalence rates in normal populations, and commensurate with rates of comorbidity observed in schizophrenia. Although clinical diagnosis of OCD was not associated with later conversion to psychosis, OCS severity in UHR youth was associated with more acute symptomatic presentation, including more severe depression and suicidality.
Keywords: Prodrome, Ultra-High-risk, Psychosis, Obsessive-Compulsive, Anxiety, Psychosocial Functioning
1. Introduction
The link between obsessive-compulsive symptomatology and psychosis has been noted since the early 20th century (Gordon, 1926; Stengel, 1945). Prevalence rates for obsessive-compulsive disorder (OCD) as high as 30% have been reported in schizophrenia populations (Byerly et al., 2005), as compared to 1.2-2.4% in the normal population (Foa et al., 1995). Further, a substantial proportion of individuals with schizophrenia report clinically significant obsessive or compulsive symptoms (Bermanzohn, 1999), which may appear early in the developmental course of the illness (Eisen et al., 1997).
Persistent obsessive-compulsive symptoms (OCS) also appear to be a powerful predictor of poorer course and outcome in individuals with psychotic illness (Fenton and McGlashan, 1986). OCS in schizophrenia have been associated with poorer cognitive functioning, particularly on measures tapping prefrontal cortical integrity (Berman et al., 1998), as well as increased levels of depression (Poyurovsky et al., 2001), more severe negative symptoms (Nechmad et al., 2003), poorer social functioning (Poyurovsky et al., 2001), and higher rates of hospitalization (Samuel et al., 1993).
There are several possible explanations as to why OCD and schizophrenia might be integrally related. For example, one disorder could present as a prodrome of the other or one disorder might cause the other (Bottas et al., 2005). Additionally, some researchers (Tibbo and Warneke, 1999) have postulated that the development of OCS in schizophrenia may be associated with effects of atypical antipsychotic medications on serotonergic sites associated with basal ganglia functioning, suggesting that OCS may be the consequence of treatment for some individuals with schizophrenia rather than an aspect of the disorder itself.
In order to explore the potential role of OC symptoms in the developmental period preceding psychosis onset, this study aims to investigate the presentation and effects of OCS on clinical course in youth who are putatively prodromal for psychosis. The prevalence of OC symptoms in this sample and their relationship to diagnostic outcome were explored both categorically, using the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 2002), as well as dimensionally, using a quantitative self-report measure of OC symptom severity, the Padua Inventory (Sanavio, 1988). The associations between OC symptomatology, clinical symptom severity, psychosocial functioning, and medication treatment history were examined to determine if OCS have a notable impact upon clinical course in UHR youth or affect subsequent diagnostic outcome. Finally, we describe OC symptoms that have been observed within this adolescent sample in order to illustrate the manner in which such symptoms may manifest during the prodromal period.
2. Methods
2.1 Participants
Participants were 64 UHR youth and 26 non-prodromal comparison (NPC) youth, between the ages of 12-22, enrolled in an ongoing investigation of adolescents at high clinical risk for developing psychosis. UHR youth were referred by local mental health providers or school staff, or were self-referred in response to advertisements or the CAPPS website. NPC youth were recruited from a community sample via advertising and were age matched to the UHR sample. Participants completed follow-up assessments 11 months [Mean (±SD) = 10.93 (10.71)], on average, after initial ascertainment. Table 1 provides details on demographics, clinical presentation, and medication use at the time of assessment.
Table 1.
Participant demographics by diagnostic group
| Characteristic | UHR OCD+ N = 13 | UHR OCD- N = 51 | NPC N = 26 |
|---|---|---|---|
| Age at examination, mean (± SD), [range] | 15.58 (2.03), [12.67-18.08] | 16.68 (2.39), [12.25-21.75] | 17.67 (2.33), [13.75-21.50] |
| Female Gender, n (%) | 6 (46%) | 19 (37%) | 15 (58%) |
| Caucasian, n (%) | 8 (62%) | 28 (56%) | 12 (46%) |
| Level of Education, mean (± SD), [range] | 9.23 (1.88), [6-12] | 9.86 (2.12), [6-15] | 11.28 (2.17), [7-15] |
| Primary SIPS-defined Prodromal Status, n (%) | |||
| Brief Intermittent Psychotic Syndrome | 2 (15%) | 10 (20%) | N/A |
| Attenuated Positive Symptom Syndrome | 11 (85%) | 40 (78%) | N/A |
| Genetic Risk & Deterioration Syndrome | 0 (0%) | 1 (2%) | N/A |
| Padua Inventory a | |||
| Total score, mean (± SD), [range] | 46.56 (43.39), [6-129] | 39.95 (35.89), [0-161] | 9.76 (9.36), [0-35] |
| Factor 1: Mental Control, mean (±SD), [range] | 13.56 (13.46), [0-38] | 15.38 (14.09), [0-49] | 2.14 (2.39), [0-10] |
| Factor 2: Contamination, mean (±SD), [range] | 12.11 (10.45), [2-29] | 7.38 (7.28), [0-27] | 4.57 (5.24), [0-20] |
| Factor 3: Checking, mean (±SD), [range] | 6.56 (8.06), [0-22] | 4.43 (5.36), [0-26] | 0.90 (1.30), [1-3] |
| Factor 4: Motor Control, mean (±SD), [range] | 3.22 (5.31), [0-16] | 2.48 (3.50), [0-15] | 0.29 (0.64), [1-2] |
| Clinical Characteristics of Sample | |||
| Converted at Follow-up, n (%) | 0 (0%) | 11 (22%) | N/A |
| SIPS Positive Symptom Total, mean (±SD), [range] | 12.46 (4.01), [5-19] | 11.51 (4.70), [1-22] | 1.58 (1.88), [0-6] |
| SIPS Negative Symptom Total, mean (±SD), [range] | 13.00 (5.39), [3-22] | 13.12 (7.55), [2-30] | 1.23 (1.77), [0-7] |
| SIPS Disorganized Symptom Total, mean (±SD), [range] | 6.54 (3.99), [2-15] | 5.90 (3.13), [0-15] | 1.0 (1.30), [0-5] |
| SIPS General Symptom Total, mean (±SD), [range] | 8.46 (3.76), [2-13] | 7.53 (4.32), [0-16] | 0.88 (1.11), [0-3] |
| BPRS Depression score, mean (±SD), [range] | 3.08 (1.75), [1-6] | 3.08 (1.71), [1-7] | 1.16 (0.38), [1-2] |
| BPRS Suicidality score, mean (±SD), [range] | 2.00 (1.00), [1-4] | 1.72 (1.13), [1-5] | 1.00 (0.0), [1-1] |
| BDI Total Score, mean (± SD), [range] | 14.25 (14.14), [2-38] | 14.78 (11.03), [1-42] | 3.59 (3.61), [0-14] |
| Global Social Functioning Scale score, mean (± SD), [range] | 6.00 (1.16), [4-7] | 5.62 (1.63), [2-8] | 8.72 (0.84), [6-10] |
| Global Role Functioning Scale score, mean (±SD), [range] | 5.31 (1.84), [3-8] | 5.22 (1.85), [1-9] | 8.48 (1.12), [4-10] |
| GAF score, mean (± SD), [range] | 47.62 (5.36), [40-58] | 44.51 (14.16), [18-70] | 81.54 (10.31), [48-95] |
| Treatment Characteristics | |||
| History of Hospitalization, n (%) | 4 (31%) | 22 (43%) | N/A |
| Current Use of Any Medication, n (%) | 9 (69%) | 35 (71%) | N/A |
| Current Atypical Antipsychotic Use1, n (%) | 4 (31%) | 21 (41%) | N/A |
| Past Atypical Antipsychotic Use, n (%) | 5 (39%) | 21 (41%) | N/A |
| Current SSRI Use1, n (%) | 8 (62%) | 20 (39%) | N/A |
| Past SSRI Use, n (%) | 6 (46%) | 25 (49%) | N/A |
Sample sizes for Padua Inventory analyses are as follows: UHR OCD+ n=9; UHR OCD- n=40, NPC n=2. Data reported in table is not transformed.
Atypical Antipsychotic medications included: Aripiprazole, Ziprasidone, Risperidone, Olanzapine, and Quetiapine.
Participants were screened with the Structured Interview for Prodromal Syndromes [SIPS, (McGlashan, 2001)] for the presence of one of three prodromal syndromes, based on attenuated subthreshold psychotic symptoms, transient psychotic symptoms, or a substantial drop in social/role functioning in conjunction with a diagnosis of schizotypal personality disorder or presence of a first-degree relative with a psychotic disorder. UHR participants were excluded if they met DSM-IV (1994) criteria for an Axis I schizophrenia-spectrum diagnosis based upon SCID-I/P (First et al., 2002) interview. NPC youth did not meet DSM-IV criteria for a psychiatric disorder as determined by SCID-I/P interview, have a first-degree family history of a psychotic disorder, or meet criteria for any of the three prodromal states defined above. Additional exclusion criteria for all participants included the presence of a neurological disorder, drug or alcohol abuse or dependence within the past 6 months, or Full Scale IQ below 70. All interviews were conducted by M.A. or Ph.D. level psychologists who had participated in an in-depth “gold-standard” training program regarding the administration and scoring of the SIPS (Miller et al., 2003) and SCID-I/P (Ventura et al., 1998). Detailed information on recruitment, inclusion criteria, inter-rater reliability, and case consensus procedures are described elsewhere (Meyer et al., 2005). All participants completed informed consent or assent for the study and were compensated for their participation in all assessments. Parental informed consent for minors was also obtained. Study protocol and informed consent procedures were approved by the UCLA Institutional Review Board.
2.2 Measures
In addition to completing the SIPS and SCID-I/P diagnostic interviews, participants were asked to complete the Padua Inventory [PI, (Sanavio, 1988)], a 60-item self-report questionnaire assessing common obsessions and compulsions in different areas of routine daily function. Ratings are based upon the degree of disturbance, from 0 (Not at all Disturbing) to 4-points (Very Much Disturbing). Participants’ responses on the PI were grouped, based upon a factor analysis of the PI in normal youth (Sternberger and Burns, 1990), to yield a Total score and four Factor scores: Mental Control (F1) assesses difficulty controlling undesirable thoughts, decision making, and rumination; Contamination (F2) assesses concerns about dirt and germs as well as repetitive cleaning behaviors; Checking (F3) assesses repetitive checking behaviors; and Motor Control (F4) assesses urges or worries about losing control of one’s own behavior in response to thoughts or impulses.
PI Total and Factor scores were examined in relation to concurrent clinical and psychosocial measures. The SIPS Positive and Negative Symptom scales were used as indicators of clinical symptom severity. The SIPS Positive Symptom scale assesses unusual thought content, suspiciousness, perceptual disturbances/hallucinations, grandiosity, and disorganized communication. Symptoms of anhedonia, avolition, flat affect, decreased role functioning, and decreased verbal comprehension/ abstraction are captured by the SIPS Negative Symptom scale. The Global Functioning Social [GFS, (Auther et al., 2006)] and Global Functioning Role [GFR, (Niendam et al., 2006)] scales provide clinician-rated assessments of social and work/school functioning, respectively, on two 10-point Likert scales, which are scored independently of symptom severity (Cornblatt et al., 2007). Severity of participants’ current depression and suicidality were assessed by clinician ratings on the Brief Psychiatric Rating Scale [BPRS, (Lukoff et al., 1986)] as well as participant self-reported depression on the Beck Depression Inventory [BDI, (Beck et al., 1988)]. UHR participants were considered “converted to psychosis” if they received a diagnosis of a schizophrenia-spectrum disorder, mood disorder with psychotic features or Psychosis NOS at follow-up.
2.3 Statistical Analysis
All analyses were conducted using SPSS12.0 software (2003, Chicago, IL). Data were checked for significant departures from normality. Due to significant skew in the distribution of Padua Inventory Total and Index Scores as well as the BDI Total Score, a square root transformation was used to improve normality for these variables. The demographic variables of age, gender, and parental education were examined to determine if these variables showed a significant relationship to DSM-IV OCD diagnosis or PI Total score. If a significant relationship was found between a demographic variable and a variable of interest, that demographic variable was then entered as a covariate in subsequent analyses. Due to the number of comparisons, we adopted a more conservative threshold for significance, with alpha set at p<0.01.
To provide a categorical analysis of OC symptom severity, information gathered from the SCID-I/P interview was used to calculate rates of DSM-IV OCD diagnoses within the sample. Youth who were rated as having clinically significant obsessions or compulsions without meeting full DSM-IV criteria for OCD (i.e. met SCID-I/P threshold criteria for all symptoms within the obsession OR compulsion subsection and experienced signification distress or impairment) were categorized as ‘subthreshold OCD’ and were included within the OCD+ group for the purposes of this analysis. Chi-square analysis was utilized to examine differences in rates of conversion to psychosis for OCD+ and OCD- UHR participants. Differences between groups with regard to history of hospitalization, as well as current or past use of atypical antipsychotic and selective serotonin reuptake inhibitor (SSRI) medications, were also examined. Analysis of covariance (ANCOVA) was used to compare UHR youth with (OCD+) and without (OCD-) diagnoses on measures of symptom severity on the normalized Padua Inventory (Total Score), SIPS, BPRS, normalized BDI, GSF, and GRF, after controlling for the effects of demographic variables.
To assess the effects of OC symptoms from a dimensional perspective, PI Total and Index Scores were compared across UHR and NPC youth using analysis of covariance (ANCOVA). In the presence of a significant overall main effect of group on the PI Total Score, post-hoc ANCOVA were used to examine differences between UHR and NPC youth on the PI Total and Index scores. ANCOVA was also used to examine whether PI Total score severity differed as a function of history of psychiatric hospitalization, current or past use of atypical antipsychotic medications, current or past use of SSRI medications, or conversion to psychosis over the follow-up period. Partial correlations, controlling for the effects of demographic variables, were used to examine the relationship between Padua Total score and clinical symptoms (SIPS Positive and Negative symptoms, BPRS Depression and Suicidality, BDI Total Score) and current psychosocial functioning (GFS, GFR) within the UHR sample.
4. Results
Sixty-four UHR and 26 NPC youth completed the SCID interview. Forty-nine (77%) of the UHR and 21 (81%) of the NPC youth completed the Padua Inventory. While 4% of PI non-completers discontinued the study before finishing their questionnaires, 18% of non-completers failed to complete the Padua despite completion of other study procedures. However, completers and non-completers of the Padua Inventory did not differ on any demographic variables. The rates of Padua non-completion did not differ significantly between the NPC, OCD+ and OCD- groups [χ2(2, n=90)=0.70, p=0.71]. Four OCD+ adolescents in the UHR sample did not complete the PI during their participation in the study. However, these UHR individuals did not differ significantly from the remaining UHR participants who did complete the PI on any demographic, clinical, or psychosocial variables of interest (p>0.19 for all comparisons).
Across the whole sample, the normalized PI Total score did not significantly differ based on parental education [Completed college vs. Less than college, F(1,67)=0.58, p=0.45] or gender [39% female; F(1, 68)=0.14, p=0.71]. However, the normalized PI Total score was negatively associated with age [r=-0.23, p=0.058]; thus, age was entered as a covariate in subsequent analyses. Within the UHR group, the rate of OCD diagnosis did not differ as a function of age [F(1, 62)=2.30, p=0.14], parental education [χ2(1, n=61)=2.65, p=0.10], or gender [χ2(1, n=64)=0.35, p=0.56].
Twenty percent (n=13) of the UHR participants were coded as OCD+, with 14% (n=9) receiving a DSM-IV diagnosis of OCD and 6% (n=4) considered subthreshold OCD. Notably, there was a trend toward difference in rates of conversion to psychosis, as none of the OCD+ youth converted to psychosis over the 11 month, on average, follow-up period [χ2(1, n=64)=3.39, p=0.06; Conversion rate=0% in OCD+ group vs. 22% in OCD- group]. However, OCD+ youth did not differ significantly from OCD- youth on any measures of clinical symptom severity, psychosocial functioning, treatment with atypical antipsychotic or SSRI medication, or rates of hospitalization (p>0.13 for all comparisons).
As shown in Figure 1, ANCOVA revealed significant differences between the three participant groups on the normalized PI Total score [F(2, 66)=8.30, p=0.001] after controlling for the effects of age. While PI Total Score did not significantly differ between the OCD+ (n=9) and OCD-(n=40) groups [F(1,46)=0.15, p=0.71], the PI Total score for each UHR group differed significantly from NPC youth [NPC (n=21) vs. OCD+, F(1,27)=15.10, p=0.001; NPC vs. OCD-, F(1,58)=15.71, p<0.001], suggesting that UHR youth, as a whole, reported more OCS than their demographically matched peers.
Figure 1.
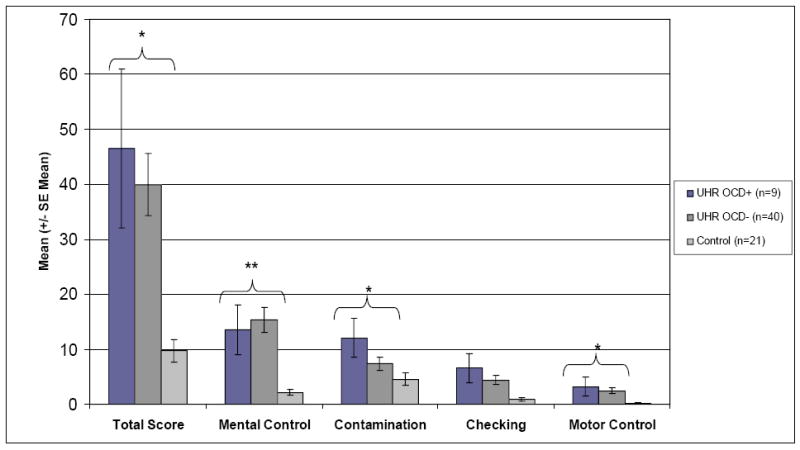
Participants’ mean (± SE mean) scores on the Padua Inventory according to diagnostic group. [* p<0.01, ** p<0.001]
As the OCD+ and OCD- groups did not differ significantly on the normalized PI Total or Index scores (p>0.13 for all comparisons), they were combined into one UHR group for analysis of the four Index scores. Post-hoc examination of the normalized PI Index scores revealed that UHR participants, after controlling for age, reported significantly more OC symptoms related to Mental Control [UHR mean(SD)=15.04(13.86) vs NPC mean(SD)=2.14(2.39); F(1,67)=21.86, p<0.001], Checking [UHR mean(SD)=4.82(5.91) vs NPC mean(SD)=0.90(1.30); F(1, 67)=10.98, p=0.001] and Motor Control [UHR mean(SD)=2.61(3.84) vs NPC mean (SD)=0.29(0.64); F(1, 67)=10.78, p=0.002] when compared to NPC participants. However, no significant difference between UHR and NPC youth was noted on the Contamination Factor [UHR mean(SD)=8.24(8.04) vs. NPC mean(SD)=4.57(5.24); F(1, 67)=2.63, p=0.11].
After controlling for the effects of age, normalized PI Total score showed a significant association with severity of SIPS Positive Symptoms [r=0.40, p=0.005] and a trend association with SIPS Negative Symptoms [r=0.26 p=0.08] within the UHR sample. Furthermore, PI Total Score was significantly associated with increased self-reported symptoms of depression on the BDI [r=0.63, p<0.001] and a trend association with clinician-rated level of suicidality [BPRS Suicidality, r=0.32, p=0.04] for UHR individuals. Within the UHR sample, PI Total Score was not associated with clinician-rated level of depression [BPRS Depression, r=0.18, p=0.25] or current psychosocial functioning [GFS, r=-0. 04, p=0.80; GFR, r=-0.02, p=0.92] after controlling for age. UHR participants’ PI Total Score also did not differ significantly based upon history of hospitalization [F(1,47)=0.21, p=0.65], conversion to psychosis [F(1,47)=0.02, p=0.88], use of atypical antipsychotic treatment [AA Current F(1,47)= 0.20, p=0.66; AA Past F(1,47)=0.76, p=0.39], or use of SSRI medication [SSRI Current F(1,47)= 0.59, p=0.45; SSRI Past F(1,47)=0.30, p=0.58].
5. Discussion
Consistent with prior studies of first-episode and chronic schizophrenia, this study reveals a high rate of obsessive-compulsive disorder in UHR youth (14-20%), when compared to the prevalence of 1-2% in the general population. Previous studies have suggested that OC symptoms may precede the onset of psychosis (Eisen et al., 1997), and our findings highlight that significant obsessive-compulsive symptoms can present as part of the prodromal picture in youth that are at clinical high-risk for psychosis. However, when examined from a DSM-IV categorical perspective, UHR youth meeting full or subthreshold OCD criteria did not differ from UHR participants without OCD on measures of clinical symptoms, psychosocial functioning, rates of hospitalization, or use of atypical antipsychotic or SSRI medication. While several studies have noted an association between co-morbid OCD and poorer course and outcome in schizophrenia (Berman et al., 1998; Eisen et al., 1997; Nechmad et al., 2003; Poyurovsky et al., 2001; Tibbo et al., 2000), our findings suggest that a diagnosis of OCD in the prodromal period is not clearly associated with such poor outcomes.
Of greatest interest, none of the UHR youth with DSM diagnoses of OCD in this sample converted to full-blown psychotic illness. This result suggests that OCD may not represent a prodrome to psychosis per se and such UHR individuals with significant OCD symptoms may in fact represent a subset of false-positives over the follow-up period. However, a proportion of individuals within this sample have not progressed through the period of highest risk, which is the initial 12 months after ascertainment (Yung et al., 2004), and some OCD+ individuals in this sample may convert to psychosis over a longer follow-up period. the risk for conversion declines progressively after the first year (Cannon et al., 2008), only a small proportion of this sample would be expected to convert after the 12 month follow-up. Nevertheless, replication of these findings in larger samples and long-term follow-up of UHR OCD+ individuals are needed to confirm these findings.
In contrast, when the effects of obsessive-compulsive symptoms were examined from a dimensional perspective via the Padua Inventory, results showed that UHR youth overall endorse a significant number of OC symptoms on the Padua Inventory when compared to normal youth, albeit at a level below that which is typically reported by individuals with OCD (Sternberger and Burns, 1990). Irrespective of OCD diagnosis, UHR adolescents reported significant concerns related to various aspects of OC symptomatology at ascertainment, including difficulty controlling their thoughts, repetitive checking behaviors, and worries about losing control of their behavior in response to thoughts or impulses. While severity of self-reported OC symptoms on the Padua Inventory was significantly associated with increased clinical severity, including level of depression and suicidality as well as increased severity of positive and negative symptoms, OC symptoms were not associated with increased rates of subsequent conversion to psychosis, rates of hospitalization, medication use, or impaired psychosocial functioning.
Preliminary follow-up data collected from 31 UHR individuals within 19 months, on average, after ascertainment (SD=10.55, Range = 4-34 months) suggest that self-reported OCS on the Padua Inventory remained stable over time (r=0.66, p<0.001) irrespective of DSM diagnosis of OCD. While this pattern of stability was observed for UHR individuals that did not convert (N=25, r=.69, p<0.001), this association was not significant for the small sample of UHR individuals that converted over the follow-up period (N=6, r=0.54, p=0.27). Irrespective of conversion, UHR participants tended to show a decline in self-reported OCS over the follow-up period [UHR Non-converters Baseline PI Total Mean(SD)= 42.76 (33.84), Follow-up PI Total Mean(SD)= 27.00 (28.00); UHR Converters Baseline PI Total Mean(SD)= 47.50 (30.19), Follow-up PI Total Mean(SD)= 38.33 (23.61)]. Therefore, it remains unclear whether the persistence of self-reported OCS over time may be associated with changes that contribute to conversion. Precise examination of the temporal course OCS in relation to prodromal symptomatology in larger samples is required to fully understand the role that OCS may play in relationship to other symptoms over the course of the prodromal period. Furthermore, the effects of psychosocial treatment on OCS and prodromal symptom severity should be examined in future analyses.
Overall, the current findings demonstrate that the experience of OC symptoms by UHR youth at the point of identification can contribute to higher rates of self-reported distress, which may exacerbate depression and subthreshold psychotic symptoms or increase risk for suicide. However, it is important to note that data for the Padua Inventory were missing for 4 of the 13 OCD+ individuals which further limited statistical power. While our use of the Padua Inventory allowed us to ascertain important information on OC symptoms through a brief self-report questionnaire format, future studies should include detailed interviews of such symptoms (e.g. YBOCS, (Goodman et al., 1989) as they are more commonly used in studies of OCD symptomatology in the United States.
A variety of OC symptoms were described by the UHR youth within our sample. A qualitative analysis of participant reports revealed that OC symptoms in adolescents at clinical high risk for psychosis manifest in two ways, either as being related or unrelated to sub-psychotic symptomatology. During the SCID interview, a subset of UHR youth (n=10) within our sample reported “classic” OC symptoms that were unrelated to their subthreshold psychotic symptoms, such as fears of contamination associated with repetitive hand washing (n=3) or compulsive behaviors like counting, organizing or checking rituals (n=7). In contrast, a separate set of UHR youth (n=3) described OC symptoms that were tied to their sub-psychotic beliefs; for example, one UHR participant reported compulsive checking behaviors that were associated with paranoid fears, such as a need to compulsively check the house for camera or recording devices. Similarly, one UHR individual reported compulsive behaviors associated with delusional thinking, such as a need to inflict pain on themselves in response to sub-psychotic beliefs that they are guilty and deserve punishment. These examples illustrate the symptomatic complexity that is present in youth who demonstrate early clinical risk indicators for psychosis. OC symptoms were only rated on the SIPS if they were closely tied with psychotic or sub-psychotic ideation, which was the case for a small minority of participants (n=3). Therefore, the association between self-reported OCS on the Padua and higher levels of SIPS positive symptoms in this investigation likely reflects a true relationship between the severity of OCS and prodromal symptoms, rather than an artifact of assessment methodology.
While we did not find that the presence of OC symptoms was clearly associated with higher risk of later conversion to psychosis, these symptoms were associated with reports of increased distress and suicidal ideation. Thus, it is important to assess for subthreshold psychotic-like symptoms in youth who present with clinically significant obsessive-compulsive symptoms in order to understand the complete clinical picture and determine the most appropriate form of treatment.
We found substantially higher rates of OCD in UHR youth relative to the general population. Yet, our results also demonstrate that the dimensional view of OC symptoms assessed by the Padua Inventory may capture aspects of symptom severity that can be obscured when looking through the categorical lens of the DSM-IV. While the presence of OC symptoms was not associated with diagnostic outcome in UHR youth, such symptoms appear to contribute significantly to the experience of significant psychological distress in these individuals. Furthermore, these findings highlight that it is essential for clinicians to fully explore the symptom picture when presented with a youth who is reporting OC symptoms, as subthreshold psychotic symptoms may be lingering underneath, thereby contributing to increased clinical and functional impairment.
Acknowledgments
The authors would like to thank the following individuals for their contributions to this project: Cindy Akin, M.A., Rachel Loewy, Ph.D., Sabrina Lux, Stephanie Meyer, Ph.D., Jennifer Johnson, Ph.D., Adrienne Gallet, Sandra DeSilva, Ph.D., and Jamie Zinberg, M.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Auther AM, Smith CW, Cornblatt BA. Global Functioning: Social scale (GFS) Zucker Hillside Hospital; Glen Oaks, NY: 2006. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Berman I, Merson A, Viegner B, Losonczy MF, Pappas D, Green AI. Obsessions and compulsions as a distinct cluster of symptoms in schizophrenia: a neuropsychological study. J Nerv Ment Dis. 1998;186:150–156. doi: 10.1097/00005053-199803000-00003. [DOI] [PubMed] [Google Scholar]
- Bermanzohn PC. Prevalence and Prognosis of Obsessive-Compulsive Phenomena in Schizophrenia: A Critical View. Psychiatr Ann. 1999;29:508–512. [Google Scholar]
- Bottas A, Cooke RG, Richter MA. Comorbidity and pathophysiology of obsessive-compulsive disorder in schizophrenia: is there evidence for a schizo-obsessive subtype of schizophrenia? J Psychiatry Neurosci. 2005;30:187–193. [PMC free article] [PubMed] [Google Scholar]
- Byerly M, Goodman W, Acholonu W, Bugno R, Rush AJ. Obsessive compulsive symptoms in schizophrenia: frequency and clinical features. Schizophr Res. 2005;76:309–316. doi: 10.1016/j.schres.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JL, Beer DA, Pato MT, Venditto TA, Rasmussen SA. Obsessive-compulsive disorder in patients with schizophrenia or schizoaffective disorder. Am J Psychiatry. 1997;154:271–273. doi: 10.1176/ajp.154.2.271. [DOI] [PubMed] [Google Scholar]
- Fenton WS, McGlashan TH. The prognostic significance of obsessive-compulsive symptoms in schizophrenia. Am J Psychiatry. 1986;143:437–441. doi: 10.1176/ajp.143.4.437. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Foa EB, Kozak MJ, Goodman WK, Hollander E, Jenike MA, Rasmussen SA. DSM-IV field trial: obsessive-compulsive disorder. Am J Psychiatry. 1995;152:90–96. doi: 10.1176/ajp.152.1.90. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Gordon A. Obsessions in their relation to psychoses. Am J Psychiatry. 1926;82:647–659. [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the Expanded Brief Psychiatric Rating Scale (BPRS) Schizophr Bull. 1986;12:594–602. [Google Scholar]
- McGlashan TH. Structured Interview for Prodromal Syndromes (SIPS) Yale University; New Haven: 2001. [Google Scholar]
- Meyer SE, Bearden CE, Lux SR, Gordon JL, Johnson JK, O’Brien MP, Niendam TA, Loewy RL, Ventura J, Cannon TD. The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. J Child Adolesc Psychopharmacol. 2005;15:434–451. doi: 10.1089/cap.2005.15.434. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Nechmad A, Ratzoni G, Poyurovsky M, Meged S, Avidan G, Fuchs C, Bloch Y, Weizman R. Obsessive-compulsive disorder in adolescent schizophrenia patients. Am J Psychiatry. 2003;160:1002–1004. doi: 10.1176/appi.ajp.160.5.1002. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, Cannon TD. Global Functioning: Role scale (GFR) University of California, Los Angeles; Los Angeles: 2006. [Google Scholar]
- Poyurovsky M, Hramenkov S, Isakov V, Rauchverger B, Modai I, Schneidman M, Fuchs C, Weizman A. Obsessive-compulsive disorder in hospitalized patients with chronic schizophrenia. Psychiatry Res. 2001;102:49–57. doi: 10.1016/s0165-1781(01)00238-4. [DOI] [PubMed] [Google Scholar]
- Samuel J, Nesdadt G, Wolyniec P, Adler L, Liang K, Pulver A. Obsessive-compulsive symptoms in schizophrenia. Schizophr Res. 1993;9:139. [Google Scholar]
- Sanavio E. Obsessions and compulsions: the Padua Inventory. Behav Res Ther. 1988;26:169–177. doi: 10.1016/0005-7967(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Stengel E. A Study on some Clinical Aspects of the Relationship Between Obsessional Neurosis and Psychotic Reaction Types. J Ment Sci. 1945;91:166–187. [Google Scholar]
- Sternberger LG, Burns GL. Obsessions and compulsions: psychometric properties of the Padua Inventory with an American college population. Behav Res Ther. 1990;28:341–345. doi: 10.1016/0005-7967(90)90087-y. [DOI] [PubMed] [Google Scholar]
- Tibbo P, Kroetsch M, Chue P, Warneke L. Obsessive-compulsive disorder in schizophrenia. J Psychiatr Res. 2000;34:139–146. doi: 10.1016/s0022-3956(99)00048-5. [DOI] [PubMed] [Google Scholar]
- Tibbo P, Warneke L. Obsessive-compulsive disorder in schizophrenia: epidemiologic and biologic overlap. J Psychiatry Neurosci. 1999;24:15–24. [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]


