Abstract
PHYSIOL BEH Exposure to estrogens during critical developmental periods and in adulthood affects sex differences in the brain. We examined the roles of estradiol (E2) and phytoestrogens, and their interactions, on potential sex differences in brain. We used aromatase knockout (ArKO) mice, which cannot produce endogenous estrogens, along with wild type (WT) littermates. Mice were gestated, raised and maintained on a diet either rich in phytoestrogens or a diet virtually void of soy-derived phytoestrogens. Adult males and females were gonadectomized and received implants filled with 17-β-estradiol to induce progestin receptors (PR), while controls received empty implants. Mice were sacrificed five days later and brain sections containing the posterodorsal medial amygdala (MePD) were processed for PR immunoreactivity. Activation of sex differences in PR required adult E2 treatment. A diet high in phytoestrogens was required for expression of sex differences in PR after E2 treatment. Our data underscore the important contribution of dietary phytoestrogens for the development of sex differences in PR-ir in the adult mouse medial amygdala. We hypothesize that both aromatization of androgens to estrogens and dietary sources of additional estrogens are part of the normal requirement for sex differences in the rodent brain.
Keywords: phytoestrogens, environmental estrogens, endocrine disrupting compounds, progestin receptor, medial amygdala, sex differences, sexual differentiation, soy
Endogenous estrogens act during development and in adults to activate expression of reproductive and other social behaviors (1–3). In contrast, phytoestrogens are biologically active non-steroidal molecules found primarily in soy products, legumes and whole-grains, they have been show to modulate aspects of reproduction including behavior (4–8). Phytoestrogens act via estrogen receptors (ERα and β) and thus activate many of the same neural circuits activated by endogenous estrogens (4, 5, 9). In recent years, increasing numbers of experiments have examined neuroendocrine effects of phytoestrogen intake as high concentrations of these compounds are increasingly being used as supplements in many consumer products (10, 11). In addition, standard soy-derived commercial laboratory rodent diets contain high concentrations of isoflavones (12). Therefore, in addition to endogenously produced estrogens, phytoestrogens are present in both human and animal diets during development and adulthood and need to be considered in studies of hormone actions.
To study phytoestrogens in an animal model that should be exquisitely sensitive to estrogens, we utilized the aromatase knockout (ArKO) mouse (13). The mutants carry targeted disruptions in both the transcriptional and translational sites of the Cyp19 gene, do not produce aromatase cytochrome P450 and are unable to convert testosterone to estradiol. The ArKO mouse does, however, express the classic estrogen receptors (ERα, ERβ) and responds to exogenous estrogen administration (13). Subtle disruptions to sperm production are noted in the ArKO testes and can be rescued by a diet high in phytoestrogens (7). In addition ArKO females treated with hormones to prime lordosis in adulthood show lower levels of behavior than wild type littermates, but only when they are raised on a phytoestrogen rich diet (14). To separate effects of phytoestrogens and endogenous estrogens, we selected two laboratory chows. One has been shown to result in plasma phytoestrogen levels in rodents equivalent to those seen either in human populations that consume large amounts of soy (phyto-rich) the other diet contain little soy (phyto-free) (12, 15).
Here we test the hypothesis that endogenous estrogens and phytoestrogens have synergistic actions on estrogen-induced progestin receptor in the mouse brain. We focused our analysis on the medial posterodorsal amygdala (MePD) for three reasons. First, this region contains PR, ERα, and ERβ (16, 17). Second, the MePD volume is sexually dimorphic and highly plastic (18–20) and lastly, this region is important for a number of hormone-dependent social behaviors in several rodent species (19, 21, 22). Our results show that the ability of E2 to induce PR immunoreactivity in this region is influenced by sex and E2 exposure prior to adulthood, however, both factors interact with dietary phytoestrogens.
Materials and Methods
Animals and Treatments
Mice were produced using heterozygous breeding pairs. Each member of the pair had one normal and one disrupted Cyp19 gene (13). At the time of these studies mice had been backcrossed for at least four generations with C57BL/6J mice. Mice were housed in a 12:12 light:dark cycle and given food and water ad libitum. One group of animals (and their dams) was fed a phytoestrogen-free diet (phyto-free; Harlan Teklad Global Diet 2014) that does not contain alfalfa or soybean meal, sources of coumestrol and isoflavones, respectively. The second group of animals was raised on a diet containing 600μg of total dietary phytoestrogens/g of food (phyto-rich; Harlan Teklad Global Diet 8604; (15). By monitoring food intake in a separate group of WT animals, we found that on average males (n=9) consumed 3.43 ± 0.03 g food/day. When isoflavone consumption was calculated for animals eating the phyto-rich diet, using the range supplied by the manufacturer (427–565 μg/g food), intake ranged from 1.465–1.938 mg isoflavone/day. Using the range provided for the phyto-free diet (5–15.5 μg/g food) isoflavone intake was calculated at 0.0171–0.0532 mg isoflavone/day.
Mice were genotype by PCR amplification of tail DNA (14). Adult mice (age 45–90 days of age) were used in this study. Each mouse was gonadectomized one week prior to receiving hormone treatment. Mice received Silastic implants (1.98 I.D. × 3.17mm O.D.) containing either 50μg 17-β-estradiol dissolved in 25μl sesame oil, or empty implants. Implants were positioned subcutaneously in the midscapular region and animals were sacrificed five days after implantation. The following 16 groups were formed. WT mice on phyto-free diet with blank implants (n=8 males, n=8 females), WT mice on phyto-rich diet with blank implants (n=11 males, n=7 females), WT mice on phyto-free diet with E2 implants (n=9 males, n=9 females), WT mice on phyto-rich diet with E2 implants (n=9 males, n=7 females), ArKO mice on phyto-free diet with blank implants (n=9 males, n=6 females), ArKO mice on phyto-rich diet with blank implants (n=10 males, n=9 females), ArKO mice on phyto-free diet with E2 implants (n=11 males, n=8 females), and ArKO mice on phyto-rich diet with E2 implants (n=12 males, n=11 females).
At the time of sacrifice, mice were deeply anesthetized with an overdose of sodium pentobarbital and brains were rapidly removed and fixed via immersion in 5% acrolein (23). Following overnight immersion in 0.1M phosphate buffer containing 30% sucrose, brains were frozen and serial coronal sections (30μm) collected through the forebrain and stored in cryoprotectant at −20°C. Consecutive sections were divided into four vials, one vial (1/4 of the brain) was processed for immunocytochemical analysis of PR.
Immunocytochemistry
Sections were removed from cryoprotectant and rinsed in 0.2M Tris Buffered Saline (TBS, 5×10min) prior to a 30 minute incubation in NaBH4 and a 10 minute incubation in 0.3% H2O2, with 3, 10 minute rinses in between. Tissue was incubated at 4°C for 48 hours in a primary antibody directed against the hinge region of the progestin receptor (H-928, 0.2mg/ml; StressGen Biotechnologies Carp., Victoria, British Columbia, Canada). Next, brain tissue was incubated in biotinylated horse anti-mouse secondary antibody (Vector Laboratories, 1:500). Following additional rinses and a one-hour incubation in an avidin-biotin complex (Vector, 1:1000), tissue was stained using a nickel intensified diaminobenzidine (DAB) solution (0.25% nickel ammonium sulfate and 0.05% DAB) activated by 0.001% hydrogen peroxide. Brain sections from each group were included in every run to control for intra-run variability. All incubation times were held constant between runs.
Image analysis and statistics
Immunoreactivity was quantified in the best matched, unilateral section from each brain using Metamorph Image Analysis (Universal Imaging West Chester, PA). As illustrated previously (24), the area quantified was the posterodorsal medial amygdala (MePD, −1.82 mm; Fig 46 in (25). The observer was “blind” to the group designations of the sections used for the analysis. Relationships among groups were assessed first with a four-way analysis of variance test (ANOVA) with genotype, hormone treatment, sex and diet as factors. Since it is well known that E2 is required to induce PR in the brain, and no differences between any other factors were noted in brains from control (blank implant) mice, we conducted further analyses on brains from mice exposed to E2 in adulthood. When main effects or interactions were noted, differences among groups were analyzed using Fisher’s LSD Multiple-Comparison tests. Significance was reported at p<0.05 or less.
Results
Analysis of all four factors (implant, sex, food, and genotype) revealed a strong main effect of implant. Animals that received E2 for five days before brains were collected had significantly more PR-ir cells in the MePD than gonadectomized controls (F(1,142)=173.01, p<0.00001; Table 1 and Figure 1). A trend was noted for a sex differences with males having more PR-ir than females (F(1,142)=3.61, p<0.06). No main effect of food or genotype were detected (F(1,142)=3.19, 0.22, respectively). Interactions between sex and food (F(1,142)=3.98, p<0.05) and genotype and food (F(1,142)=4.78, p<0.03) were detected. Males consuming the phyto-rich chow had more PR-ir cells than animals in any of the other groups and WT mice of both sexes had more PR-ir when they consumed phyto-rich versus phyto-free chow (p<0.05). No sex by genotype interaction was detected (F(1,142)=1.68).
Table 1.
Mean ± SEM PR-ir neurons (N per group) in the MePD of mouse brains.
| Diet: | Phytoestrogen Rich Diet | Phytoestrogen Free Diet | ||
|---|---|---|---|---|
| Treatment: | Blank | E2* | Blank | E2* |
| WT Males | 37.9±14 (11) | 390.1±58 (9) | 10.4±4 (8) | 226.4±28 (9) |
| WT Females | 10.1±3 (7) | 253.6±50 (7) | 6.6±3 (8) | 173.7±46 (9) |
| ArKO Males | 31.6±11 (10) | 274.9±44 (12) | 9.0±3 (8) | 227.4±42 (11) |
| ArKO Female | 33.2±17 (9) | 168.4±34 (11) | 28.5±10 (6) | 270.9±44 (8) |
Significant main effect of hormone treatment, E2>Blank.
Figure 1.
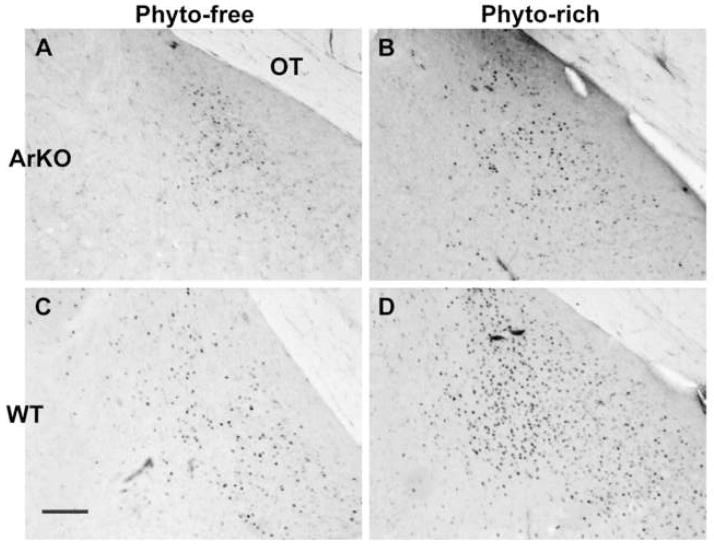
Photomicrographs of PR-ir cells in the medial posterodorsal amygdala of aromatase knockout (ArKO) male mice (A, B) and WT male littermates (C, D). Males were castrated and one week later treated with estradiol for five days prior to sacrifice. Panels A and C show tissue from mice fed Phytoestrogen (Phyto) -free chow. Panels B and D illustrate tissue from males fed a Phyto-rich chow. Scale bar=100μm. OT = optic tract.
To isolate the roles of genotype, sex and food we asked if these factors had any influence on baseline levels of PR-ir. A three-way analysis of these variables restricted to brains of control animals (no E2 implants) revealed no effects of sex or genotype (F(1,65)=0.1, 1.33 respectively). We noted a trend for an effect of diet (F(1,65)=3.41, p<0.07) with animals on the phyto-rich diet having more PR-ir cells than animals on the phyto-free chow. We did not find any interactions between sex and food, genotype and food or sex and genotype (F(1,65)=1.68, 0.02, 2.67 respectively).
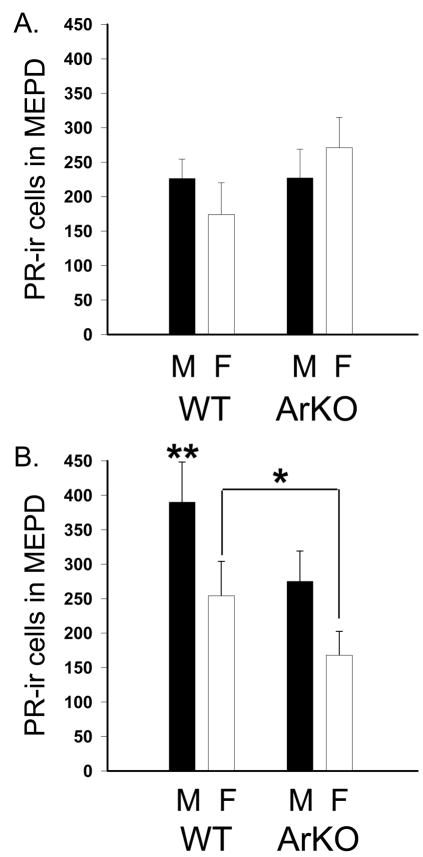
In contrast, all the same significant effects that we noted in the full data set were present in the analysis of data from the E2 implanted mice only. This demonstrates that the effects of sex, food and genotype are enabled when E2 is given. A main effect of sex (F(1,75)=4.87, p<0.03) was due to more PR-ir cells in the MePD of males than females (Table 1 and Figure 2). No main effects of genotype or food were found (F(1,75)=0.31, 1.52 respectively). An interaction between sex and food and another between genotype and food were observed (F(1,75)=4.25, 4.35, respectively p<0.045). As described above, the sex by food interaction was due to males consuming the phyto-rich chow which had more PR-ir cells than animals in any of the other groups. The genotype by food interaction was caused by the significant difference between WT mice that consumed phyto-rich versus phyto-free chow (p<0.05). No interaction between sex and genotype was detected (F(1,75)=1.50).
Figure 2.
Mean (+SEM) PR-ir cell numbers with in the medial posterodorsal amygdala (MePD) of wild type (WT) and aromatase knockout (ArKO) mice. All mice were gonadectomized and one week later treated with estradiol for 5 days prior to sacrifice. In panel A data from mice on a phytoestrogen reduced diet are shown. In panel B are data from mice consuming a phytoestrogen rich diet. Black histograms represent males (M) and white histograms represent females (F). *When consuming phyto-rich food females have significantly fewer PR immunoreactive (-ir) neurons induced by estradiol than do males, p<0.05. **Significantly more PR-ir neurons were present in MePD of the WT males on phytoestrogen rich chow as compared with all other groups.
Discussion
Here we report a sex difference in PR-ir in the mouse MePD. As in many other regions, this dimorphism in the MePD depends on developmental and/or activational E2 (18, 26–32). In addition we now show that dietary phytoestrogens are involved. The sex difference in PR-ir is eliminated by either depriving males of phytoestrogens via a diet low in these compounds, or by disruption of the aromatase enzyme gene, Cyp19. Estradiol-regulated PR expression has previously been shown to be sexually dimorphic in several rodent species (32–34). The direction of the sex difference varies with the age of the animal and the region of the brain examined. Neonatal male rats and mice express higher perinatal PR-ir cell numbers in the medial preoptic area (mPOA) than females (30, 35). This sex difference may be caused by higher circulating levels of testosterone in male as compared with female neonates (36). The sex difference in mice is eliminated by disruption of the ERα gene (35). In adults, E2-mediated PR induction is greater in females than males in the ventromedial nucleus (VMN) and the medial preoptic area (mPOA) (32, 33). This report is the first we know of to document a sex difference in the MePD in PR-ir cells in response to adult treatment with E2. However others have shown elegantly in several rodent species that the MePD is highly plastic during puberty and in adulthood; responding to changes in photoperiod and behavioral interactions (37–43)
Dietary phytoestrogens enhance the effects of E2 on PR protein induction; this effect was noted in WT males, but not in the ArKO males. Since E2 treated female mice in all genotype and diet groups showed statistically equivalent levels of PR induction, which were comparable to the ArKO males, we speculate that the ability to respond differentially to the phyto-rich versus phyto-free diet requires exposure to E2 some time prior to adulthood during development. It is at this time that ERβ activation can have defeminizing actions in brain (44). The maximal PR induction response is only observed in adult WT males in which dietary phytoestrogens were available throughout life. This suggests that phytoestrogens cannot completely compensate for endogenous E2 produced by aromatization in the male brain and vice versa. Partial compensation is also noted in males. When E2 treatment is given to ArKO male mice fed the phyto-rich diet their PR-ir cell numbers are similar to WT males fed the phyto-free chow. This result suggests that phytoestrogens and E2 work synergistically to maximize plasticity in response to E2 in the adult male MePD. This finding is similar to a study done in the ArKO testes in which maintenance on a phytoestrogen rich diet was able to partially reverse infertility (7).
The MePD is an interesting area to examine. Recent studies demonstrate that it is highly plastic, sexually dimorphic, and more asymmetric in males than females (40, 43, 45, 46). We did not count neurons in our study by any method other than PR-ir. Data from rats have demonstrated more neurons and glia in the male MePD versus the females (43). It is possible that the enhanced PR-ir we noted in male brains was simply a reflection of more neurons in the male MePD as compared with the female. However in a strain of mice which we did not use here, no sex differences in cell numbers were noted, but the volume of the MePD was greater in males likely due to large cell somata (42). Photoperiod, a predictor of the breeding season for Siberian hamsters, can modulate both androgen levels and the size of the MePD however co-habitation with females can block the effect of short days on the MePD (47). Laboratory mice are not photoperiodic, however the presence of phytoestrogens in food may likewise signal the spring and suitable breeding conditions. Only a few studies have assessed variability in phytoestrogenicity over time in plants but they do document seasonal changes (48–50).
The role of phytoestrogens in the MePD has not been examined, but other brain regions have been studied. In rats, volumes of the sexually dimorphic nucleus of the preoptic area (SDN-POA) are equivalent in males and females when the animals are raised on a phytoestrogen low chow, but a sex difference is present in adulthood in rats on a standard chow (containing a high level of phytoestrogens) (51). In addition females have a larger anteroventral periventricular (AVPV) nucleus than males. In males diet significantly affects this region with males on low phytoestrogen food having smaller volumes than males on phyto-rich diet. In addition to neural effects, phytoestrogens affect behavior. Male rats typically perform better than females at visual spatial memory (VSM) tasks requiring the use of reference, but not working, memory. Dietary phytoestrogens reverse the direction of this sex difference (15). Phytoestrogens produced anxiolytic effects in both male and female rats (52). Furthermore, female rats treated with an isoflavone supplement display significantly reduced female receptivity (5). Taken together, these data show that phytoestrogen consumption can result in a physiological change in both brain and behavior.
The MePD is part of the chemosensory pathway that transduces sexually relevant olfactory information (53). Male rats exposed to an anti-aromatase drug during perinatal development do not display their typical olfactory preferences for females (54). In ArKO mice olfactory abilities detected with a liquid olfactometer are comparable to WT males, but ArKO females have enhanced abilities to distinguish between urine from females primed with E2 and progesterone from those primed with E2 only (55). While the direct connection between these behaviors and the MePD has not been shown, it is known that exposure to opposite sex chemosignals stimulates c-fos immunoreactivity in the MePD (56, 57). It is not known if these responses are affected by phytoestrogen consumption.
Phytoestrogens can also affect receptivity in adult female mice. Female ArKO mice had previously been shown to express lower levels of receptivity than WT littermates, and this led to the hypothesis that prepubertal exposure to estradiol is needed for complete feminization of this behavior (58). However when females raised in a manner identical to the present experiment, consuming phytoestrogen-rich versus phyto-free diets, were tested for receptivity only ArKO females on phyto-rich chow had impaired receptivity (14). Thus phytoestrogens may masculinize female behaviors. Moreover this action is likely mediated by ERβ. Estrogen receptor β knockout males treated in adulthood with priming steroids display higher levels of lordosis than wild type control males (59). Female mice treated for the first three days after birth with either estradiol or a specific ERβ agonist, but not an ERα agonist, have lower lordosis quotients in adult hood than controls (44). Interestingly when adjacent sections from the male brains used in this study were examined for numbers of ERα and ERβ-ir cells in the MePD an effect was seen only for ERβ. Males treated with estradiol and raised on a phytoestrogens rich chow had elevated ERβ positive cells as compared with males that received the low phytoestrogens chow (Kudwa and Rissman, unpublished). Taken together these results suggest that phytoestrogens affect ERβ dynamics in this region.
In humans, the amount of soy supplements routinely ingested by mothers, and subsequently by their infants, is increasing. It is likely that these soy supplements are delivering phytoestrogens to developing infants and in particular to the central nervous system (6). Considering that phytoestrogens can affect neural development and plasticity in adult rodents it is important for us to determine how these dietary supplements act in humans.
Acknowledgments
The authors would like to thank Aileen Wills and Savera Shetty for technical assistance. We are also grateful to Dr. Jin Ho Park for constructive comments on this paper. This work was supported by NIH grant R01 MH57759 (EFR). AEK was supported by an NIH F31 MH070092.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–42. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 2.Hutchison JB, Wozniak A, Beyer C, Karolczak M, Hutchison RE. Steroid metabolising enzymes in the determination of brain gender. J Steroid Biochem Mol Biol. 1999;69:85–96. doi: 10.1016/s0960-0760(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–63. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 4.Jacob DA, Temple JL, Patisaul HB, Young LJ, Rissman EF. Coumestrol antagonizes neuroendocrine actions of estrogen via the estrogen receptor alpha. Exp Biol Med (Maywood) 2001;226:301–6. doi: 10.1177/153537020122600406. [DOI] [PubMed] [Google Scholar]
- 5.Patisaul HB, Dindo M, Whitten PL, Young LJ. Soy isoflavone supplements antagonize reproductive behavior and estrogen receptor alpha- and beta-dependent gene expression in the brain. Endocrinology. 2001;142:2946–52. doi: 10.1210/endo.142.7.8241. [DOI] [PubMed] [Google Scholar]
- 6.Patisaul HB. Phytoestrogen action in the adult and developing brain. J Neuroendocrinol. 2005;17:57–64. doi: 10.1111/j.1365-2826.2005.01268.x. [DOI] [PubMed] [Google Scholar]
- 7.Robertson KM, O’Donnell L, Simpson ER, Jones ME. The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact significantly on testis function. Endocrinology. 2002;143:2913–21. doi: 10.1210/endo.143.8.8957. [DOI] [PubMed] [Google Scholar]
- 8.Weber KS, Setchell KD, Stocco DM, Lephart ED. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol. 2001;170:591–9. doi: 10.1677/joe.0.1700591. [DOI] [PubMed] [Google Scholar]
- 9.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 10.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Walfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S11. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 11.Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A. Phyto oestrogens: where are we now? Br J Nutr. 1998;79:393–406. doi: 10.1079/bjn19980068. [DOI] [PubMed] [Google Scholar]
- 12.Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–47. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- 13.Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 14.Kudwa AE, Boon WC, Simpson ER, Handa RJ, Rissman EF. Dietary phytoestrogens dampen female sexual behavior in mice with a disrupted aromatase enzyme gene. Behav Neurosci. 2007;121:356–361. doi: 10.1037/0735-7044.121.2.356. [DOI] [PubMed] [Google Scholar]
- 15.Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 16.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Willkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 17.Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- 18.Rood BD, Murray EK, Laroche J, Yang MK, Blaustein JD, De Vries GJ. Absence of progestin receptors alters distribution of vasopressin fibers but not sexual differentiation of vasopressin system in mice. Neuroscience. 2008;154:911–921. doi: 10.1016/j.neuroscience.2008.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43:336–346. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 20.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cushing BS, Perry A, Musatov S, Ogawa S, Papademetriou E. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. J Neurosci. 2008;28:10399–10403. doi: 10.1523/JNEUROSCI.1928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- 24.Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene disrupted mice. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- 26.Lauber AH, Romano GJ, Pfaff DW. Sex difference in estradiol regulation of progestin receptor mRNA in rat mediobasal hypothalamus as demonstrated by in situ hybridization. Neuroendocrinology. 1991;53:608–61327. doi: 10.1159/000125781. [DOI] [PubMed] [Google Scholar]
- 27.Kudwa AE, Harada N, Honda SI, Rissman EF. Effects of organisational oestradiol on adult immunoreactive oestrogen receptors (alpha and beta) in the male mouse brain. J Neuroendocrinol. 2007;19:767–772. doi: 10.1111/j.1365-2826.2007.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudwa AE, Gustafsson JA, Rissman EF. Estrogen receptor beta modulates estradiol induction of progestin receptor immunoreactivity in male, but not in female, mouse medial preoptic area. Endocrinology. 2004;145:4500–4506. doi: 10.1210/en.2003-1708. [DOI] [PubMed] [Google Scholar]
- 29.Bodo C, Kudwa AE, Rissman EF. Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147:415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- 30.Quadros PS, Lopez V, De Vries GJ, Chung WC, Wagner CK. Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J Neurobiol. 2002;51:24–32. doi: 10.1002/neu.10040. [DOI] [PubMed] [Google Scholar]
- 31.Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2003;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- 32.Temple JL, Fugger HN, Li X, Shetty SJ, Gustafsson J, Rissman EF. Estrogen receptor beta regulates sexually dimorphic neural responses to estradiol. Endocrinology. 2001;142:510–513. doi: 10.1210/endo.142.1.8054. [DOI] [PubMed] [Google Scholar]
- 33.Blaustein JD, Ryer HI, Feder HH. A sex difference in the progestin receptor system of guinea pig brain. Neuroendocrinology. 1980;31:403–409. doi: 10.1159/000123110. [DOI] [PubMed] [Google Scholar]
- 34.Quadros PS, Goldstein AY, De Vries GJ, Wagner CK. Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol. 2002;14:761–767. doi: 10.1046/j.1365-2826.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- 35.Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ. Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor alpha expression. J Neurobiol. 2001;47:176–182. doi: 10.1002/neu.1025. [DOI] [PubMed] [Google Scholar]
- 36.vom Saal FS. Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J Anim Sci. 1989;67:1824–1840. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- 37.Nishizuka M, Arai Y. Organizational action of estrogen on synaptic pattern in the amygdala: implications for sexual differentiation of the brain. Brain Res. 1981;213:422–426. doi: 10.1016/0006-8993(81)90247-x. [DOI] [PubMed] [Google Scholar]
- 38.Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- 39.Johansen JA, Jordan CL, Breedlove SM. Steroid hormone masculinization of neural structure in rats: a tale of two nuclei. Physiol Behav. 2004;83:271–277. doi: 10.1016/j.physbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
- 42.Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008;1190:115–12143. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol. 2008;506:851–85944. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- 44.Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci. 2005;25:10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol. 2007;501:904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- 47.Cooke BM, Hegstrom CD, Keen A, Breedlove SM. Photoperiod and social cues influence the medial amygdala but not the bed nucleus of the stria terminalis in the Siberian hamster. Neurosci Lett. 2001;312:9–12. doi: 10.1016/s0304-3940(01)02173-5. [DOI] [PubMed] [Google Scholar]
- 48.Booth NL, Overk CR, Yao P, Totura S, Deng Y, Hedayat AS, Bolton JL, Pauli GF, Farnsworth NR. Seasonal variation of red clover (Trifolium pratense L. Fabaceae) isoflavones and estrogenic activity. J Agric Food Chem. 2006;54:1277–1282. doi: 10.1021/jf052927u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro C, Tiritan ME, Rocha E, Rocha MJ. Seasonal and Spatial Distribution of Several Endocrine-Disrupting Compounds in the Douro River Estuary, Portugal. Arch Environ Contam Toxicol. 2009;56:1–11. doi: 10.1007/s00244-008-9158-x. [DOI] [PubMed] [Google Scholar]
- 50.Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW. Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem. 2002;50:4861–4866. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 51.Lephart ED, Rhees RW, Setchell KD, Bu LH, Lund TD. Estrogens and phytoestrogens: brain plasticity of sexually dimorphic brain volumes. J Steroid Biochem Mol Biol. 2003;85:299–309. doi: 10.1016/s0960-0760(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 52.Patisaul HB, Blum A, Luskin JR, Wilson ME. Dietary soy supplements produce opposite effects on anxiety in intact male and female rats in the elevated plus-maze. Behav Neurosci. 2005;119:587–594. doi: 10.1037/0735-7044.119.2.587. [DOI] [PubMed] [Google Scholar]
- 53.Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210:557–560. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- 54.Bakker J, Van Ophemert J, Slob AK. Sexual differentiation of odor and partner preference in the rat. Physiol Behav. 1996;60:489–494. doi: 10.1016/s0031-9384(96)80023-0. [DOI] [PubMed] [Google Scholar]
- 55.Wesson DW, Keller M, Douhard Q, Baum MJ, Bakker J. Enhanced urinary odor discrimination in female aromatase knockout (ArKO) mice. Horm Behav. 2006;49:580–586. doi: 10.1016/j.yhbeh.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wersinger SR, Rissman EF. Oestrogen receptor alpha is essential for female-directed chemo-investigatory behaviour but is not required for the pheromone-induced luteinizing hormone surge in male mice. J Neuroendocrinol. 2000;12:103–110. doi: 10.1046/j.1365-2826.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- 57.Pierman S, Douhard Q, Bakker J. Evidence for a role of early oestrogens in the central processing of sexually relevant olfactory cues in female mice. Eur J Neurosci. 2008;27:423–431. doi: 10.1111/j.1460-9568.2007.06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann N Y Acad Sci. 2003;1007:251–262. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- 59.Kudwa AE, Bodo C, Gustafsson J, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc Natl Acad Sci U S A. 2005;102:4608–12. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]