Abstract
The present study addressed the question whether neural activity in left lateral parietal cortex is modulated by amount of information recollected. In two experiments (one using fMRI and the other ERPs), subjects first studied pairs of pictures presented for either 1 or 6 s. They then performed a standard “Remember/Know” recognition memory test in which the old items comprised one of the pictures from each studied pair. In both experiments, a surprise posttest indicated that subjects recollected more details about the study presentation of the items presented for the longer duration. In the fMRI experiment, recollection‐ and familiarity‐based recognition elicited activity in distinct cortical networks. Additionally, recollection‐related activity in left inferior parietal cortex was of greater magnitude for test items presented for 6 s than for 1 s. In the ERP study the “left‐parietal old/new effect”—a putative correlate of successful recollection—was likewise modulated by amount of information retrieved. Together, these findings provide further support for dual‐process models of recognition memory and add weight to the proposal that retrieval‐related activity in left inferior parietal cortex reflects processes supporting the online representation of retrieved episodic information. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: episodic memory, recognition memory, remember‐know, event‐related potential, episodic buffer
INTRODUCTION
Numerous functional neuroimaging studies have implicated the left posterior parietal cortex in recognition memory [for reviews see Ciaramelli et al., 2008; Skinner and Fernandez, 2007; Vilberg and Rugg, 2008; Wagner et al., 2005]. Activity in this region has been reported to dissociate according to whether recognition judgments are made on the basiws of recollection, when contextual information from a study episode is recovered, as opposed to familiarity, when an item is recognized without contextual retrieval [e.g., Wheeler and Buckner, 2004; Yonelinas et al., 2005; see also Vilberg and Rugg, 2008]. Although recollection‐sensitive left parietal activity tends to be located in and slightly posterior to the angular gyrus (Brodmann Area (BA) 39/19), familiarity‐sensitive regions are located more superiorly in the vicinity of the intraparietal sulcus (IPS; BA 7). Current evidence suggests that the left parietal region associated with familiarity is actually responding to something akin to the salience of the eliciting test items rather than directly reflecting a memory signal [Vilberg and Rugg, 2008], but the functional significance of recollection‐related left inferior parietal activity remains unclear.
In a recent study [Vilberg and Rugg, 2007], we attempted to adjudicate between two competing hypotheses regarding the role of the left inferior parietal cortex in episodic retrieval. We asked whether the region supports the maintenance of retrieved information, as initially suggested by Wilding and Rugg [1996], or whether it instead acts to reorient attention toward the contents of recollection, as was suggested by Rugg and Henson [2002; see also Wagner et al., 2005]. Specifically, we addressed the question whether recollection‐sensitive left inferior parietal activity is sensitive to the amount of information recollected in response to a recognition test item. We argued that if this region plays a role in the representation or maintenance of the recollected information, activity should be modulated by amount of information retrieved (analogous to the “load” effects reported for regions held to support storage of information in working memory; e.g., Braver et al., 1997]. By contrast, if the region acts merely to reorient attention to retrieved content, left inferior parietal activity might be expected to be insensitive to the amount of information recollected, because attentional reorienting is usually conceived of as an all‐or‐none phenomenon. Our experimental procedure used a modified Remember/Know paradigm. This gave subjects the option to make one of two different kinds of “remember” response: “R2” responses were employed to indicate that recollection of the test item included the identity of the item co‐presented on the study trial with or without the recollection of any additional contextual study details, whereas “R1” responses signified recollection of contextual details excluding the studied associate. The conclusions drawn on the basis of this procedure rely on the assumption that R2 responses signify the recollection of more information than R1 responses. Our findings indicated that recollection‐selective activity in left inferior lateral parietal cortex (BA 39/19) was modulated by amount of information recollected, supporting the view that this region plays a role in the representation of recollected information.
Although these results would seem to rule against the possibility that the left inferior parietal cortex acts to reorient attention toward the products of recollection, it remains possible that the amount‐dependent modulation of activity in this region was a reflection of processes downstream of recollection, rather than directly reflecting variation in the resources required to represent different amounts of information. In our previous study (see earlier), participants were required to respond differentially according to how much information they could recollect, thus requiring the on‐line evaluation of recollected content in order to meet the demands of the retrieval task. This is in contrast to a standard Remember/Know test, in which a recollection‐based response requires no evaluation of retrieved content beyond that needed to establish that content of some kind was retrieved. Thus, it remains to be determined whether recollection‐related left inferior parietal activity is modulated by amount of information recollected in the absence of the demand for a differential response based on evaluation of retrieved content. If this region does indeed contribute to the maintenance of the products of recollection, its activity should be modulated by amount of information retrieved regardless of task demands.
Converging evidence from fMRI and event‐related potential (ERP) studies of episodic memory retrieval have suggested that the “left parietal old/new effect,” a putative ERP correlate of recollection that is maximal over the left parietal scalp, may be the electrophysiological correlate of recollection‐related left inferior parietal activity [for reviews see Friedman and Johnson, 2000; Rugg and Curran, 2007]. In a companion experiment to the previously described fMRI study [Vilberg et al., 2006], we reported that the amplitude of the left parietal old/new effect was modulated by the amount of information recollected, mirroring the fMRI findings of Vilberg and Rugg [2007]. Thus, it was of interest in the present study to determine whether recollection‐sensitive left inferior parietal activity, and the left parietal old/new ERP effect, are both modulated by our manipulation of amount of retrieved information.
The current study consisted of parallel fMRI and ERP experiments in which a study task manipulation was employed (exposure duration of 1 vs. 6 s) to enable test trials to be segregated according to the amount of information recollected without the need for differential responding on the basis of retrieved content. It is well established that increased study duration results in increases in estimates of recollection [as well as familiarity; see e.g., Yonelinas, 2002]. On the basis of a behavioral pilot study, we expected that both the probability of recollection and the amount of information recollected would be greater for items associated with 6 s study exposures than 1 s exposures. This expectation was confirmed (see Results). In each experiment, subjects underwent two study‐test cycles. At study, subjects encoded pairs of object images flanking a central background scene. At test, they performed a standard Remember/Know recognition memory test on individual object images. We predicted that these recollection‐sensitive left parietal fMRI and ERP effects would be modulated by amount of information recollected, with greater effects for remembered items studied for 6 s than for remembered items studied for 1 s.
MATERIALS AND METHODS
Experiment 1 (fMRI)
Subjects
Subjects were right‐handed, native English speakers aged between 18 and 23. A total of 20 individuals (eight females) took part in the experiment. In accordance with the requirements of the UCI Institutional Review Board, which approved the study, all subjects gave informed consent prior to participating. Two subjects were excluded from all analyses due to inadequate behavioral performance (response omissions on more than 20 trials, and correct rejection accuracy <70%, respectively).
Stimuli
Stimuli were color photographs of objects and outdoor scenes. The pool of object images used to create stimulus lists was composed of 408 pictures drawn from the same pool employed by Smith et al. [2004]. Allocation of object images to experimental conditions was randomized on a subject‐specific basis. For each subject, 288 object images were selected to be presented at study, 144 in each of two study sessions. An additional 90 images were selected to serve as new test stimuli, 45 in each of two test sessions. Twenty other objects were selected from the stimulus pool to be used in a short practice session. For all subjects, an identical set of four outdoor scenes was used during the study sessions. These scenes were selected from a pool previously used by Johnson and Rugg [2007].
Object images were presented in pairs on a gray background at study. The members of each stimulus pair were presented in different quadrants of the display monitor, flanking a centrally located outdoor scene image. Outdoor scenes subtended 11.9° of visual angle. The maximum vertical and horizontal visual angle subtended by an object image was 6.0°. All location combinations of object images occurred with equal probability. Each of the four outdoor scene images were presented an equal number of times during study. One hundred forty‐four of the 288 study object pictures were selected to serve as old items at test, 72 per test session. Old test items were sampled equally from the four possible study locations. Two buffer trials were added to the beginning of each study list, and three buffer trials were added to the beginning of each test list.
Procedure
Subjects completed a short practice session outside of the scanner prior to beginning the first study session. The practice session consisted of one study and one test practice block of 8 and 12 trials, respectively. Subjects received instructions on how to perform each task just prior to its administration. After practice, participants were positioned in the scanner and remained there for the duration of the two study‐test cycles.
Study trials consisted of the presentation of two object images surrounding an outdoor scene for a duration of either 1 or 6 s, followed by a blank screen for 1 s. The assignment of display duration was randomized across study trials for each participant, with the requirement that half of the displays were presented for 6 s and half for 1 s. The study task was to visualize an interaction between the two object images, and to continue to visualize this interaction for the duration of the display. After completing each study session, subjects were given a 5–10 min break during which task instructions were read by the experimenter to the subjects over headphones. Each test trial consisted of the presentation of a central red fixation cross (+) for 567 ms, followed by a centered test picture for 516 ms, followed by a white fixation cross (+) for 2517 ms. At test, old and new stimuli were randomly interspersed with null events (45 in each test block). During these null trials, a red fixation cross was displayed for 567 ms, followed by a white fixation cross for 3032 ms. Posttest assessment of what was recollected during the second test session occurred outside of the scanner ∼10 min after completion of the final test session.
The test task employed a standard Remember/Know procedure. Three responses were possible: New, Know (K), and Remember (R). Instructions on how to use the response categories as well as examples of when to use them were both read by the subjects and verbally explained. Instructions for Know and Remember responses were identical to those used by Gallo et al. [2001], with the exception that the instructions were altered to accommodate the use of pictures. Subjects were instructed to use the Know option when nothing could be recollected about the picture's study occurrence, but the subject was nonetheless confident that the test picture had been studied. The Remember option was to be used when some specific aspect of what happened or was experienced at the time of initial study could be recollected. Subjects were instructed to use the New response when they judged that a test picture was new or when they were uncertain whether the test picture was old.
Subjects were instructed to use the index and middle fingers of one hand to make K and R responses, respectively, and the index finger of the other hand to respond New. Hand assignment was randomized across subjects. Two keypads were used to make responses in the scanner. A 5‐min break was given between the end of the first test session and the start of the second study session.
After the second test session had been completed, subjects were asked to verbally report the basis of their judgments for each of the remembered images (those given R responses) from the second test. Each image was displayed on the screen, and the subject's narrative was recorded by the experimenter. Participants were directed to list all details from the study episode which had been recollected at the time of the initial test, including for example, the test item's pairmate, the background scene, the location of the test item, the location of the pairmate, the duration of the display, and the scenario created by the participant to link the test item with its pairmate.
fMRI data acquisition
High‐resolution T1‐weighted anatomical images (240 × 240 matrix, 1 mm3 voxels) and blood oxygenation level‐dependent (BOLD), T2*‐weighted echoplanar functional images (SENSE factor of 2, flip angle 70°, 80 × 80 matrix, FOV = 24 cm, TR = 2,000 ms, TE = 30 ms) were acquired using a 3T Philips Achieva MRI scanner equipped with an eight channel receiver head coil (Philips Medical Systems, Andover, MA). Three hundred and thirteen volumes were acquired during each test session. Each volume comprised 30 slices oriented parallel to the AC‐PC line (thickness 3 mm, 1 mm inter‐slice gap, 3 mm3 voxels) acquired in a descending sequence. The first five volumes of each session were discarded to allow equilibration of tissue magnetization.
fMRI data analysis
Statistical Parametric Mapping (SPM5, Wellcome Department of Imaging Neuroscience, London, UK), run under Matlab R2006a (The Mathworks, USA) was used for fMRI data analysis. Functional imaging timeseries were subjected to realignment (to the first retained volume), concatenation across session, reorientation, spatial normalization to a standard EPI template [based on the Montreal Neurological Institute (MNI) reference brain; Cocosco et al., 1997], and smoothing with an 8 mm FWHM Gaussian kernel. Analysis was performed using a General Linear Model (GLM) in which a delta function was used to model neural activity at stimulus onset. These functions were convolved with the canonical hemodynamic response function (HRF) and its temporal and dispersion derivatives to model the BOLD response [Friston et al., 1998]. The analyses of the parameter estimates of the temporal and dispersion derivatives added little to the findings obtained with the canonical HRF, and therefore are not reported. Seven event‐types (R hits for items studied for 1 s [R1], R hits for items studied for 6 s [R6], K hits for items studied for 1 s [K1], K hits for items studied for 6 s [K6], Misses [M], and Correct Rejections [CRs], along with events of no interest such as buffer trials, and trials with incorrect or omitted responses) were modeled. The model also included as covariates the across‐scan mean and six regressors representing motion‐related variance (three for rigid‐body translation and three for rotation). An AR(1) model was used to estimate and correct for nonsphericity of the error covariance [Friston et al., 2002]. The GLM was used to obtain parameter estimates representing the activity elicited by the events of interest.
A statistical threshold of P < 0.001, uncorrected, with an extent threshold of 5 contiguous voxels was employed for principal contrasts. Contrasts employed as exclusive masks were thresholded at P < 0.05. Contrasts employed as inclusive masks were thresholded at P < 0.01. For the purpose of visualization of the findings, Caret software [Van Essen et al., 2001] was used to map cortical regions of interest onto inflated fiducial brains via average fiducial mapping onto the PALS‐B12 atlas [Van Essen, 2002, 2005] in SPM2 space.
Experiment 2 (ERP)
Subjects
A total of 24 (12 females) individuals took part in the experiment. Of these, eight were excluded from analyses due to the contribution of insufficient artifact‐free ERP trials (fewer than 15) to one or more of the critical response categories (remember hits for test items studied for 1 s, remember hits for test items studied for 6 s, and correct rejections). Thus, data from a total of 16 participants are reported.
Stimuli
Stimuli in the ERP experiment were identical to those employed in the fMRI experiment with the exception of their visual angle—the maximum vertical and horizontal visual angle subtended by an object image was 3.4°, and the maximum vertical and horizontal visual angle subtended by the outdoor scene images was 6.7°.
Procedure
The procedure employed in the ERP experiment was identical to that of the fMRI experiment except that the timing of the test displays varied slightly. The duration of the red fixation cross was 600 ms, followed by a centered test picture for 507 ms, a white fixation cross for 2,507 ms, and a blank screen between trials for 507 ms. No null trials were presented in the ERP experiment. The electrode cap was applied just prior to beginning the first study block, and was removed after completion of the posttest. A keypad was used to make responses.
EEG recording
EEG was recorded (Contact Precision Instruments, London, UK) from 37 silver/silver chloride electrodes, 31 of which were arranged in an elasticated cap (Easycap, Germany) according to the International 10–20 system [American Electroencephalographic Society, 1994], with the remaining electrodes positioned above and below the left eye, on the outer canthus of each eye, and over each mastoid process. Data were acquired continuously at test at a sampling rate of 256 Hz and an amplifier bandpass of 0.01–40 Hz. Electrode impedances were kept below 5 kΩ. EEG was digitally filtered offline with a bandpass of 0.1–19 Hz (3dB points), epoched from 102 ms prestimulus to 1,946 ms poststimulus onset, downsampled to 125 Hz, and referenced to linked mastoids. Trials containing artifact resulting from eye movement other than blinks or from excessive baseline drift were rejected. A linear regression method was used to correct blink artifacts [see e.g., Henson et al., 2004].
RESULTS
Experiment 1
fMRI analyses were restricted to trials associated with correct behavioral responses (hits and correct rejections) and misses. Behavioral results are reported only for those participants who were included in the fMRI analyses.
Behavioral performance
Test
Overall correct rejection rate was 92.7%, against a false alarm rate of 6.1%. Table I shows the proportion of old and new items attracting each category of response. Estimates of familiarity were calculated after correcting K responses in accordance with the independence assumption [Yonelinas and Jacoby, 1995] ((P(F) = ((P(K|old)/(1 − P(R|old)) − ((P(K|new)/(1 − P(R|new))) for both K1 and K6 trials. Corrected recollection estimates (P(hit) − P(false alarm)) for R1 and R6 trials were also calculated. Corrected estimates of familiarity were 0.36 and 0.54 for K1 and K6 trials, respectively, a difference which was significant, t(17) = 6.93, P < 0.0001. Likewise, corrected recollection estimates for R1 and R6 trials significantly differed (0.28 vs. 0.54, respectively), t(17) = 11.09, P < 0.0001.
Table I.
Percentage of old and new items attracting each category of response in the fMRI experiment
| Item | Remember | Know | New |
|---|---|---|---|
| Old: 1 s study | 28.8 | 29.3 | 40.7 |
| Old: 6 s study | 55.5 | 26.3 | 17.7 |
| New | 1.3 | 4.8 | 92.7 |
Table II shows the mean RTs for test responses. A repeated‐measures ANOVA on the R1, R6, K1, K6, M1 (old items studied for 1 s which were afforded New responses), M6 (old items studied for 6 s which were afforded New responses), and CR RTs revealed a significant effect of response type, F(2.8, 48.1) = 21.48, P < 0.0001. Pairwise tests indicated that CR RTs were significantly shorter than those for the other response types, all P < 0.01. Additionally, R1, R6, M1, and M6 RTs were significantly shorter than K1 and K6 RTs, all P < 0.001. R6 and R1 RTs did not significantly differ from one another, nor did K1 and K6, nor M1 and M6 RTs.
Table II.
Mean response latency (ms) by response category in the fMRI experiment
| Item | Remember | Know | New |
|---|---|---|---|
| Old: 1 s study | 1310 (68) | 1515 (57) | 1238 (70) |
| Old: 6 s study | 1298 (65) | 1528 (61) | 1245 (125) |
| New | 1300 (80)a | 1704 (69)b | 1087 (33) |
Standard error in parentheses.
8 subjects contributed to this measure.
14 subjects contributed to this measure.
Posttest recall
Participants reported a greater number of correct details from the study episode for R6 than for R1 trials (means of 2.4 vs. 1.7 details, respectively), t(17) = 5.94, P < 0.001.
fMRI findings
We first sought to identify regions where activity varied along a single dimension of “memory strength.” We then identified regions where activity was selectively associated with either recollection or familiarity through whole‐brain voxel‐wise analyses of the responses elicited by the different classes of test item collapsed over the study exposure manipulation, that is, “R,” “K,” and “M” items. We then identified recollection‐sensitive regions that were additionally sensitive to amount of information retrieved, as operationalized by the contrast between activity elicited by items endorsed as recollected after 6 vs. 1 s study exposures. Finally, we asked whether activity in any familiarity‐sensitive region was modulated by study‐exposure duration.
Memory strength
We determined whether any regions were activated in a manner concordant with a memory strength signal using the same general approach as in our prior study [Vilberg and Rugg, 2007; see also Gonsalves et al., 2005]. Specifically, we searched for regions where activity varied with memory strength by inclusively masking R > K, K > M, and M > CR contrasts, and separately, R < K, K < M, and M < CR contrasts,1 in each case using thresholds for the individual contrasts of P < 0.015 (to give in each case a conjoint one‐tailed threshold of ∼P < 0.0005, according to Fisher's procedure; Lazar et al., 2002]. These analyses revealed a single 17‐voxel cluster in left dorsolateral prefrontal cortex (BA 9/46; center of mass: x = −47, y = 21, z = 26; see Fig. 1) where activity increased with increasing strength.2 The rationale for the selection of the contrasts employed in these analyses is that, according to single‐process, strength‐based models of recognition memory, average item strength varies from correct rejections (lowest strength), to misses, to K judgments, to R judgments [Dunn, 2004; Gonsalves et al., 2005]. At the request of a referee, who expressed the concern that inclusion of correct rejections might obscure monotonic strength effects because of a “novelty response” elicited by these items, we also searched for strength‐sensitive regions by inclusively masking just the R vs. K and K vs. M contrasts. Because these contrasts are not orthogonal, they were individually thresholded at P < 0.0005, one‐sided. No regions were identified where activity decreased with increasing “strength.” This procedure revealed two small regions in prefrontal cortex (cluster sizes of 5 and 6 voxels), and a larger cluster in superior parietal cortex (cluster size of 40 voxels; center of mass: x = −34, y = −72, z = 45), where activity increased with increasing strength (see Figure 1).
Figure 1.
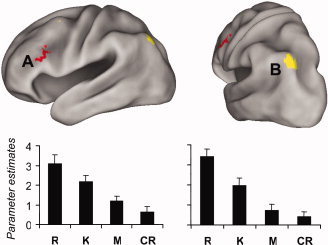
Top: Memory‐strength sensitive regions showing (red) increasing activity from CR to R, and (yellow) increasing activity from M to R. Bottom: Mean parameter estimates (and standard errors) of activity in the center of mass of A (left; MNI coordinates: −47, 21, 26) and B (right; MNI coordinates: −34, −72, 45).
Recollection vs. familiarity
Following the approach adopted in our previous study [Vilberg and Rugg, 2007], familiarity‐sensitive regions were identified by exclusively masking the K > M contrast with the R > K contrast. Likewise, regions selectively sensitive to recollection were identified by exclusively masking the R > K contrast with the K > M contrast. The resulting familiarity‐sensitive regions included medial and left lateral prefrontal cortex, left intraparietal sulcus, and precuneus. Recollection‐sensitive regions included left inferior parietal cortex, medial occipito‐parietal cortex, left fusiform gyrus, left dorsal and ventral prefrontal cortex, and right precentral gyrus. Additionally, two clusters within the medial temporal lobe, likely falling within left and right parahippocampal cortex, were also recollection‐sensitive. These familiarity‐ and recollection‐sensitive regions are illustrated in Figure 2 (see Table III for coordinates).
Figure 2.
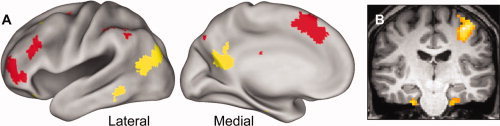
A: Regions of the left hemisphere selectively sensitive to recollection (yellow) and familiarity (red). Effects (thresholded at P < 0.001, exclusively masked at P < 0.05) are mapped onto inflated fiducial brains (see Methods). B: Coronal section (based on a representative subject's anatomical image) illustrating recollection effects in bilateral parahippocampal cortex (thresholded for display purposes at P < 0.005).
Table III.
Regions selectively sensitive to familiarity and recollection
| Region | BA | HM | Location | Peak Z (# voxels) |
|---|---|---|---|---|
| Regions selectively sensitive to familiarity | ||||
| Medial frontal gyrus | 6/8 | LR | −6, 33, 39 | 5.06 (335) |
| Middle frontal gyrus | 10 | L | −36, 51, 9 | 4.32 (161) |
| 9/46 | L | −48, 27, 33 | 5.03 (66) | |
| 6/9 | L | −54, 9, 36 | 3.66 (10) | |
| Inferior frontal gyrus | 45 | L | −39, 21, −12 | 3.36 (7) |
| Superior frontal gyrus | 6 | L | −27, −3, 60 | 3.70 (10) |
| Insula | L | −33, 15, 0 | 3.34 (5) | |
| Caudate | R | 12, 12, 0 | 3.40 (8) | |
| L | −12, 3, 12 | 4.16 (22) | ||
| Globus pallidus | L | −15, 3, −3 | 3.57 (5) | |
| Thalamus | R | 9, 0, 9 | 3.31 (5) | |
| Cingulate gyrus | 23 | LR | 0, −18, 27 | 3.36 (20) |
| Lateral parietal cortex | 40 | R | 48, −30, 42 | 4.04 (51) |
| 40 | L | −48, −48, 51 | 4.46 (129) | |
| 40 | R | 45, −48, 51 | 4.27 (15) | |
| 7/19 | R | 42, −72, 45 | 3.52 (9) | |
| Precuneus | 7 | R | 12, −66, 33 | 3.65 (14) |
| Cuneus | 7/19 | LR | −3, −81, 42 | 4.09 (33) |
| Cerebellum | R | 36, −63, −36 | 3.39 (7) | |
| Regions selectively sensitive to recollection | ||||
| Inferior frontal gyrus | 47 | L | −36, 30, −21 | 3.80 (11) |
| Middle frontal gyrus | 46 | L | −27, 27, 54 | 3.64 (16) |
| Precentral gyrus | 4 | R | 39, −21, 57 | 4.58 (106) |
| Anterior medial temporal lobe | R | 21, −15, −36 | 4.01 (10) | |
| Parahippocampal cortex | L | −24, −24, −30 | 3.60 (7) | |
| R | 24, −27, −30 | 3.47 (9) | ||
| Fusiform gyrus | 37 | L | −63, −51, −12 | 3.73 (44) |
| Posterior cingulate cortex | 23 | LR | −6, −54, 18 | 4.12 (148) |
| Lateral parietal/occipital cortex | 39/19 | L | −51, −75, 21 | 4.50 (281) |
Z‐values refer to peak activated cluster.
BA, brodmann area; HM, hemisphere; L, left; R, right.
Amount of information recollected
We identified recollection‐sensitive regions that were additionally sensitive to amount of information retrieved by contrasting activity elicited by R6 and R1 items (thresholded at P < 0.01), inclusively masking this contrast by the R > K contrast (P < 0.001), and then exclusively masking with the K > M contrast (P < 0.05). The conjoint significance of the R6 > R1 and R > K contrasts (which are orthogonal) was estimated as P < 0.0001 by Fisher's procedure [Lazar et al., 2002]. As illustrated in Figure 3, this analysis revealed a single 11‐voxel cluster on the border of inferior parietal (BA39) and occipital (BA19) cortex (peak coordinates = −30, −84, 39; Z = 2.87). The effect survived small volume correction for False Discovery Rate [Genovese et al., 2002; P < 0.05] within a 5 mm radius sphere centered on the peak of our previously identified left parietal, amount‐sensitive region [Vilberg and Rugg, 2007]. The complementary contrast, searching for recollection‐sensitive regions where activity was greater for R1 than R6 items, revealed no significant clusters, even when the threshold for the R1 > R6 contrast was reduced to P < 0.05.
Figure 3.
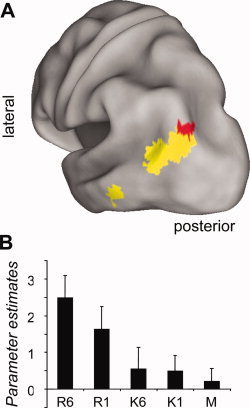
A: Recollection‐sensitive region in the left lateral inferior parietal cortex where activity is also sensitive to amount of information retrieved. Regions in yellow are those identified by the recollection sensitive contrast (see text), whereas those in red are a subset of the recollection‐sensitive regions wherein R6 > R1 (thresholded for display purposes at P < 0.05). B: Mean parameter estimates (and standard errors) of activity in the peak voxel of the amount‐sensitive region.
An additional set of contrasts was performed to determine whether any of the recollection‐sensitive regions identified above were also sensitive to differences between K6 and K1 trials. This was accomplished by inclusively masking the K6 > K1 contrast (P < 0.01) in one case, and the K1 > K6 contrast in the other, with the R > K contrast (P < 0.001; and exclusively masking by K > M at P < 0.05). These analyses failed to identify any significant clusters.
Familiarity‐related modulations
We assessed whether activity in familiarity‐sensitive regions was also sensitive to the study history of test items by inclusively masking the K6 > K1 contrast with the familiarity‐sensitive contrast described earlier (K > M at P < 0.001, exclusively masked with R > K at P < 0.05). This comparison, as well as the complementary contrast of K1 > K6, failed to yield any significant effects. This remained the case when the thresholds for the K6 > K1 and K1 > K6 contrasts were reduced to P < 0.05.
Experiment 2
ERP analyses were restricted to trials associated with correct behavioral responses (hits and correct rejections). Few participants had sufficient (15+) artifact‐free K1 and K6 hit trials, or M6 and M6 trials, to permit analyses of these trial types. Thus, the ERP analyses presented below are restricted to trials associated with R1 hits, R6 hits, and correct rejections. Behavioral results are reported for those participants whose data were included in ERP analyses, although even for these subjects, too few subjects made sufficient K or miss responses to permit meaningful analysis of RTs.
Behavioral performance
Test
Overall correct rejection rate was 87%, against a false alarm rate of 11%. Table IV shows the proportion of old and new items attracting each category of response. Estimates of recollection and familiarity, corrected for independence as described earlier, varied according to the duration of study exposure (0.30 vs. 0.50 for K1 and K6 trials, t(15) = 3.62, P < 0.005; and 0.45 vs. 0.73 for R1 and R6 trials, t(15) = 10.64, P < 0.0001). Table V shows the mean RTs at test. A repeated‐measure ANOVA on the R1, R6, and CR RTs revealed no effect of trial type, however, given our prior findings of differences between RTs associated with different amounts of recollection [see Vilberg et al., 2006], we wished to address the issue directly in the present data. A pairwise t‐test indicated that R6 RTs were indeed significantly shorter than R1 RTs, P < 0.025. Because of the potential impact of differences in the variance of the RT distributions on the grand averaged ERP waveforms, we additionally utilized pairwise contrasts to determine whether the variance associated with R6 RTs differed from that for R1 RTs. These contrast revealed that the variance associated with R6 RTs was significantly smaller than that for R1 RTs, P < 0.01.
Table IV.
Percentage of old and new items attracting each category of response in the ERP experiment
| Item | Remember | Know | New |
|---|---|---|---|
| Old: 1 s study | 48.8 | 20.3 | 29.2 |
| Old: 6 s study | 76.8 | 13.8 | 7.7 |
| New | 4.0 | 7.4 | 86.9 |
Table V.
Mean response latency (ms) by response category in the ERP experiment
| Item | Remember | Know | New |
|---|---|---|---|
| Old: 1 s study | 1178 (71) | 1569 (79) | 1335 (82) |
| Old: 6 s study | 1133 (80) | 1649 (93) | 1439 (125)a |
| New | 1264 (151)b | 1718 (145)c | 1187 (61) |
Standard error in parentheses.
15 subjects contributed to this measure.
10 subjects contributed to this measure.
14 subjects contributed to this measure.
Posttest recall
As in the fMRI experiment, subjects correctly reported more details for old items afforded remember responses which were displayed for 6 s than those which were displayed for 1 s (means of 1.8 vs. 1.5 details, respectively), t(15) = 2.53, P < 0.05.
ERP findings
As noted earlier, the ERP analyses were restricted to trials associated with correct Remember and New responses (referred to as R6, R1, and New trials). Grand average ERP waveforms from four representative scalp locations are shown in Figure 4. Across subjects, the mean number of trials (and range) included in these ERPs were 37 (20–57), 24 (15–42), and 49 (28–72) for R6, R1, and New responses, respectively. Visual inspection of the waveforms reveals generic old/new effects as well as a parietal old/new effect which is of seemingly greater amplitude for R6 than R1 trials.
Figure 4.
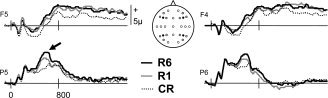
Grand average ERP waveforms from four representative scalp locations (shown as filled black circles) for the R6 hit, R1 hit, and correct rejection trial types. The left parietal old/new effect is indicated by the arrow. Electrode locations used in the initial ANOVA of the ERP data (see text) are displayed as filled circles.
To determine whether the left parietal old/new effect was modulated by amount of information retrieved, an ANOVA was conducted on the mean ERP amplitudes for the R6, R1, and New trials for the 500–800 ms poststimulus latency region (the same latency region employed to quantify the parietal old/new effect in our previous study; Vilberg et al., 2006]. The electrode locations used in this global ANOVA are indicated in Figure 4. The electrodes were grouped by hemisphere and further factored into anterior/posterior location. The initial ANOVA employed the factors of trial type, hemisphere, anterior/posterior location, and site. Only those effects involving the factor of trial type are reported. In the event of a significant effect involving this factor, follow‐up pairwise comparisons were performed between trial types using the same factors as those employed in the global ANOVA. Greenhouse‐Geisser corrected degrees of freedom were used for all ANOVAs.
Results of the initial and subsidiary ANOVAs are summarized in Table VI. There was a significant main effect of trial type in the initial ANOVA. Subsidiary ANOVAs contrasting trial types with one another revealed that R6 ERPs were more positive‐going than both R1 and New ERPs (see Fig. 4). When analyses were restricted to parietal electrode sites, both the R1 vs. New and R6 vs. New contrasts revealed trial type by hemisphere interactions, F(1, 15) = 5.41, P < 0.05 and F(1, 15) = 5.74, P < 0.05, respectively. Additionally, both the R1 vs. R6 and R6 vs. New contrasts revealed main effects of trial type, F(1, 15) = 7.37, P < 0.025 and F(1, 15) = 7.27, P < 0.025, respectively. Further restricting analyses to left parietal electrode sites revealed main effects of trial type for the R1 vs. New [F(1, 15) = 6.69, P < 0.025], R6 vs. New [F(1, 15) = 14.34, P < 0.025], and R6 vs. R1 [F(1, 15) = 7.59, P < 0.025] contrasts, with R6 waveforms being more positive‐going than R1 waveforms, which were themselves more positive‐going than New waveforms. Figure 5 illustrates the scalp topographies of these effects.
Table VI.
ANOVA table for the 500–800 ms latency range
| Contrast | Effect | Statistic |
|---|---|---|
| R1/R6/New | TT | F(1.6, 23.3) = 3.91, P < 0.05 |
| TT/S | F(2.2, 33.1) = 4.30, P < 0.025 | |
| R1/R6 | TT | F(1, 15) = 5.61, P < 0.05 |
| TT/S | F(1.4, 21.0) = 6.37, P < 0.025 | |
| TT/AP/S | F(2.0, 30.3) = 3.90, P < 0.05 | |
| R1/New | TT/AP/S | F(2.0, 30.4) = 3.13, P < 0.06 |
| R6/New | TT | F(1, 15) = 5.89, P < 0.05 |
| TT/HM | F(1, 15) = 4.81, P < 0.05 | |
| TT/S | F(1.3, 18.9) = 6.96, P < 0.025 |
TT, Trial type; AP, anterior/posterior; HM, hemisphere; S, site.
Figure 5.
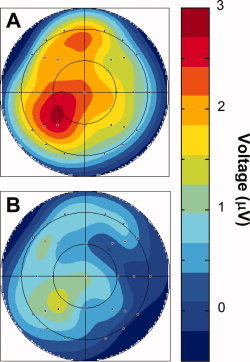
Topographic voltage plots of the A: R6 hit—correct rejection and B: R1 hit—correct rejection subtractions for the 500–800 ms latency region. Note the common amplitude scale.
An additional analysis was performed to rule out the possibility that modulation of the left parietal old/new effect could be accounted for by variation in the timing of the associated behavioral responses. As mentioned previously, R6 RTs were both significantly faster and less variable than R1 RTs. By removing the slowest 10% of the R1 trials from the ERP averages of each subject, we were able to nullify these differences in mean RTs and their standard deviations. These newly formed ERP averages for R1 trials were then contrasted with R6 trials over the 500–800 ms poststimulus‐onset interval at left parietal scalp sites. This analysis revealed that the waveforms for R6 trials remained more positive‐going than those for R1 trials [main effect of trial type, F(1, 15) = 14.35, P < 0.005]. Thus, the difference in the magnitude of the left parietal old/new effect elicited on R1 and R6 trials is not attributable to differences between these item types in the mean or variance of the associated RTs.
DISCUSSION
Consistent with prior findings [see Yonelinas, 2002 for review], the manipulation of study duration affected estimates of recollection and familiarity in both experiments. Additionally, the outcome of the posttests demonstrated that, as anticipated, more information was recollected in association with test items studied for the longer of the two durations. This result validates our procedure for segregating test trials according to the amount of information recollected. Because our subjects were unaware of the existence of the posttest when making their initial Remember/Know/New judgments, they had no reason to further evaluate the products of retrieval beyond what was required to detect the occurrence of recollection. Differences in the fMRI BOLD signals or ERPs elicited by recollected items associated with the two study exposure durations are therefore unlikely to reflect postretrieval processes engaged by the requirement to differentially classify the items according to recollected content.
The present fMRI findings add weight to prior claims that recollection and familiarity have dissociable neural correlates [Henson et al., 1999; Montaldi et al., 2006; Vilberg and Rugg, 2007; Wheeler and Buckner, 2004; Yonelinas et al., 2005]. Regions identified as recollection‐ and familiarity‐sensitive overlapped with those reported previously, particularly in left lateral parietal cortex where familiarity engaged a more superior region than did recollection [Vilberg and Rugg, 2007; Wheeler and Buckner, 2004; Yonelinas et al., 2005; see Vilberg and Rugg, 2008 for review]. Recollection‐related activity was also identified in left dorsal prefrontal and fusiform cortex, as well as in bilateral parahippocampal cortex. These findings are also consistent with prior reports implicating these regions in successful recollection [for reviews see Diana et al., 2007; Skinner and Fernandez, 2007].
Together with these previous results, the present fMRI findings demonstrating that recollection and familiarity are associated with anatomically distinct cortical regions lend strong support to dual‐process models of recognition memory, which posit that recognition judgments are based upon two qualitatively distinct memory signals [e.g., Mandler, 1980; Yonelinas, 2002]. These models can be contrasted with single‐process models which propose that recognition judgments are based upon an evaluation of a unidimensional strength variable [e.g., Donaldson, 1996; Dunn, 2004; Wixted, 2007]. Under the single‐process account, one might expect to find regions where activity varies continuously across different levels of strength [e.g., Gonsalves et al., 2005]. As in our prior study [Vilberg and Rugg, 2007], we searched for such regions by inclusively masking contrasts between recognition judgments that, according to single process models, were ordered by increasing or decreasing strength. Consistent with our previous findings, we were unable to find any regions where activity declined with increasing strength. Moreover, we found only one small cluster in prefrontal cortex (in a different region from that identified in the prior study) that demonstrated increased activity across the full range of relevant test items (i.e., items accorded correct rejections through to items endorsed as Remembered). Together with our previous results, the present findings offer little evidence for the existence of regions where retrieval‐related activity scales according to the construct of unidimensional memory strength.
A subsidiary analysis that discounted the activity elicited by correct rejections revealed a region in posterior left superior parietal cortex where activity was enhanced both for K judgments relative to misses, and for R judgments relative to K judgments (see Fig. 1). This region abuts both the more anterior parietal region where activity was modulated by item familiarity and the more inferior, recollection‐sensitive region (cf. Figs. 1 and 2). Its proximity to these two regions raises the possibility that its apparent sensitivity to memory strength is a reflection of separate but spatially overlapping neuronal populations that share the properties of one or other of their functionally distinct neighbors. Regardless of the functional significance of the seemingly strength‐related activity in this parietal region, the relative paucity of areas demonstrating such activity stands in marked contrast to the extensive cortical regions where retrieval‐related activity was segregated according to whether it was associated with recollection or familiarity (see Fig. 2). As aforementioned, the existence of the functionally dissociable regions offers strong support for the proposal that recollection and familiarity are qualitatively, and not merely quantitatively, distinct forms of memory.
The primary objective of the fMRI experiment was to determine whether recollection‐sensitive activity in left inferior lateral parietal cortex would show sensitivity to amount of information recollected when postretrieval demands were minimized. As predicted, a region on the border of BAs 39 and 19 was sensitive to both recollection and amount of information retrieved, demonstrating greater activity for recollected items studied for 6 than for 1 s. Crucially, this region overlapped with the amount‐sensitive lateral parietal region identified in our prior fMRI study [Vilberg and Rugg, 2007; see Fig. 6]. As is discussed in more detail below, we interpret this finding as further evidence that left inferior lateral parietal cortex supports the on‐line representation or maintenance of recollected content.
Figure 6.
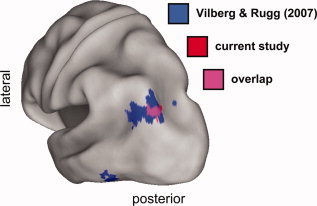
Overlay of left hemisphere recollection‐sensitive regions also sensitive to amount recollected in the current fMRI experiment (red) and that of Vilberg and Rugg [2007; blue]. Magenta demarcates the area of overlap between the two studies. In each case, regions were identified by the inclusive mask of the contrast identifying the effect of amount recollected (thresholded for display purposes at P < 0.05) and the overall recollection effect (R > K, P < 0.001, exclusively masked by K > M, P < 0.05).
Our proposal that recollection‐sensitive inferior parietal cortex is sensitive to amount of recollected information is subject to an important caveat.3 The two fMRI studies providing the most direct evidence in favor of this proposal [the current study and Vilberg and Rugg, 2007] both employed visually complex stimuli as study and test items, raising the possibility that the findings reflect modulation of a visually responsive neural population rather than a region sensitive to recollection more generically. This possibility garners support from the locus of these amount‐sensitive effects: Although belonging to the extensive inferior lateral parietal region identified as recollection‐sensitive by the R > K contrast (see Fig. 2), the region identified as amount‐sensitive occupies only its posterior aspect, extending into a region of lateral occipital cortex (Figs. 3 and 4) previously identified as supporting visual imagery [e.g., Mellet et al., 2000; Newman et al., 2005]. That said, it is noteworthy that recollection‐sensitive effects have been reported in much the same region (BA39/19) in studies employing words rather than pictures as study and test items [Henson et al., 1999; Woodruff et al., 2005; see also Wilding, 2000 for an example of the modulation of the parietal old/new ERP effect in a study employing exclusively verbal materials]. Resolution of this issue will require research along similar lines to the present study, but with the use of a wider range of experimental materials.
Analogous to our prior study, no clusters were identified where recollection‐sensitive activity was greater for items associated with the recollection of relatively less information (recollected items studied for 1 vs. 6 s). Although caution is of course necessary in the interpretation of null findings, the failure to find significant effects in this “reversed” contrast weighs against the possibility that the information retrieved in the two cases varied qualitatively, rather than quantitatively [cf. Vilberg and Rugg, 2007]. Given the former scenario, significant differences would be expected for both directions of the contrast. We also failed to detect differences in the activity of recollection‐sensitive (or any other) regions for “K6” vs. “K1” items. This suggests that while the manipulation of exposure duration may have modulated the probability of later familiarity‐based recognition judgments, it did not affect the nature or strength of the memory signal supporting these judgments.
In contrast to our prior study [Vilberg and Rugg, 2007], we failed to identify any extra‐parietal regions where activity was modulated according to amount of information recollected. This may suggest that the regions identified in the previous study, which included left fusiform cortex and right precentral gyrus, were supporting processes downstream of retrieval. For instance, the sensitivity of left fusiform cortex to amount of recollection may have reflected the need to attend to recollected visual information in order to fulfill the demands of the retrieval task which, unlike in the present case, required differential responding on the basis of what had been recollected. It is also possible, however, that the present failure to detect extra‐parietal effects of amount of information recollected simply reflects a lack of statistical power. Notably, our prior study employed a sample of 28 subjects, in contrast to the 18 subjects contributing to the present fMRI dataset. Clearly, further investigation is needed to elucidate the functional roles of these and other extra‐parietal, recollection‐sensitive regions.
In addition to fMRI data, we obtained complementary ERP data using the same experimental procedure. This allowed us to assess whether the functional parallels observed previously between recollection‐related left inferior parietal activity and the left parietal ERP old/new effect [e.g., Herron et al., 2003, 2004; Vilberg and Rugg, 2007; Vilberg et al., 2006; Woodruff et al., 2006; Yonelinas et al., 2005] extended to the present method for modulating amount of information recollected. Consistent with our prior results [Vilberg et al., 2006; see also Wilding, 2000], the left parietal old/new effect was modulated by amount of information recollected, demonstrating a greater magnitude for recollected items studied for 6 s than for 1 s. This finding adds to the evidence suggesting that left lateral parietal fMRI recollection effects and the left parietal ERP old/new effect reflect common neural and functional processes [Rugg and Curran, 2007]. As discussed by Vilberg and Rugg 2008, the significance of these findings goes beyond the mere identification of a putative generator of the ERP effect. To the extent that the fMRI and ERP effects are indeed reflections of the same neural processes, the timing of the ERP effect indicates that these processes onset sufficiently early to play a direct role in recollection‐based memory judgments, a conclusion that cannot be drawn on the basis of fMRI findings alone.
CONCLUSIONS
We sought to determine whether recollection‐sensitive activity in left parietal cortex is modulated by amount of information recollected in a conventional Remember/Know recognition memory test. As indexed by both fMRI and ERPs, parietal recollection effects were greater in magnitude when elicited by test items triggering recollection of more versus less information. For the reasons discussed in detail elsewhere [Vilberg and Rugg, 2007, 2008], we interpret these findings as evidence in favor of the proposal that left inferior lateral parietal cortex contributes to the representation of recollected information, and against the alternative proposal that the region supports the reorienting of attention to retrieved information [see Ciaramelli et al., 2008; Rugg and Henson, 2002; Wagner et al., 2005]. By this argument, in addition to any role played by lateral parietal cortex in supporting the temporary buffering of modality‐specific information [e.g., Baldo and Dronkers, 2006], part of this region also supports a similar function in respect of the supra‐modal information that constitutes the products of successful recollection [see also Vilberg and Rugg, 2007]. Thus, left inferior parietal cortex may contribute to something akin to the “episodic buffer” proposed by Baddeley [2000] as an interface between working and episodic memory [Vilberg and Rugg, 2008].
Acknowledgements
KV was supported by NIMH National Research Service Award MH14599‐27 and by NSF Graduate Research Fellowship Award D/DGE‐0234621.
Footnotes
We employed inclusively masked pair‐wise contrasts to identify strength‐sensitive regions in order to constrain the results to voxels where activity varied monotonically with strength, conforming to what is predicted by single‐process models. Regression‐based approaches such as “parametric analysis” [Friston, 1997] are markedly over‐inclusive in this respect, identifying many voxels where activity varies non‐monotonically with strength [see Vilberg and Rugg, 2007].
We also searched for strength‐sensitive effects by inclusively masking the contrasts of CR vs. M; M vs. K1; K1 vs. K6; K6 vs. R1; R1 vs. R6, each thresholded at P < 0.05, on the grounds that a single‐process model might predict strength to be ordered across the CR (lowest), Miss, K1, K6, R1 and R6 (highest) trial types. No voxels were identified by these additional analyses, even when the CR trial type was excluded from the masking procedure (that is, M vs. K1; K1 vs. K6; K6 vs. R1; R1 vs. R6).
We thank an anonymous reviewer for bringing this possibility to our attention.
REFERENCES
- Baddeley A ( 2000): The episodic buffer: A new component of working memory? Trends Cogn Sci 4: 417–423. [DOI] [PubMed] [Google Scholar]
- Baldo JV,Dronkers NF ( 2006): The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology 20: 529–538. [DOI] [PubMed] [Google Scholar]
- Braver TS,Cohen JD,Nystrom LE,Jonides J,Smith EE,Noll DC ( 1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E,Grady C,Moscovitch M ( 2008): Top‐down and bottom‐up attention to memory: A hypothesis (Atom) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia 46: 1828–1851. [DOI] [PubMed] [Google Scholar]
- Cocosco C,Kollokian V,Kwan RS,Evans( 1997): A Brainweb: Online interface to a 3D MRI simulated brain database. Neuroimage 5: S425. [Google Scholar]
- Diana RA,Yonelinas AP,Ranganath C ( 2007): Imaging recollection and familiarity in the medial temporal lobe: A three‐component model. Trends Cogn Sci 11: 379–386. [DOI] [PubMed] [Google Scholar]
- Donaldson W ( 1996): The role of decision processes in remembering and knowing. Mem Cognit 24: 523–533. [DOI] [PubMed] [Google Scholar]
- Dunn JC ( 2004): Remember‐know: A matter of confidence. Psychol Rev 111: 524–542. [DOI] [PubMed] [Google Scholar]
- Friedman D,Johnson J Jr ( 2000): Event‐related potential (ERP) studies of memory encoding and retrieval: A selective review. Microsc Res Tech 51: 6–28. [DOI] [PubMed] [Google Scholar]
- Friston KJ ( 1997): Imaging cognitive anatomy. Trends Cogn Sci 1: 21–27. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Fletcher PC,Josephs O,Holmes A,Rugg MD,Turner R ( 1998): Event‐related fMRI: Characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Glaser DE,Henson RN,Kiebel S,Phillips C,Ashburner J ( 2002): Classical and Bayesian inference in neuroimaging: Applications. Neuroimage 16: 484–512. [DOI] [PubMed] [Google Scholar]
- Gallo DA,Roediger HL,McDermott KB ( 2001): Associate false recognition occurs without strategic criterion shifts. Psychon Bull Rev 8: 579–586. [DOI] [PubMed] [Google Scholar]
- Genovese CR,Lazar NA,Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gonsalves B,Kahn I,Curran T,Norman KA,Wagner AD ( 2005): Memory strength and repetition suppression: Multimodal imaging of medial temporal cortical contributions to recognition. Neuron 47: 751–761. [DOI] [PubMed] [Google Scholar]
- Henson RN,Rugg MD,Shallice T,Josephs O,Dolan RJ ( 1999): Recollection and familiarity in recognition memory: An event‐related functional magnetic resonance imaging study. J Neurosci 19: 3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN,Rylands A,Ross E,Vuilleumeir P,Rugg MD ( 2004): The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. Neuroimage 21: 1674–1689. [DOI] [PubMed] [Google Scholar]
- Herron JE,Quayle AH,Rugg MD ( 2003): Probability effects on event‐related potential correlates of recognition memory. Cogn Brain Res 16: 66–73. [DOI] [PubMed] [Google Scholar]
- Herron JE,Henson RNA,Rugg MD ( 2004): Probability effects on the neural correlates of retrieval success: An fMRI study. Neuroimage 21: 302–310. [DOI] [PubMed] [Google Scholar]
- Johnson JD,Rugg MD ( 2007): Recollection and the reinstatement of encoding‐related cortical activity. Cereb Cortex 17: 2507–2515. [DOI] [PubMed] [Google Scholar]
- Lazar NA,Luna B,Sweeney JA,Eddy WF ( 2002): Combining brains: A survey of methods for statistical pooling of information. Neuroimage 16: 538–550. [DOI] [PubMed] [Google Scholar]
- Mandler G ( 1980): Recognizing: The judgment of previous occurrence. Psychological Review 87: 252–271. [Google Scholar]
- Mellet E,Tzourio‐Mazoyer N,Bricogne S,Mazoyer B,Kosslyn SM,Denis M ( 2000): Functional anatomy of high‐resolution visual mental imagery. J Cogn Neurosci 12: 98–109. [DOI] [PubMed] [Google Scholar]
- Montaldi D,Spencer TJ,Roberts N,Mayes AR ( 2006): The neural system that mediates familiarity memory. Hippocampus 16: 504–520. [DOI] [PubMed] [Google Scholar]
- Newman SD,Klatzky RL,Lederman SJ,Just MA ( 2005): Imagining material versus geometric properties of objects: An fMRI study. Cogn Brain Res 23: 235–246. [DOI] [PubMed] [Google Scholar]
- Rugg MD,Curran T ( 2007): Event‐related potentials and recognition memory. Trends Cogn Sci 11: 251–257. [DOI] [PubMed] [Google Scholar]
- Rugg MD,Henson RNA ( 2002): Episodic memory retrieval: An (event‐related) functional neuroimaging perspective In Parker AE,Wilding EL, Bussey T, editors. The Cognitive Neuroscience of Memory Encoding and Retrieval. Psychology Press, 3–37. [Google Scholar]
- Skinner EI,Fernandez MA ( 2007): Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia 45: 2163–2179. [DOI] [PubMed] [Google Scholar]
- Smith APR,Dolan RJ,Rugg MD ( 2004): Event‐related potential correlates of the retrieval of emotional and nonemotional context. J Cogn Neurosci 16: 1–17. [DOI] [PubMed] [Google Scholar]
- Van Essen DC ( 2002): Windows on the brain. The emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol 12: 574–579. [DOI] [PubMed] [Google Scholar]
- Van Essen DC ( 2005): A population‐average, landmark‐ and surface‐based (PALS) atlas of human cerebral cortex. Neuroimage 28: 635–662. [DOI] [PubMed] [Google Scholar]
- Van Essen DC,Dickson J,Harwell J,Hanlon D,Anderson CH,Drury HA ( 2001): An integrated software system for surface‐based analyses of cerebral cortex. J Am Med Inform Assoc 41: 1359–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL,Rugg MD ( 2007): Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia 45: 2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL,Rugg MD (in press): Memory retrieval and the parietal cortex: A review of evidence from a dual‐process perspective. Neuropsychologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL,Moosavi RF,Rugg MD ( 2006): The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Res 1122: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD,Shannon BJ,Kahn I,Buckner RL ( 2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453. [DOI] [PubMed] [Google Scholar]
- Wheeler ME,Buckner RL ( 2004): Functional‐anatomic correlates of remembering and knowing. Neuroimage 21: 1337–1349. [DOI] [PubMed] [Google Scholar]
- Wilding EL ( 2000): In what way does the parietal ERP old/new effect index recollection? Int J Psychophysiol 35: 81–87. [DOI] [PubMed] [Google Scholar]
- Wilding EL,Rugg MD ( 1996): An event‐related potential study of recognition memory with and without retrieval of source. Brain 119: 889–905. Erratum on 1416. [DOI] [PubMed] [Google Scholar]
- Wixted JT ( 2007): Dual‐process theory and signal‐detection theory of recognition memory. Psychol Rev 114: 152–176. [DOI] [PubMed] [Google Scholar]
- Woodruff CC,Johnson JD,Uncapher MR,Rugg MD ( 2005): Content‐specificity of the neural correlates of recollection. Neuropsychologia 43: 1022–1032. [DOI] [PubMed] [Google Scholar]
- Woodruff CC,Hayama HR,Rugg MD ( 2006): Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Res 1100: 125–135. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP ( 2002): The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang 46: 441–517. [Google Scholar]
- Yonelinas AP,Jacoby LL ( 1995): The relation between remembering and knowing as bases for recognition: Effects of size congruency. J Mem Lang 34: 622–643. [Google Scholar]
- Yonelinas AP,Otten LJ,Shaw KN,Rugg MD ( 2005): Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25: 3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]


