Abstract
OBJECTIVE
Pro-inflammatory cytokines of placental or systemic origin are thought to play a central role in the pathophysiology of preeclampsia. We sought to estimate the fractional excretion of tumor necrosis factor (TNF)-α in relationship to proteinuria in women with severe preeclampsia.
METHODS
In a cross-sectional study, we evaluated the serum and urine levels of TNF-α in 45 women diagnosed with severe preeclampsia (mean±SEM, gestational age: 29.1±0.5 weeks). Forty-five healthy pregnant control women matched for parity, maternal and gestational age at recruitment (30.1±0.4 weeks) served as control. Urinary concentrations were normalized to creatinine. The fractional excretion of the TNF-α was interpreted in relationship to those of total proteins and that of soluble fms-like tyrosine kinase-1 (sFlt-1).
RESULTS
We found that preeclamptic women had significantly higher serum TNF-α concentrations compared to controls (mean ± SEM, preeclampsia: 1.39±0.09 vs. control: 0.93±0.07 pg/mL, P<0.001). In contrast, urinary levels of TNF-α were significantly decreased in preeclampsia compared with healthy controls (median [interquartile range], preeclampsia: 0.26 [0.10–0.91] vs. control: 0.58 [0.21–1.29] pg/mg creatinine, P=0.003), even though the hypertensive women had higher levels of proteinuria. In contrast to sFlt-1, urinary TNF-α did not correlate with the degree of proteinuria. Additionally, in preeclampsia the fractional excretion of TNF-α was significantly lower (preeclampsia: 1.92 [0.46–4.20] vs. control: 7.2 [2.44–12.07] percent, P<0.001).
CONCLUSION
The fractional excretion of TNF-α is significantly reduced in women with severe preeclampsia, despite proteinuria. The decreased clearance and altered renal excretion of this cytokine may contribute to the exaggerated inflammatory response observed in preeclampsia.
INTRODUCTION
Hypertensive disorders are a leading cause of maternal and perinatal morbidity and mortality, worldwide.1,2,3 Preeclampsia complicates between 5 to 9% of all pregnancies.4 Regrettably, given the potentially grave prognosis of severe preeclampsia and our inability to precisely determine its cause, the best treatment option remains delivery, regardless of gestational age.5
The early pathophysiologic events leading to preeclampsia remain an enigma.6 For several years, we and others have concentrated our attention to understanding the role of circulating and urinary anti-angiogenic factors (soluble fms-like tyrosine kinase-1 [sFlt-1]) and other placental markers (inhibin, activin, monocyte chemoattractant protein-1) in the pathogenesis of preeclampsia.7,8,9,10,11 The urinary concentrations of two such markers, sFlt-1 and inhibin A, were significantly increased in women with preeclampsia and urinary levels correlated directly with both serum concentrations and the degree of proteinuria.8,9 The dramatic increase in maternal serum and urinary levels of these markers in women with severe preeclampsia could reflect increased placental production, glomerular leakage, altered re-uptake, or endogenous renal production.8,9
Tumor necrosis-α (TNF-α) is a pro-inflammatory cytokine with a pleiotropic effect on the immune system, tissue homeostasis, embryonic development, and placentation.7,12,13,14 This cytokine plays a vital role in the regulation of inflammation because it affects the release of other pro-inflammatory cytokines (IL-1, IL-6, monocyte chemoattractant protein-1) via both positive and negative feedback mechanisms.11,15,16 Compelling evidence suggests that when released in large amounts, TNF-α induces excessive coagulation, enhanced activation and injury of the vascular endothelium, and, as noted, induces trophoblast apoptosis and impedes trophoblast invasion.17,18,19
A current theory holds that increased decidual TNF-α levels increase decidual cell expression of a number of monocyte/macrophage chemotractant factors which lead to the recruitment and activation of macrophages in the placental bed.20,21 In vitro studies demonstrate that macrophage-associated TNF-α induces apoptosis of extravillous trophoblasts. 22 Beyond its stimulation of decidual monocyte/macrophage trafficking, TNF-α can exert direct effects on trophoblast to stimulate apoptosis and inhibit trophoblast migration. 23 The resultant defective placentation is followed by the systemic release of cytotoxic factors which damage the maternal vascular endothelium.24,25,26 Although TNF-α could also be an “effect” of the pathophysiological changes associated with severe preeclampsia, the significance of the above mentioned model is predicated by studies which demonstrated that in preeclampsia widespread damage and dysfunction of the maternal endothelium, placental oxidative stress and aberrant lymphocyteTh1/Th2 cytokine balance [interleukin (IL)-2, IL-12, IL-15, IL-18, interferon (IFN)-γ, tumor necrosis factor (TNF)-α vs. IL-4, IL-10, transforming growth factor (TGF)], induces an exaggerated maternal systemic inflammatory response characterized by hypertension and proteinuria.27,28,29,30
Given these protean TNF-α effects it seems reasonable to propose that increased decidual and systemic TNF-α expression plays an important role in initiating and maintaining the pathophysiologic mechanism of this specific pregnancy-related syndrome. Further, because TNF-α is a small molecule [17 kilodaltons (kDa)], the degree of kidney damage which characterizes severe preeclampsia may be an important regulator of the systemic TNF-α levels. We sought to test the hypothesis that, in women with severe preeclampsia the fractional excretion of this pro-inflammatory cytokine is increased in direct relationship with proteinuria.
METHODS
Participants and sample collection
We conducted a cross-sectional study using time matched serum and urine samples collected from 90 women pregnant with singletons enrolled at Yale-New Haven Hospital from June 2004 to May 2007. The Yale University Human Investigation Committee approved our study protocol and written consent was obtained from all of the participants. Subjects were recruited from women evaluated or admitted to the Labor and Birth unit or the antepartum High and Low Risk units. The decision to recommend admission or delivery of the fetuses was made by the primary physician, independent of our research protocol. The study group consisted of 45 pregnant women diagnosed with severe preeclampsia. The control group consisted of 45 women matched one to one for maternal and gestational age at enrollment that appeared healthy by clinical and laboratory standards at the time of enrollment. Control women were selected from our data base prior to evaluation of their serum or urine TNF-α levels. None of the controls were excluded from the final analysis. Exclusion criteria for enrollment were preexisting proteinuria and/or hypertension, active labor, clinical symptoms suggestive of viral or bacterial infection, known or suspected congenital malformation, and isolated intrauterine growth restriction (IUGR). All control women had a pregnancy course uncomplicated by preeclampsia.
Gestational age was established based on last menstrual period and/or early ultrasound evaluations (<20 weeks of gestation) in all cases.31 Severe preeclampsia was defined based on the American College of Obstetricians and Gynecologists criteria: gestational age > 20 weeks, blood pressure of 160 mm Hg systolic or higher or 110 mm Hg diastolic or higher on 2 occasions at least 6 hours apart, and/or proteinuria of at least 5 g in a 24-hour urine specimen or 3+ or greater on 2 random urine samples collected at least 4 hours apart.5 Other elements of the diagnosis included: IUGR (<10th percentile), cerebral or visual disturbances (headache, visual changes), epigastric or right upper-quadrant pain, pulmonary edema or cyanosis, oliguria (urinary output less than 500 mL/24 h), impaired liver function, and thrombocytopenia (<100,000 cells/µL).5
All biological samples (serum and urine) were collected contemporaneous to clinical assessment or admission, as previously described.8 The urine sample (5–10 mL) was collected in a sterile container (“clean catch” method or bladder catheterization if performed for clinical indications). Blood samples were collected by venipuncture, prior to intravenous administration of fluids and allowed to clot. Serum and urine samples were spun at 3000 × g at 4°C for 20 minutes, and the supernatant aliquoted and immediately stored at −80°C until analyzed.
Immunoassay procedures
ELISA assays for TNF-α were performed according to the manufacturer’s instructions using a high sensitivity design (R&D Systems, Minneapolis, MN, www.rndsystems.com). Serum and urine samples were assayed in duplicate with a capture monoclonal antibody directed against human TNF-α. The assay detects the total amount of TNF-α in the sample composed of free TNF-α and that bound to soluble receptors. Incubation protocols were performed followed by washings and readings at 490 nm with background correction at 650 nm using a VERSAmax™ microplate reader with Softmax Pro 3.1.1 software (Molecular Devices, Sunnyvale, CA). The mean minimal detectable concentration in the assays for TNF-α reported by the manufacturer was 0.106 pg/mL. The intra- and inter-assay coefficients of variation were <3.1% and 7.1%, respectively. Free sFlt-1 was measured by ELISA (R&D Systems) in the same urine aliquot as previously described.8
Other biochemical estimates
Total protein concentration in serum and urine was measured using a bicinchoninic acid/cupric sulphate reagent (BCA kit, Pierce, Rockford, Il). Creatinine levels were measured in the same aliquot used for immunoassays using a colorimetric assay (Stanbio Laboratory, Boerne, TX) against standard curves derived from known concentrations. To correct for diurnal variations in urine concentration urine levels of TNF-α, sFlt-1 and total protein were normalized to urine creatinine and expressed per mg creatinine.
Fractional excretion indicators (clearance ratios) were calculated using the formula: (urine/plasma analyte concentration) ÷ (urine/plasma creatinine concentration) × 100. The fractional excretion of a substance represents the proportion of the substance excreted in the urine compared with that filtered by the glomeruli. It is generally reported relative to creatinine clearance since creatinine is neither reabsorbed nor significantly secreted. Calculations of fractional excretion help to understand whether increased serum levels of an analyte are due to increased production or decreased excretion.
Statistical analysis
Data were tested for normality using the Kolmogorov-Smirnov method and reported as either mean with standard error (for normally distributed data) or as median and interquartile range [IQR] (for non-normally distributed data). Comparisons were performed using paired t-test, Wilcoxon's signed rank test and McNemar's chi-square test as appropriate. For immunoassay results logarithmic transformations were applied before statistical comparisons were performed. Relationships between variables (correlations) were explored using Spearman’s Rank order correlations. Comparison between strength of correlations was achieved based on z statistic.32 Statistical analysis was performed with SigmaStat version 2.03 (SPSS, Inc., Chicago, IL), SPSS version 14.0 (SPSS, Inc., Chicago, IL) and MedCalc (Broekstraat, Belgium) softwares. A P value of less than 0.05 was considered to indicate statistical significance. Sample size calculations were based on prior published data on urinary levels of sFlt-1.8,10 It was estimated that 17 patients in each group would be necessary to detect differences equal to the standard deviation observed for urinary sFlt-1 concentrations (normalized to creatinine) with 80% power and a confidence coefficient of 95% using an unpaired t-test. For our case-control study design, a sample size of 45 per group would allow detecting differences equal to twice the standard deviation with 90% power and an alpha of 0.05.
RESULTS
The demographic, clinical and outcome characteristics of the women in two groups are presented in Table 1. There was no difference in maternal age, race, gravidity, parity or gestational age at the time of sample collection. In the severe preeclampsia group, 11 women (24%) had IUGR and 12 (26%) had HELLP syndrome. Twenty-five (56) of the preeclamptic women had neurological manifestations (persistent headache, visual changes, hyperreflexia) including eclampsia (n=3). As expected, women with severe preeclampsia had higher blood pressure values and were significantly proteinuric by both urine dipstick and 24-hour urine protein excretion. All preeclamptic women had an indicated premature delivery which in 96% of cases occurred at <34 weeks while the controls delivered at a median gestational age of 38 [37–39] weeks.
Table 1.
Demographic, clinical and outcome characteristics of women enrolled in the study
| Variable | Severe Preeclampsia N=45 | Control N=45 | P value |
|---|---|---|---|
| Maternal characteristics at enrollment | |||
| Age, years † | 27 [20–35] | 28 [22–31] | 0.618 |
| Non-caucasian race | 26 [58] | 22 [49] | 0.556 |
| Weight, kg ‡ | 88 ± 3 | 85 ± 5 | 0.324 |
| Gravidity † | 2 [1–3] | 2 [1–3] | 0.706 |
| Parity † | 0 [0–1] | 1 [0–1] | 0.612 |
| Nulliparity, n [%] § | 26 [58] | 20 [44] | 0.239 |
| Gestational age, weeks ‡ | 29.1 ± 0.5 | 30.1 ± 0.4 | 0.147 |
| Systolic blood pressure, mmHg ‡ | 169 ± 3 | 109 ± 3 | <0.001 |
| Diastolic blood pressure, mmHg ‡ | 102 ± 2 | 65 ± 2 | <0.001 |
| Proteinuria - urinary dipstick - | 3 [2–4] | 0 [0–0] | <0.001 |
| 24h-protein excretion, grams/24h † | 2.5 [1.3–4.1] | NA | NA |
| Neurological manifestations | 25 [56] | 0 [0] | <0.001 |
| IUGR, n [%] § | 11 [24] | 0 [0] | 0.003 |
| HELLP, n [%] § | 12 [27] | 0 [0] | 0.002 |
| Outcome characteristics | |||
| Gestational age at delivery, weeks † | 31 [28–33] | 38 [37–39] | <0.001 |
| Delivery <34 weeks, n [%] § | 43 [96] | 0 [0] | <0.001 |
| Indicated delivery, n [%] § | 45 [100] | 0 [0] | <0.001 |
| Enrolment-to-delivery interval, days † | 1 [0–2] | 59 [47–85] | <0.001 |
Data presented as median [interquartile range] and analyzed by Signed-Rank tests.
Data presented as mean ± standard error and analyzed by paired t-tests.
Data presented as n (%) and analyzed by McNemar’s tests.
We found that women with preeclampsia had significantly lower serum protein (preeclampsia: 6.3 ± 0.2 vs. control: 7.4 ± 0.2 g/dL, P<0.001) and higher serum creatinine levels (preeclampsia: 0.89 ± 0.05 vs. control: 0.63 ± 0.04 mg/dL, P<0.001). Furthermore, our measurements of the protein-to-creatinine ratio confirmed significant differences in total protein concentration between preeclamptic and non-preeclamptic women in the same urine aliquot used for assessment of TNF-α excretion (preeclampsia: 11.2 [7.0–17.3] vs. control: 7.5 [6.9–10.0] mg/mgc, P=0.003). Similarity, we determined that urinary output of sFlt-1 was markedly elevated in preeclampsia (114.6 [47.9–190.0] vs. control: 11.2 [4.4–17.6] pg/mg creatinine, P<0.001). This data confirms the correct clinical classification of the women in the two groups and demonstrates that our study was adequately powered to detect biologically relevant differences related to preeclampsia.
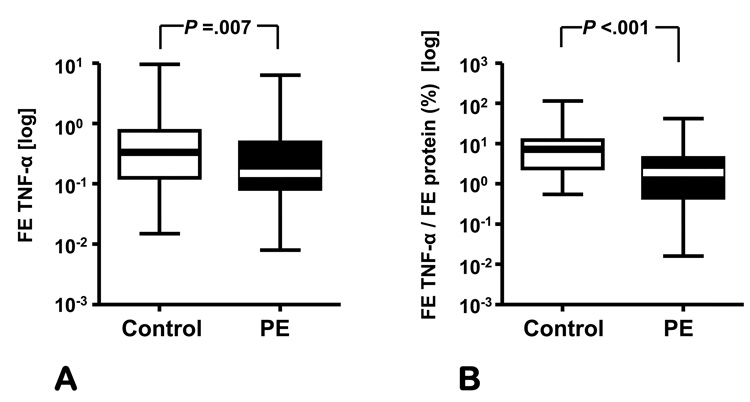
In our study, preeclampsia was associated with elevated serum TNF-α (preeclampsia: 1.39 ± 0.09 vs. control: 0.93 ± 0.07 pg/mL, P<0.001) (Figure 1A) and sFlt-1 (preeclampsia: 2661.9 ± 164.2 vs. control: 384.3 ± 65.3 pg/mL, P<0.001) concentrations. However, the urinary levels of TNF-α were significantly decreased between groups (preeclampsia: 0.26 [0.10–0.91] vs. control: 0.58 [0.21–1.29] pg/mg creatinine, P = .003 (Figure 1B) despite the increase in proteinuria in preeclamptic group. Furthermore, the fractional excretion of TNF-α in women with preeclampsia was also lower than that of normal women (preeclampsia: 0.15 [0.09–0.47] vs. control: 0.33 [0.13–0.74], P=0.007) (Figure 2A). We found out that when normalized for the fractional excretion of total proteins, women with severe preeclampsia had a significantly lower excretion of TNF-α compared to normal controls (preeclampsia: 1.92 [0.46–4.20] vs. control: 7.20 [2.4–12.10] percent, P<0.001).
Figure 1. Serum and urine levels of TNF-α in preeclampsia.
Serum (A) and urine TNF-α (B) concentration (normalized for urinary creatinine) in women with severe preeclampsia (PE) versus pregnant controls. The data (in logarithmic format) is presented as percentiles with median. The ends of the boxes define the 25th and 75th percentiles, the line inside the box defines the median and the whiskers show the largest and smallest values. Statistical comparisons were performed with Student t-tests after logarithmic transformation of data. Urinary levels were normalized to creatinine concentration and expressed per mg creatinine (mgc).
Figure 2. Fractional excretion of TNF-α in preeclampsia.
Fractional excretion (FE) of TNF-α (A) expressed as clearance ratio (A) and relative to the fractional excretion of total proteins (B) in women with severe preeclampsia (PE) versus pregnant controls. The data (in logarithmic format) is presented as percentiles with median. The ends of the boxes define the 25th and 75th percentiles, the line inside the box defines the median and the whiskers show the largest and smallest values. Statistical comparisons were performed with Student t-tests after logarithmic transformation of data.
To further explore the relationships between proteinuria and TNF-α renal clearance, we studied in parallel the elimination of another factor, sFlt-1, whose serum levels were also increased by preeclampsia. We established that in non-preeclamptic (control) pregnancies, there was a significant and similar strength of correlation between the urinary elimination of total proteins and that of TNF-α (R=0.531, P<0.001) and sFlt-1 (r=0.331, P=0.027, z-statistic TNF-α vs. sFlt-1: 1.13, P=0.256) (Figure 3A). In contrast, in preeclamptic pregnancies only the elimination of sFlt-1 correlated with the extent of proteinuria (r=0.619, P<0.001) while that of TNF-α did not (r=−0.174, P=0.251) (Figure 3B) rendering a significant difference in the strength and/or direction of correlation of urinary the sFlt-1 elimination versus that of TNF-α (z-statistic: 4.12, P<0.001).
Figure 3. Relationships of TNF-α, sFlt-1 and total protein excretion.
Correlation analyses between the urinary elimination of total proteins and that of sFlt-1 and TNF-α in normal (A) versus severe preeclamptic pregnancies (B). All values were normalized to urine creatinine and expressed per mg creatinine (mgc). The thick lines represent the linear regression between proteinuria and urinary sFlt-1 (circles) or TNF-α (squares) in logarithmic format. The 95% confidence intervals are shown by the respective dotted lines.
DISCUSSION
Our findings demonstrate that the fractional excretion of the inflammatory cytokine TNF-α is significantly reduced in women with severe preeclampsia, despite proteinuria. Therefore, we propose this impaired urinary clearance may contribute to the observed and previously reported elevation in circulating levels of TNF-α in women with severe preeclampsia. 4,27,28
The TNF-α molecule is synthesized as a 26 kDa membrane-associated protein which is enzymatically cleaved into the soluble 17 kDa cytokine. The actions of TNF-α are mediated by two distinct cell-surface receptors (Type 1, a 55 kDa protein and Type 2 a 75 kDa protein). Soluble forms of TNF-α receptors (sTNFp55 and sTNFp75) have been identified as representing truncated forms generated by shedding of the extracellular domains of the respective surface receptors. Both types of soluble receptors are able to bind and antagonize the biological activity of TNF-α in an effort to modulate the cellular effects of this potent cytokine.33 Previous findings demonstrated that, in preeclampsia, both types of soluble TNF-α receptors are increased in the maternal circulation in addition to TNF-α.34 It is important to note that normal human urine is known to contain TNF-α-binding activity which has been attributed to excretion of the soluble receptors.35 In normal pregnancy, urinary levels of sTNFp55 and sTNFp75 were assayed in the range of 1 ng/mL,36 a level which is 500-fold in excess to the highest urinary TNF-α readout in our cohort (<2 pg/mL). This observation led us prefer an immunoassay that quantifies total rather than free TNF-α immunoreactivity. By taking this approach, we were able to examine the renal clearance of the TNF-α molecule and minimize the possibility that our immunoassay results are modified by concurrent changes in the TNF-α soluble receptors.
Compelling clinical and experimental evidence confirmed that in highly inflammatory states such as sepsis, increased TNF-α concentration was associated with reduced survival.37 Efforts have therefore concentrated on therapeutic strategies to effectively remove TNF-α from circulation in hopes of promoting recovery and increasing survival.38 Studies aimed to evaluate the role of the kidney in clearance of the inflammatory cytokines of septic patients reveal that the kidney removes some pro-inflammatory cytokines from plasma at the onset of the disease as long as the diuresis is maintained.39 However, in advanced sepsis characterized by acute renal failure and oliguria, the fractional excretion of several inflammatory cytokines (including TNF-α) drops and as a consequence their plasma concentration rises. 39
In humans, the role of the kidney in controlling cytokine homeostasis is well-established.40 For instance, in non-proteinuric systemic inflammatory states (such as after cardiac surgery), the kidney preferentially filters smaller pro-inflammatory molecules (generally <20 kDa) and less readily filters the larger anti-inflammatory cytokines (>20 kDa) and soluble cytokine receptors.40 Moreover, the filtered pro-inflammatory cytokines are not excreted intact in the urine, but are absorbed in the proximal renal tubules and denatured by intracellular proteolytic mechanisms.41 Because previous studies demonstrated that the kidney is the main catabolic organ of TNF-α, it seems reasonable to propose that a proximal tubular injury, even subclinical in nature, would result in an impaired ability to inactivate reabsorbed TNF-α and would contribute to the elevated systemic TNF-α levels.40 From this perspective, our results are novel. Additionally, while the initiating injury in preeclampsia is generally regarded as placental in origin, our findings underscore the potential importance of the kidney in propagating the systemic disturbances in preeclamptic women.
The pathophysiologic events responsible for the increase in urinary protein excretion in preeclampsia are complex and not entirely understood.42 Possible increases in glomerular capillary pressure, alterations in the size and/or charge selectivity of the glomerular filter, compromised proximal tubular reabsorption are the most often posited mechanisms.42 Although the possible change in charge selectivity of the glomerular barrier in preeclampsia is still a subject of active debate, studies have shown that despite 100-fold increases in urinary albumin excretion, fractional excretion of neutral dextrans of comparable molecular weight was decreased.42 This loss of charge selectivity in preeclampsia could thus explain why the relative excretion of TNF-α is reduced while excretion of total proteins and sFlt-1 increases. Further studies are warranted to describe and clarify the mechanisms responsible for altered kidney handling of this potent cytokine in preeclampsia, and to evaluate if similar mechanisms apply in renal dysfunction patients with and without chronic proteinuria.
In summary, preeclampsia is associated with reduced TNF-α clearance compared to other proteins, despite increased systemic levels.
ACKNOWLEDGEMENT
The authors thank the residents, nurses, and faculty at Yale University who assisted with collection of the samples.
Supported by the Department of Health and Human Services, NIH/NICHD RO1-HD047321-01 (IAB), RO3-HD50249-02 (CSB) and R01-HL070004-03 (CJL).
Footnotes
Financial Disclosure: The authors have no potential conflicts of interest to disclose.
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. government.
REFERENCES
- 1.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Tuffnell DJ, Jankowicz D, Lindow SW, Lyons G, Mason GC, Russell IF, Walker JJ. Yorkshire Obstetric Critical Care Group. Outcomes of severe pre-eclampsia/eclampsia in Yorkshire 1999/2003. BJOG. 2005;112:875–880. doi: 10.1111/j.1471-0528.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 3.Menzies J, Magee LA, Li J, MacNab YC, Yin R, Stuart H, Baraty B, Lam E. Preeclampsia Integrated Estimate of RiSk (PIERS) Study Group. Instituting surveillance guidelines and adverse outcomes in preeclampsia. Obstet Gynecol. 2007;110:121–127. doi: 10.1097/01.AOG.0000266977.26311.f0. [DOI] [PubMed] [Google Scholar]
- 4.Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–28. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 5.ACOG Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 6.Baumwell S, Karumanchi SA. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106:c72–c81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 7.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Express placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhimschi CS, Magloire L, Funai E, Norwitz ER, Kuczynski E, Martin R, et al. Fractional excretion of angiogenic factors in women with severe preeclampsia. Obstet Gynecol. 2006;107:1103–1113. doi: 10.1097/01.AOG.0000207698.74104.4f. [DOI] [PubMed] [Google Scholar]
- 9.Hamar BD, Buhimschi IA, Sfakianaki AK, Pettker CM, Magloire LK, Funai EF, et al. Serum and urine inhibin A but not free activin A are endocrine biomarkers of severe pre-eclampsia. Am J Obstet Gynecol. 2006;195:1636–1645. doi: 10.1016/j.ajog.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Buhimschi CS, Norwitz ER, Funai E, Richman S, Guller S, Lockwood CJ, Buhimschi IA. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol. 2005;192:734–741. doi: 10.1016/j.ajog.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Gollapudi S. Molecular mechanisms of TNF-alpha-induced apoptosis in naive and memory T cell subsets. Autoimmun Rev. 2006;5:264–268. doi: 10.1016/j.autrev.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura K, Kawamura N, Kumagai J, Fukuda J, Tanaka T. Tumor necrosis factor regulation of apoptosis in mouse preimplantation embryos and its antagonism by transforming growth factor alpha/phosphatidylionsitol 3-kinase signaling system. Biol Reprod. 2007;76:611–618. doi: 10.1095/biolreprod.106.058008. [DOI] [PubMed] [Google Scholar]
- 14.Cartwright JE, Balarajah G. Trophoblast interactions with endothelial cells are increased by interleukin-1beta and tumour necrosis factor alpha and involve vascular cell adhesion molecule-1 and alpha4beta1. Exp Cell Res. 2005;304:328–336. doi: 10.1016/j.yexcr.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Eigler A, Sinha B, Hartmann G, Endres S. Taming TNF: strategies to restrain this proinflammatory cytokine. Immunol Today. 1997;18:487–492. doi: 10.1016/s0167-5699(97)01118-3. [DOI] [PubMed] [Google Scholar]
- 16.Kast RE. Tumor necrosis factor has positive and negative self regulatory feedback cycles centered around cAMP. Int J Immunopharmacol. 2000;22:1001–1006. doi: 10.1016/s0192-0561(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 17.Serin IS, Ozcelik B, Basbug M, Kilic H, Okur D, Erez R. Predictive value of tumor necrosis factor alpha (TNF-α) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2002;100:143–145. doi: 10.1016/s0301-2115(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 18.Anim-Nyame N, Gamble J, Sooranna SR, Johnson MR, Steer PJ. Microvascular permeability is related to circulating levels of tumour necrosis factor-alpha in pre-eclampsia. Cardiovasc Res. 2003;58:162–169. doi: 10.1016/s0008-6363(02)00844-1. [DOI] [PubMed] [Google Scholar]
- 19.Williams MA, Farrand A, Mittendorf R, Sorensen TK, Zingheim RW, O'Reilly GC, et al. Maternal second trimester serum tumor necrosis factor-alpha-soluble receptor p55 (sTNFp55) and subsequent risk of preeclampsia. Am J Epidemiol. 1999;149:323–329. doi: 10.1093/oxfordjournals.aje.a009816. [DOI] [PubMed] [Google Scholar]
- 20.Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, et al. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- 23.Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers GM, Taylor RN, Roberts JM. Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells. Am J Obstet Gynecol. 1988;159:908–914. doi: 10.1016/s0002-9378(88)80169-8. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed A, Rahman M, Zhang X, Acevedo CH, Nijjar S, Rushton I, et al. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Mol Med. 2000;6:391–409. [PMC free article] [PubMed] [Google Scholar]
- 27.Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522–8530. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- 29.Sargent IL, Borzychowski AM, Redman CW. NK cells and human pregnancy--an inflammatory view. Trends Immunol. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–555. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadlock FP, Deter RL, Harrist RB, Park SK. Computer assisted analysis of fetal age in the third trimester using multiple fetal growth parameters. J Clin Ultrasound. 1983;11:313–316. doi: 10.1002/jcu.1870110605. [DOI] [PubMed] [Google Scholar]
- 32.Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 2nd ed. Boston: Houghton Mifflin Company; 1988. [Google Scholar]
- 33.Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunez-Gonzalez JR, Sanabria-Vera CJ, Romero-Adrian T. Measurement of the serum concentrations of tumor necrosis factor alpha and its soluble receptors in normal and pre-eclamptic pregnant patients. Invest Clin. 2001;42:171–181. [PubMed] [Google Scholar]
- 35.Arntzen KJ, Liabakk NB, Jacobsen G, Espevik T, Austgulen R. Soluble tumor necrosis factor receptor in serum and urine throughout normal pregnancy and at delivery. Am J Reprod Immunol. 1995;34:163–169. doi: 10.1111/j.1600-0897.1995.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 36.Austgulen R, Liabakk NB, Brockhaus M, Espevik T. Soluble TNF receptors in amniotic fluid and in urine from pregnant women. J Reprod Immunol. 1992;22:105–116. doi: 10.1016/0165-0378(92)90009-s. [DOI] [PubMed] [Google Scholar]
- 37.Gordon AC, Lagan AL, Aganna E, Cheung L, Peters CJ, McDermott MF, et al. TNF and TNFR polymorphisms in severe sepsis and septic shock: a prospective multicentre study. Genes Immun. 2004;5:631–640. doi: 10.1038/sj.gene.6364136. [DOI] [PubMed] [Google Scholar]
- 38.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29(7 Suppl):S121–S125. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- 39.Graziani G, Bordone G, Bellato V, Finazzi S, Angelini C, Badalamenti S. Gruppo di Studio Trattamenti depurativi in area critica of the Italian Society of Nephrology. Role of the kidney in plasma cytokine removal in sepsis syndrome: a pilot study. J Nephrol. 2006;19:176–182. [PubMed] [Google Scholar]
- 40.Gormley SM, McBride WT, Armstrong MA, McClean E, MacGowan SW, Campalani G, et al. Plasma and urinary cytokine homeostasis and renal function during cardiac surgery without cardiopulmonary bypass. Cytokine. 2002;17:61–65. doi: 10.1006/cyto.2001.0972. [DOI] [PubMed] [Google Scholar]
- 41.Pessina GP, Pacini A, Bocci V, Maioli E, Naldini A. Studies on tumor necrosis factor (TNF): II. Metabolic fate and distribution of human recombinant TNF. Lymphokine Res. 1987;6:35–44. [PubMed] [Google Scholar]
- 42.Moran P, Lindheimer MD, Davison JM. The renal response to preeclampsia. Semin Nephrol. 2004;24:588–595. doi: 10.1016/s0270-9295(04)00130-5. [DOI] [PubMed] [Google Scholar]