Abstract
Mutations in Connexin50 (Cx50) cause cataracts in both humans and mice. The mechanism(s) behind how mutated connexins lead to a variety of cataracts have yet to be fully elucidated. Here, we tested whether the cataract inducing Cx50-S50P mutant interacts with wild-type Connexin43 (Cx43) to form mixed channels with attenuated function. Using dual whole-cell voltage clamp, immunofluorescent microscopy and in situ dye transfer analysis we identified a unique interaction between the mutant subunit and wild-type Cx43. In paired Xenopus oocytes, co-expression of Cx50-S50P with Cx43 reduced electrical coupling ≥90%, without a reduction in protein expression. In transfected cells, Cx50-S50P did not target to cell-cell interfaces by itself, but co-expression of Cx50-S50P with Cx43 resulted in its localization at areas of cell-cell contact. We used Cx43 conditional knockout, Cx50 knockout and Cx50-S50P mutant mice to examine this interaction in vivo. Mice expressing both Cx43 and Cx50-S50P in the lens epithelium revealed a unique expression pattern for Cx43 and a reduction in Cx43 protein. In situ dye transfer experiments showed that the Cx50-S50P mutant, but not the Cx50, or Cx43 conditional knockout, greatly inhibited epithelial cell gap junctional communication in a manner similar to a double knockout of Cx43 and Cx50. The inhibitory affects of Cx50-S50P lead to diminished electrical coupling in vitro, as well as a discernable reduction in epithelial cell dye permeation. These data suggest that dominant inhibition of Cx43 mediated epithelial cell coupling may play a role in the lens pathophysiology caused by the Cx50-S50P mutation.
Keywords: cataract, connexin, mutation, lens, gap junction, intercellular communication
Introduction
Gap junctions are dynamic intercellular channels that provide a crucial pathway for the transport of several ions and small molecules essential for the proper growth and development of various multicellular organisms [5]. All gap junctions modulate physiological processes by ionically coupling cells via the regulated exchange of ions (Ca2+, Na+, K+, Cl-) between neighboring cells; however, biochemical coupling, or the sharing of larger metabolites and signaling molecules (cAMP, cGMP, IP3) between cells, is dependent on the protein composition of these channels [13;21;23;47]. To date, the connexin gene family contains over 20 members that are expressed in an overlapping, tissue-dependent pattern [15]. Each connexin includes cytoplasmic amino and carboxyl termini as well as four transmembrane domains linked by a single cytoplasmic loop and two extracellular domains, E1 and E2, which are thought to play a role in connexon docking and channel gating (Fig. 1) [5;21]. Each connexon or hemichannel can be of uniform (homomeric) or varied (heteromeric) connexin composition, and neighboring cells may contribute either heteromeric or homomeric connexons to form full intercellular channels known as heteromeric, heterotypic, or homotypic gap junctions. Recent studies have shown that mixing the connexin composition of gap junctions changes both the permeability and electrophysiological properties of these channels [16;22;27], while mutation, removal, or genetic replacement of specific connexins can lead to distinct physiological defects and disease [40;45].
Fig. 1.
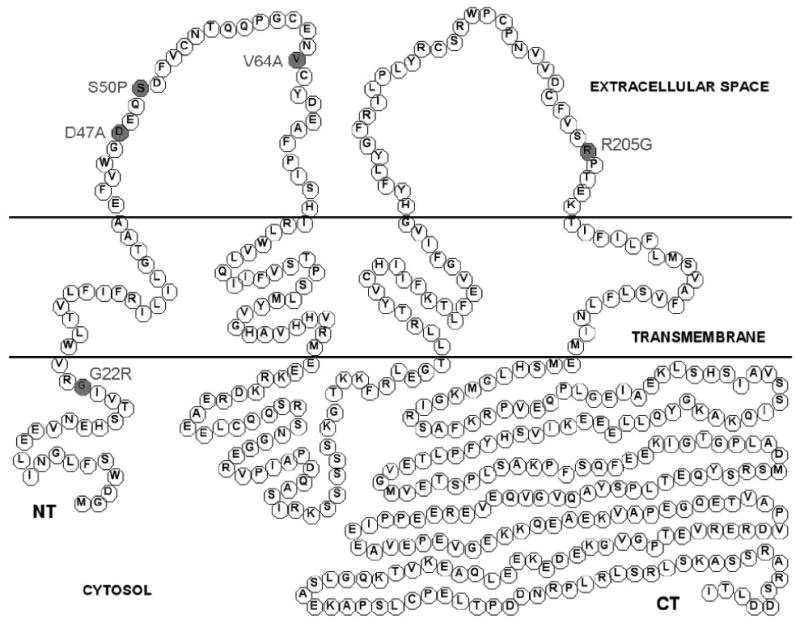
Schematic drawing of Cx50 protein containing all reported murine mutations associated with cataract. Single amino acid mutations linked to distinct cataract pathologies in mice are marked in grey.
The lens is an avascular organ dependent on gap junction-mediated intercellular communication for organ function and homeostasis. The lens contains three distinct cellular populations: a single layer of epithelial cells spanning the anterior portion of the lens, differentiated fiber cells that constitute the majority of the lens cortex, and the mature fiber cells that comprise the lens core. In the vertebrate lens, all cell types are directly coupled to neighboring cells by gap junctions [19;26]. To date, three connexins have been identified in the mammalian lens: Cx43, present in the lens epithelium; Cx46, present only in the fiber cells; and Cx50 present throughout the epithelium and lens fibers [3;28;29;42].
Genetically engineered mouse models have identified the importance of gap junction channels formed by Cx46 and Cx50, specifically their significant role in maintaining lens transparency and organ development, respectively [18;32;44]. These phenotypic anomalies were further defined by genetic replacement (knock-in) studies, which revealed that the replacement of endogenous Cx50 with Cx46 prevented cataract development, but failed to rescue the reduction in organ size caused by the loss of Cx50 [39]. On the other hand, the removal of endogenous Cx43, expressed in many other tissues throughout the body, such as the heart and brain [15], leads to cardiac malformation and neonatal death [31]. Thus, the examination of Cx43's involvement in lens development has been largely limited to the prenatal analysis of Cx43 knockout mice. Murine Cx43 knockout animals exhibit phenotypically normal lenses at embryonic day 18 [46], indicating that Cx43 is not essential for proper lens fiber cell development. However, these embryonic knockout lenses exhibited a reduction in cell-cell communication, a finding that may implicate a role for Cx43 in postnatal lens homeostasis [46].
The crucial nature of gap junctional communication in the lens was further evidenced by recent reports indicating that mutations in various connexin genes result in hereditary disease in humans and mice [13;15;47]. Autosomal dominant cataract CZP1 and CZP3 have been associated with mutations in Cx50 and Cx46, respectively [1;2;24;35;38], while genetic alterations within the Cx43 coding region lead to oculodentodigital dysplasia, ODDD [30]. These mutations provide a valuable resource for determining the molecular mechanisms behind connexin-associated genetic disease.
The distinct role of Cx50 in lens homeostasis and proper organ function is supported by research conducted on mice bearing mutations in the Cx50 coding region with distinct lens pathologies (Fig. 1, Table 1) [7-9;20;34;37;49;49;50]. A recent series of studies have partially defined a novel mutation in the first extracellular domain of Cx50, Cx50-S50P, originally identified as L1. Mutant mice homozygous for this mutation displayed ruptured, cataractous lenses resulting from a missense mutation in the Cx50 gene, causing a single amino acid substitution from serine to proline at codon 50 [49]. Analysis of Cx50-S50P mutant lenses showed that the dominant cataract pathology was associated with a deficiency in fiber cell development and posterior capsule rupture [17;49]. Further experimentation revealed that these anomalies were the result of unique interactions between endogenous wild-type Cx46 and Cx50 and the mutant Cx50-S50P subunits that altered intrinsic channel function [8]. These findings indicated that interactions between endogenous lens connexins are required for proper organ growth, function, and development in vivo.
Table 1.
Summary of all reported murine Cx50 mutations and their mutant phenotypes.
| Cx50 Mutation (Domain) |
Lens Phenotype | Reference |
|---|---|---|
| 1. D47A (E1 Loop) |
|
|
| 2. G22R (N-Term.) |
|
|
| 3. S50P (E1 Loop) |
|
|
| 4. V64A (E1 Loop) |
|
|
| 5. R205G (E2 Loop) |
|
To date the majority of lens gap junction research has focused on the relationship between fiber-fiber interactions and cataractogenesis. This manuscript provides an analysis of epithelial cell coupling in mutant lenses that develop cataract. To this end, we hypothesize that mutant Cx50-S50P subunits interact with wild-type Cx43 in the lens epithelium and functionally alter gap junctional communication. To test this hypothesis, we examined the electrophysiological properties of mixed channels containing both wild-type Cx43 and mutant Cx50-S50P subunits, using the dual whole-cell voltage clamp in combination with the paired Xenopus oocyte system. Additionally, transfected HeLa cells and isolated murine lens capsules were analyzed in order to further define the localization and expression characteristics of these proteins, and a novel technique combining whole-cell patch clamp and fluorescent microscopy was developed to qualitatively identify alterations in biochemical coupling between lens epithelial cells in situ.
Materials and Methods
Molecular cloning
Wild-type murine Cx50 was subcloned using the EcoR1 site of the pCS2+ expression vector. Mutant Cx50-S50P cDNA was generated from total mRNAs of homozygous mutant lenses by RT-PCR using a pair of primers- (sense) 5′ cgggatcctagtgagcaatgggcgac 3′ and (anti-sense) 5′ ggaattcgtcatatggtgagatcatc 3′, subcloned and sequenced using the pBSKIII and pCS2+ expression vector as previously described by DeRosa et al. for expression in Xenopus laevis, and immunofluorescent microscopy [8]. Similarly, wild-type Cx43 was obtained in the pCS2+ expression vector [33].
In vitro transcription, oocyte microinjection, and pairing
Wild-type Cx50, Cx43 and mutant Cx50-S50P plasmids were linearized using the NotI restriction site of pCS2+, and transcribed using the SP6 mMessage mMachine (Ambion). Adult Xenopus females were anesthetized, the ovaries were removed and Stage V-VI oocytes were collected after the ovarian lobes were de-folliculated in a solution of 50mg/ml collagenase B, and 50mg/ml hyaluronidase in modified Barth's medium (MB) without Ca2+. Cells were first injected with 10ng of antisense Xenopus Cx38 oligonucleotide to eliminate coupling caused by endogenous intercellular channels, and cultured overnight in MB medium containing 2 mM of CaCl2. Oligonucleotide injected oocytes were then re-injected with either wild-type Cx43, wild-type Cx50 or mutant Cx50 cRNA transcripts (5ng/cell) alone or in combination. H2O injected cells were used as a negative control. The vitelline membranes were then removed in a hypertonic solution (200mM aspartic acid, 10mM HEPES, 1mMMgCl2, 10mM EGTA, and 20mM KCl at pH 7.4), and oocytes were manually paired with the vegetal poles apposed in normal MB media.
Dual whole-cell voltage clamp
Following an overnight incubation, gap junctional coupling between oocyte pairs was measured using the dual whole-cell voltage clamp technique [4;36]. Current and voltage electrodes (1.2mm diameter, omega dot ; Glass Company of America, Millville, NJ, USA) were pulled to a resistance of 1-2MΩ with a horizontal puller (Narishige, Tokyo, Japan) and filled with solution containing 3M KCl, 10mM EGTA, and 10mM HEPES, pH 7.4. Voltage clamp experiments were performed using two GeneClamp 500 amplifiers (Axon Instruments, Foster City Calif., USA) controlled by a PC-compatible computer through a Digidata 1320A interface (Axon Instruments). The pCLAMP 8.0 software (Axon Instruments) was used to program stimulus and data collection paradigms.
For measurements of junctional conductance, cell pairs were first clamped at -40mV to eliminate any transjunctional potentials. Following this initial clamp, a single cell was subjected to alternating pulses of ± 10-20mV, while the current produced by the change in voltage was recorded in the second cell. Junctional conductance (Gj) was calculated by dividing the measured current by the voltage difference, Gj = Ij/(V1-V2).
Preparation of oocyte samples for western blots
Oocytes were collected in 1 ml of buffer containing 5 mM Tris pH 8.0, 5 mM EDTA and protease inhibitors [42] and then lysed using a series of mechanical passages through needles of diminishing caliber (20, 22, 26 Ga). Extracts were centrifuged at 1000 g at 4°C for 5 min. The supernatant was then centrifuged at 100,000 g at 4°C for 30 min. Membrane pellets were resuspended in SDS sample buffer (1μl per oocyte) and samples were separated on 10% SDS gels and transferred to nitrocellulose membranes. Blots were blocked with 5% BSA in 1× PBS for 1 h and probed with a polyclonal Cx50 antibody [42] or a monoclonal anti-mouse Cx43 antibody (Zymed) followed by incubation with appropriate alkaline-phosphatase conjugated secondary antibodies (Jackson laboratories). Band intensities were quantified using Kodak 1D Image Analysis software (Eastman Kodak, Rochester, N.Y., USA). Values from three independent experiments were normalized to the mean value of band intensity of the wild-type sample.
Transient transfection and immunocytochemical staining
HeLa cells were plated on 22 mm square coverslips and grown to 50% confluence then transiently transfected with 4-5μg of connexin DNA subcloned into the pCS2+ vector by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After overnight incubation, cells were fixed with 1% paraformaldehyde in PBS, blocked and permeabilized with 5% BSA in 1× PBS with 0.1% Tx-100. Cells were stained with either a polyclonal rabbit-anti Cx50 or a monoclonal mouse-anti Cx43 antibody (1:500 dilution) followed by incubation with secondary antibodies, Cy3 anti-mouse (IgG) and Cy2 conjugated anti-rabbit (Jackson ImmunoResearch Laboratories), respectively. For co-localization experiments cells were co-transfected with the subsequent cDNAs via the pCS2+ expression vector. Co-transfected cells were then incubated with either a polyclonal anti-Cx50 antibody or a monoclonal anti-Cx43 primary antibody, followed by treatment with both a Cy2 conjugated goat anti-rabbit (Cx50) and Cy3 goat anti-mouse secondary antibody (Cx43) at a 1:1000 concentration. Cells were viewed and photographed on an Olympus BX51 microscope using an Optronics MagnaFire digital camera. Gap junctional plaque formation was quantified by immunofluorescent microscopy. Images were photographed at 60× and areas of cell-cell contact were examined for the presence of gap junctional plaques and counted.
Genetic breeding and in situ dye permeation
Generation of ENU-mutagenized mice, mouse breeding and genome-wide linkage analysis were described previously [12]. Second generation homozygous, Cx50(S50P/S50P) mutant mice were produced according to previously described protocol [49]. The generation and phenotype of Cx50 knockout mice has been previously described by White et al. [39;44]. Cx43flox/ flox mice [6] were provided by Daniel A. Goodenough (Harvard Medical School). MLR10 Cre mice [51] were provided by Michael L. Robinson (Miami University of Ohio). Cx43flox/flox mice and MLR10 Cre mice were interbred in order to obtain Cx43flox/flox-MLR10 Cre mice which were then crossed with Cx50 knockout mice to have lens specific Cx43 and Cx50 double knockout animals.
In order to analyze biochemical coupling in murine lens epithelial cells, lens capsules were dissected and pinned down on a Slygard coated dish containing M199 medium with HEPES. Patch clamp electrodes filled with a combination of Lucifer yellow (1mg/ml) and Neurobiotin (1mg/ml) were applied to a single, central epithelial cell. After formation of a high resistance seal, the patch was opened and dyes were allowed to permeate into the epithelial cells for 4 minutes while the resting membrane voltage was monitored. Capsules were then immediately fixed in 2% paraformaldehyde for 1 hour, mounted on standard microscope slides and stained with rhodamine conjugated avidin (TRITC) (ImmunoPure) and DAPI (Vectashield mounting media). Images were captured on an Olympus BX51 microscope using an Optronics MagnaFire digital camera at 40× magnification using DAPI, TRITC, and FITC filters.
Lens capsule immunocytochemistry
Lenses were dissected, and the lens capsules were isolated and pinned as previously detailed [43]. Capsules were fixed for 1 hr with 2% paraformaldehyde in PBS and blocked with 5% BSA in PBS with 0.1% Tx-100 for 30 min. Epithelial cell gap junctions were stained with rabbit anti-Cx43 antibody or goat anti-Cx50 antibody followed by incubation with Cy3-conjugated AffiniPure Goat anti-rabbit antibody or Cy3-conjugated AffiniPure Rabbit anti-goat antibody, respectively (Jackson ImmunoResearch Laboratories, West Grove, PA). Capsules were then mounted with Vectashield with DAPI, and capsules were viewed and photographed at 60× magnification on a microscope (BX51; Olympus, Tokyo, Japan) using a digital camera (MagnaFire; Optronics, Goleta, CA).
Lens capsule western blot analysis
Wild type, Cx50-S50P mutant, Cx50KO and conditional Cx43KO lens epithelial capsules were peeled from 1-2 week old mice and homogenized in SDS sample buffer. Equal volumes of capsule protein samples from each genotype were separated on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell BioScience, Keene, NH) and were first probed with rabbit anti-Cx43 antibody. Membranes were then stripped and probed with a goat anti-Cx50 antibody. Primary antibodies were detected by peroxidase conjugated Goat anti-Rb and Rb anti-Goat secondary antibodies, respectively and developed by chemiluminescence (Santa Cruz Biotechnology). Protein standards (MagicMark; Invitrogen, Carlsbad, CA) were used as molecular weight markers.
Results
Cx50-S50P inhibits wild-type Cx43 mediated coupling in Xenopus oocytes
Recent data have shown that mutant lens connexins lead to aberrant coupling and gap junction-mediated intercellular communication. We have previously shown that Cx50-S50P fails to form functional homotypic channels [8]. To test the hypothesis that the Cx50-S50P mutation alters intercellular communication in the lens epithelium, we measured gap junctional conductance, Gj, in oocyte pairs injected with various combinations of wild-type Cx43 and Cx50 or mutant Cx50-S50P cRNAs (Fig. 2). Control oocyte pairs injected with anti-sense oligonucleotide and water showed minimal junctional conductance. Conversely, homotypic wild-type Cx43 or Cx50 channels exhibited mean conductance values of ∼29 and ∼36 μS, respectively, a significant increase in Gj over that of the background (P < 0.002, P < 0.0001, respectively Student's t-test). Oocytes co-injected with both wild-type connexin cRNAs were paired to form heteromeric channels. These channels exhibited a mean Gj of approximately 11 μS, a level of coupling significantly lower than that of either homotypic channels (P < 0.04 Cx43, P < 0.007 Cx50, Students t-test), yet over 100-fold greater than that of the background (P < 0.005, Student's t-test). At present, it is unclear why co-expression of both wild-type connexins resulted in reduced conductance. Interestingly, oocytes paired to form heteromeric gap junctions composed of wild-type Cx43 and Cx50-S50P produced insignificant levels of coupling that were indistinguishable from that of the water-injected negative controls. Additionally, the heterotypic pairing of oocytes expressing wild-type Cx43 in one cell and either water, wild-type Cx50, or mutant Cx50-S50P in the second cell failed to significantly couple cells, as levels of junctional conductance were not statistically different from that of the background, measured in heterotypic Cx43/water- injected pairs. Taken together, these results indicate that mutant Cx50-S50P subunits interact with wild-type Cx43 to functionally inhibit gap junctional coupling in vitro.
Fig. 2.
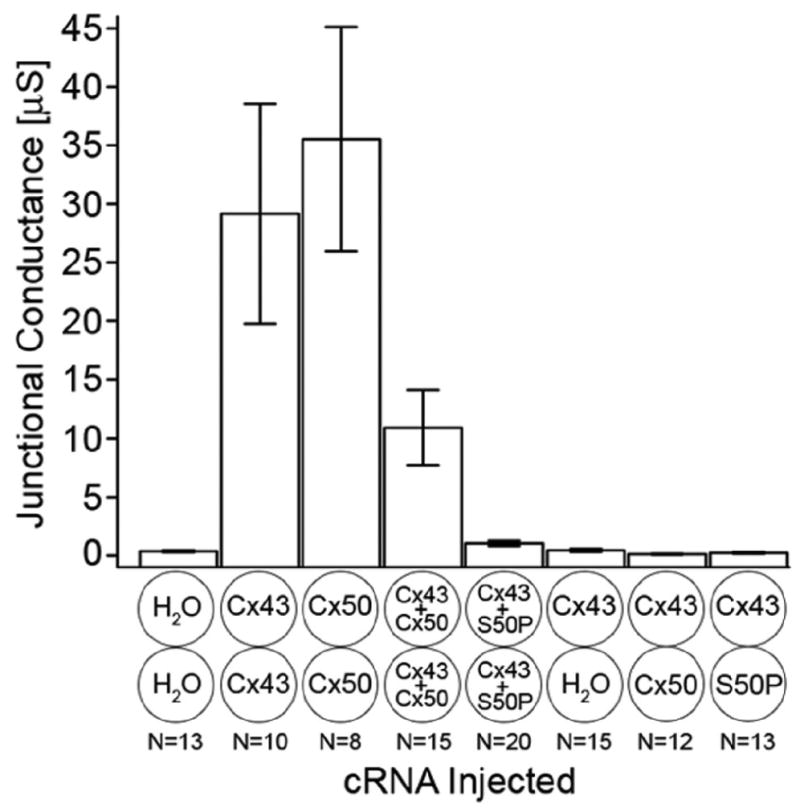
Junctional conductance measurements recorded from Xenopus oocyte pairs injected with wild-type Cx43, Cx50, and mutant Cx50-S50P transcripts alone or in combination. Cell pairs expressing wild-type Cx43 or Cx50 subunits alone formed homotypic gap junctions with mean conductance values of approximately 29 and 36 μS, respectively. Oocytes co-injected with both wild-type Cx43 and Cx50 formed functional intercellular channels with a significantly reduced level of coupling, ∼11 μS, when compared to both homotypic wild-type channels. Conversely, the co-expression of wild-type Cx43 and mutant Cx50-S50P transcripts failed to form functional gap junction channels, exhibiting a level of coupling not significantly higher than that of the background. Similarly, heterotypic cell pairs formed by pairing one oocyte expressing wild-type Cx43 with another injected with either water, wild-type Cx50, or Cx50-S50P cRNAs, failed to produce levels of coupling that were significantly higher than that of the water injected negative controls. It has previously been shown that homotypic channels comprised exclusively of mutant Cx50-S50P subunits fail to form functional intercellular channels [8]. Columns represent the mean ± SE.
Connexin expression in Xenopus oocytes
Based on our current data, we hypothesized that mutant Cx50-S50P subunits interact with wild-type Cx43 proteins to inhibit the inherent functional properties of Cx43. In order to determine if the translation of wild-type Cx43, Cx50 or mutant Cx50-S50P was impaired by the co-expression of these proteins, the production of the lens connexins in Xenopus oocytes was examined via immunoblot analysis. Oocytes injected with water, wild-type Cx43, Cx50, or Cx50-S50P cRNA alone, and cells expressing both mutant and wild-type connexins were analyzed using a monoclonal antibody specific for mouse Cx43 (Zymed) or a polyclonal antibody specific for the central cytoplasmic loop of mouse Cx50 [42]. Immunoblotting revealed a band of ∼40 kDa in samples injected with Cx43 cRNA alone or co-injected oocytes expressing Cx43 and either wild-type or mutant Cx50 (Fig. 3A). Similarly, western blots probed with Cx50 antibody detected bands of ∼60 kDa in samples injected with either Cx50 cRNA alone or co-injected with wild-type Cx43 and either wild-type Cx50 or Cx50-S50P transcripts (Fig. 3B).
Fig. 3.
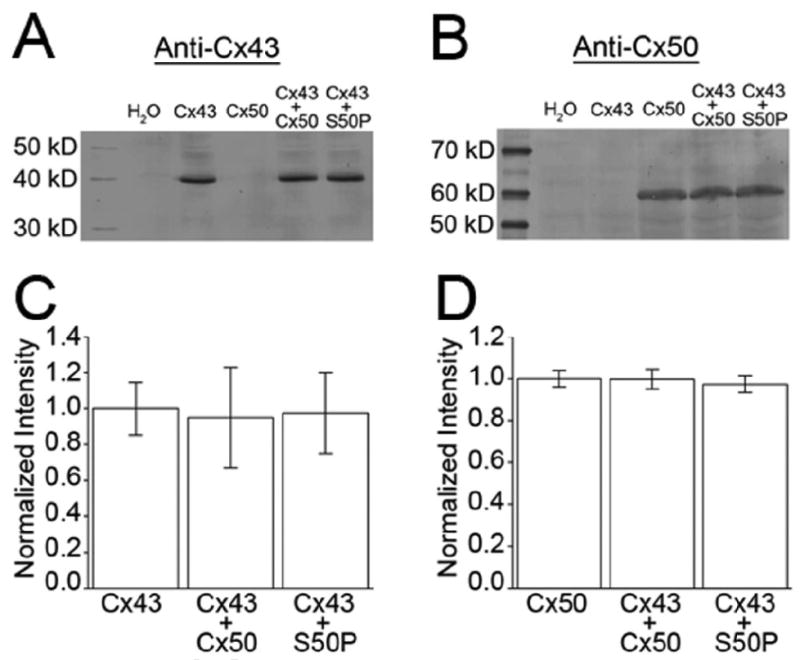
Expression of wild-type lens connexins and mutant Cx50-S50P subunits in Xenopus oocytes were analyzed by immunoblotting. Blots probed with either a monoclonal mouse anti-Cx43 (A) or a polyclonal rabbit anti-Cx50 antibody (B) showed similar levels of wild-type Cx43 and either wild-type or mutant Cx50 when expressed alone or in co-injected cells. (C, D) Band densitometry quantitatively confirmed that the total mean protein expression was not significantly different from that of either wild-type connexin. Columns represent the mean ± SD of 3 independent experiments.
Connexin expression was quantified using band densitometry on replicate immunoblots (n=3). A histogram plotting the mean band intensity values (normalized to wild-type mean intensity value) showed no statistically significant alteration in wild-type Cx43 expression regardless of whether Cx43 was expressed alone or in conjunction with wild-type Cx50 or mutant Cx50-S50P proteins (Fig. 3C) (P > 0.5, ANOVA). Similarly, samples tested for Cx50 expression exhibited nearly identical levels of Cx50 production (Fig. 3D) in samples injected with Cx50 alone or co-injected with wild-type Cx43 (P > 0.5, ANOVA). Thus, both wild-type and mutant transcripts were synthesized equally and it can be concluded that no inherent changes in connexin degradation were initiated by the co-expression of Cx50-S50P and Cx43. Additionally, any alteration in channel function, specifically reduced intercellular coupling, cannot be attributed to differences in protein expression or degradation.
Co-expression of wild-type Cx43 and Cx50-S50P restores targeting of mutant S50P subunits to gap junctions in transfected cells
In order to better understand the mechanism responsible for the loss of electrical coupling seen when wild-type Cx43 and Cx50-S50P were co-expressed, immunohistochemical studies were conducted. These experiments enabled us to determine whether the reductions in junctional conductance were the result of a failure to localize connexins to the plasma membrane efficiently. To this end wild-type Cx43 and/or Cx50-S50P were expressed in transiently transfected HeLa cells for analysis via immunofluorescent microscopy (Fig. 4).
Fig. 4.
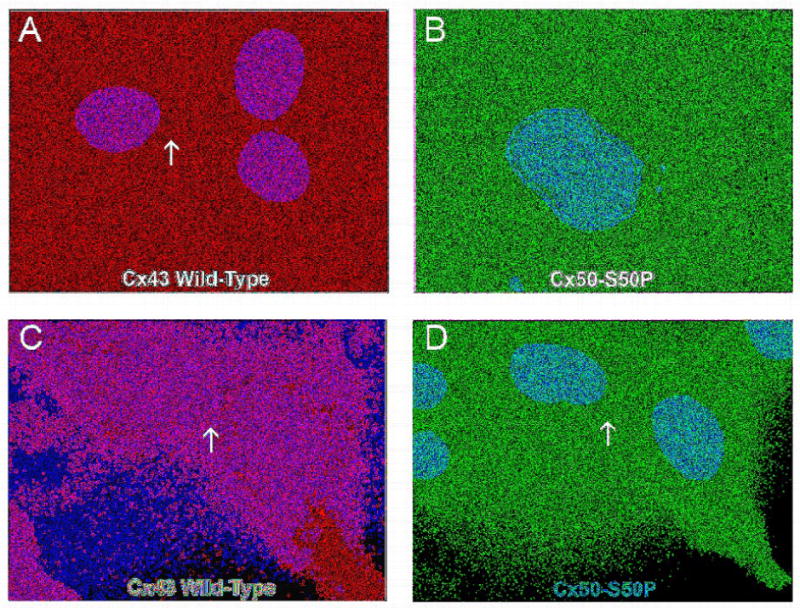
Immunofluorescent imaging of wild-type and mutant connexins in transfected HeLa cells. Transiently transfected HeLa cells expressing wild-type Cx43 (A) or Cx50-S50P (B) proteins alone were immunostained with a monoclonal mouse anti-Cx43 and polyclonal rabbit anti-Cx50 antibody, respectively, and examined by fluorescent microscopy. Merged images taken at 60× magnification displayed Cy3 staining of Cx43 (red) (A), Cy2 staining of Cx50-S50P (B), and DAPI staining of cell nuclei (blue). Overlay images showed that both wild-type and mutant connexins were efficiently translated in vitro, however only wild-type Cx43 subunits properly localized to the plasma membrane specifically at areas of cell to cell apposition (A, arrow),whereas mutant Cx50-S50P protein accumulated in subcellular locations (B). HeLa cells co-transfected with both wild-type Cx43 and Cx50-S50P (C and D) revealed that in the presence of Cx43, S50P subunits co-localize to the membrane and form junctional plaques at areas of cell-cell contact (C, Cy3 staining of Cx43, red, D, Cy2 staining of Cx50-S50P, red; arrows).
Immunofluorescent images revealed ample expression of both wild-type Cx43 (Fig. 4A and 4C) and Cx50-S50P (Fig. 4B and 4D) proteins in vitro. As expected, when expressed alone, Cx50-S50P was unable to localize plasma membrane [8], instead accumulating within the cell (Fig. 4B). Conversely, Cx43 subunits were properly targeted to the membrane at regions of cell-to-cell apposition in 80% of the cell pairs analyzed (Table 2), a finding consistent with the formation of gap junctions (Fig. 4A, arrow). Interestingly, co-transfection of HeLa cells with both wild-type Cx43 and Cx50-S50P cDNAs induced the localization of Cx50-S50P at cell-cell interfaces in 67% of the cell pairs examined (Fig. 4C and 4D, arrows, Table 2), a much higher percentage localization that seen with Cx50-S50P alone. These in vitro data support the hypothesis that the mutant Cx50-S50P protein may associate with wild-type Cx43, a phenomenon that might play a role in the observed reduction in electrical coupling. Additionally, these findings suggest that the loss of junctional coupling seen in Xenopus oocytes paired to form heteromeric Cx43/Cx50-S50P channels is not due to an inability to target to cell-cell interfaces.
Table 2.
Quantitative analysis of gap junctional plaque formation in transiently transfected HeLa cells containing wild-type Cx43 and mutant Cx50-S50P subunits alone or co-expressed.
| Connexin(s) expressed | Junctional plaques present | Total number of pairs analyzed | Percentage of gap junctions expressing connexins |
|---|---|---|---|
| Wild-type Cx43 | 16 | 20 | 80% |
| Cx50-S50P | 0 | 17 | 0% |
| Cx43 + Cx50-S50P | 12 | 18 | 67% |
Cx50-S50P mutant protein alters endogenous Cx43 expression and localization in lens epithelial cells
Based on our in vitro analysis of wild-type and mutant connexin expression we hypothesize that the co-expression of endogenous wild-type Cx43 and mutant Cx50-S50P proteins in vivo could affect protein localization in the lens epithelium. To test this hypothesis we evaluated connexin expression patterns in 1-2 week old murine lenses (Fig. 5). Immunofluorescent detection of Cx43 revealed no difference in the Cx50 knockout capsules (Fig. 5C) when compared to wild-type lenses (Fig. 5A). However, in the homozygous Cx50-S50P mutant lens (Fig. 5G) Cx43 labeling was weaker and less organized than that visualized in both wild-type (Fig. 5A) and Cx50 knockout lens (Fig. 5C) epithelial cells. As expected, the Cx50 antibody failed to detect any endogenous protein in the Cx50 knockout capsules (Fig. 5D), and Cx50 was found properly localized at the membrane in wild type (Fig. 5B), Cx50-S50P (Fig. 5H) and conditional Cx43 knockout (Fig. 5F) lenses. Note that there were occasional cells labeled for Cx43 in the conditional Cx43 knockout capsule (Fig. 5E); and this mosaicism could be caused by variable Cre activity in the lens epithelium. We were not able to test Cx50-S50P localization in lenses lacking Cx43, but would predict that it would not target to gap junctions based on the in vitro data presented above. Taken together these data indicate that the co-expression of wild-type Cx43 and mutant Cx50-S50P alter protein localization and gap junctional plaque formation via a unique interaction between the mutant and wild-type connexins in both in vitro and in vivo expression systems.
Fig. 5.
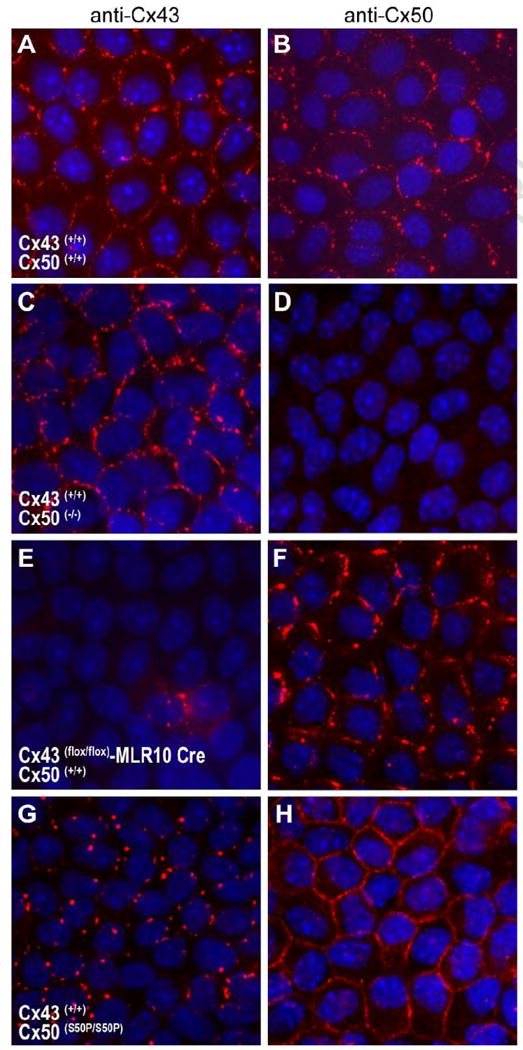
Immunofluorescent staining of the Cx43 and Cx50 lens proteins in lens epithelial cells. Lens capsules from wild-type (A, B), Cx50 knockout (C, D), conditional Cx43 knockout (E,F) and homozygous Cx50-S50P mutant (G,H) lenses were immunostained with antibodies raised against Cx43 and Cx50 and examined by fluorescence microscopy. Merged images show Cy3 staining of connexins (red) and DAPI staining of cell nuclei (blue). Discernable aberrations in endogenous connexin 43 staining were visible in the presence of mutant Cx50-S50P (G) a phenomenon not seen in the Cx50 knockout animal (C).
In order to determine if the reduction in Cx43 staining seen in homozygous Cx50-S50P mutant lens capsules was the result of reduced protein expression, western blotting experiments were conducted using the lens capsules of wild-type, Cx50-S50P mutant, Cx50 knockout, and Cx43 conditional knockout mice. For this, an equal number of lens capsules were collected, electrophoresed and probed with an anti-Cx43 antibody (Fig. 6A). Filters were then stripped and stained with an anti-Cx50 antibody (Fig. 6B). This enabled us to qualitatively analyze and compare in vivo connexin expression across all genetic conditions. Western blotting revealed Cx43 and its phosphorylated forms [28] in wild-type, Cx50-S50P homozygous and Cx50 knockout mutant lens samples whereas conditional Cx43 knockout lenses displayed greatly reduced levels of endogenous Cx43 (Fig. 6A). Similarly, western blots probed with Cx50 antibody detected ∼60 kDa bands of similar intensity in lens samples procured from either wild-type, homozygous mutant or Cx43 knockout animals (Fig. 6B). Interestingly, Cx43 expression was visibly reduced in the Cx50-S50P mutant mouse (Fig. 6A), a result which provides additional support for a unique interaction between wild-type Cx43 and Cx50-S50P proteins that may play a role in the severe pathology associated with this mutation in vivo.
Fig. 6.
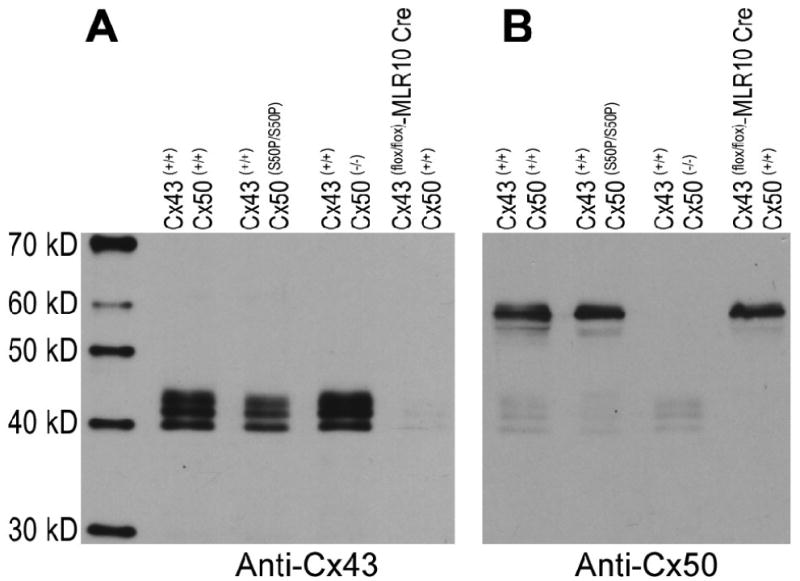
Analysis of connexin synthesis in mouse lenses. Equal volumes of the lens capsules isolated from wild-type, homozygous Cx50-S50P mutant, Cx50 knockout, and Cx43 knockout mice were probed with antibodies specific for Cx43 (A) and Cx50 (B). Cx43 expression levels were unchanged in wild-type and Cx50 knockout mice, while genetic knockout of Cx43 resulted in the loss of endogenous Cx43 production. Interestingly, a visible reduction in Cx43 protein expression was seen in homozygous mutant lenses; a phenomenon independent of the deletion of Cx50 (A). Conversely, Cx50 expression was nearly identical in wild-type, homozygous mutant and Cx43 knockout animals (B).
The Cx50-S50P cataract exhibits a more severe pathology than the Cx43 conditional or Cx50 knockout animal
Based on our in vivo and in vitro analysis of mutant channel function and protein characterization we hypothesized that the homozygous Cx50-S50P mutant mouse would produce a lens pathology distinct from that of either the Cx50 or Cx43 single knockout mice. A comparative analysis of wild-type, Cx50 knockout, Cx43flox/flox-MLR10 Cre, and Cx50-S50P homozygous mutant mouse lenses was conducted to qualitatively decipher any phenotypic changes associated with connexin diversity (Fig. 7). Adult wild-type (Fig. 7A) and Cx43 conditional knockout (Fig. 7C) mice developed clear, intact lenses with no obvious reduction in lens size, a finding that indicates Cx43 is not required for maintaining lens clarity and organ homeostasis. Conversely, the Cx50 knockout (Fig. 7B) exhibited a mild nuclear opacity, and a reduction in organ mass. The homozygous Cx50-S50P mutant mouse (Fig. 7D) developed small, ruptured lenses with a whole lens cataract and epithelial and fiber cell abnormalities, a pathophysiology more severe than that of the Cx50 knockout and Cx43flox/flox-MLR10 Cre lenses. Taken together, these data provided additional support for a unique mechanism associated with the Cx50-S50P mutant phenotype, as the homozygous mutant exhibits lens abnormalities far more severe than those caused by the simple removal of endogenous Cx43 or Cx50.
Fig. 7.
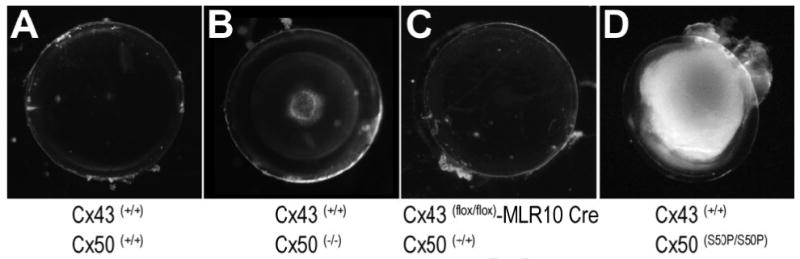
Dense cataract and posterior capsule rupture develop in homozygous Cx50-S50P mutant lenses. Wild-type lenses were clear and intact (A). Cx50 knockout developed a mild nuclear opacity (B). Cx43 knockout animals produced clear lenses that developed normally until at least 6 months of age (C). Homozygous Cx50-S50P mutant lenses revealed a whole lens cataract, primary fiber cell abnormalities and eventually progressed to posterior capsule rupture (D).
The presence of Cx50-S50P proteins drastically alters biochemical coupling in the lens epithelium
To determine whether mixing wild-type Cx43 and mutant Cx50-S50P affected gap junction mediated biochemical coupling in the lens epithelium, we have developed a method to assess junctional permeability in lens epithelial cells in situ. By using a lens capsule whole mount preparation in combination with whole-cell patch clamp electrophysiology and fluorescence microscopy, we were able to qualitatively analyze dye permeation in the epithelium of wild-type, Cx50 knockout, Cx43flox/flox-MLR10 Cre, Cx50S50P homozygous mutant and Cx43flox/flox-MLR10 Cre Cx50(-/-) mouse lenses.
Briefly, lens capsules were isolated from 1-2 week old, wild-type (containing endogenous Cx50 and Cx43), Cx50 knockout (lacking all endogenous Cx50), Cx43flox/flox-MLR10 Cre (lacking Cx43 in the lens), homozygous mutant S50P mice (containing wild-type Cx43 and only mutant Cx50-S50P protein) and Cx43flox/flox-MLR10 Cre Cx50(-/-) mouse (devoid of both Cx43 and Cx50). A single patch clamp electrode, containing both Lucifer yellow (MW 457, charge -2) and neurobiotin (MW 287, charge +1), was applied to a central epithelial cell attached to the underlying capsule. After the formation of a high resistance seal between the cell membrane and the aforementioned electrode, the patch was opened enabling the dyes to diffuse from the electrode into the donor cell. Injected capsules were then fixed and stained with rhodamine-conjugated avidin to detect neurobiotin spread, and DAPI to define cell nuclei, enabling us to simultaneously monitor the permeation of Lucifer yellow and neurobiotin throughout adjoined cells via fluorescent microscopy (Fig. 8).
Fig. 8.

In situ dye transfer assay of epithelial cell gap junctional coupling. Patch clamp electrodes were filled with a mixture of Lucifer yellow and neurobiotin and injected into a single centrally located epithelial cell. Wild-type lens capsules showed widespread coupling to neurobiotin and modest transfer of Lucifer yellow to surrounding cells (A). The loss of endogenous wild-type Cx50 channels (B) revealed similar dye spread patterns to those of the wild-type for both neurobiotin and Lucifer yellow. Conversely, Cx43flox/flox MLR10 Cre mice expressing only wild-type Cx50 protein produced a visible reduction in neurobiotin and Lucifer yellow dye transfer (C). Furthermore, homozygous mice expressing only wild-type Cx43 and Cx50-S50P mutant subunits displayed a severely diminished level of biochemical coupling for both neurobiotin and Lucifer yellow ionic dyes (D) a phenomenon that was also exhibited by Cx43, Cx50 double knockout lenses (E). These findings indicate a dominant interaction between mutant S50P subunits and wild-type Cx43 subunits that severely inhibits junctional communication in the murine lens. Injections were analyzed in 4-8 lens capsules from each genotype and representative images were chosen.
Fluorescent microscopy images revealed that wild-type epithelial cells showed extensive coupling to neurobiotin and moderate transfer of Lucifer yellow (Fig. 8A). When Cx50 was deleted (Fig. 8B), the dye permeation was largely unaffected for both dyes, indicating that biochemical dye transfer in the epithelium is governed primarily by endogenous Cx43 channels at this developmental age. This was further verified as Cx43flox/flox-MLR10 Cre lenses (Fig. 8C) showed a reduced transfer of both neurobiotin and Lucifer yellow compared to wild-type and Cx50 knockout lenses. Furthermore, examination of mice homozygous for the Cx50-S50P mutation revealed a sizeable reduction in permeability for both dyes (Fig. 8D), as both the negatively-charged Lucifer yellow and positively-charged neurobiotin were retained primarily within the donor cell a finding similar to that seen in the Cx43flox/flox-MLR10 Cre Cx50(-/-) lenses (Fig. 8E). These data suggest that the Cx50-S50P mutation can inhibit the activity of endogenous Cx43 in situ to suppress gap junctional coupling in mouse lens epithelial cells via an inhibitory interaction.
Discussion
In the current study, the co-expression of wild-type Cx43 and Cx50-S50P caused a severe reduction in gap junctional conductance in vitro, as well as a decrease in permeation to fluorescent dyes in intact lens capsules, a finding similar to that seen in the double Cx43flox/flox-MLR10 Cre/Cx50 knockout lens. Additionally, immunoblotting revealed that these inherent changes in channel function may be associated with a reduction in endogenous Cx43 expression, a phenomenon not seen in Xenopus oocytes, or transfected HeLa cells. Conversely, an alteration in protein production, increase in connexin degradation, or the inability to target to junctional plaques when co-expressed was not seen in vitro. Finally, both in vivo and in vitro immunohistochemical staining indicated that the presence of mutant Cx50-S50P subunits altered gap junctional plaque formation. Taken together, this research provides evidence for a unique interaction between the cataract-causing Cx50-S50P mutant protein and wild-type Cx43 in the murine lens. These experiments support the hypothesis that Cx50-S50P subunits functionally inhibit the electrical and biochemical properties of gap junctions containing Cx43 subunits, a phenomenon that may play a role in the lens pathophysiology of the Cx50-S50P cataract, specifically the large cystic lumen below the epithelium [49].
Research characterizing Cx43's role in lens development has been extensive, although conflicting [14;28;31;46]. To date, attempts to genetically knock out Cx43 in mice via homologous recombination have led to neonatal death, thus preventing researchers from definitively identifying Cx43's role in postnatal lens development. However, the genetic manipulation of mice has indicated that neither Cx50, nor Cx43 play a significant role in embryonic lens development, specifically in fiber cell differentiation [46]. In a conditional Cx43 KO model using a less restrictive nestin driven cre recombinase to remove Cx43 from many ocular tissues, lens development appeared normal until a failure of aqueous humor production by the ciliary body was observed [6]. Interestingly, a more recent analysis of murine lens epithelial cells revealed that Cx43 contributes to postnatal epithelial cell coupling in an age dependant manner, a finding that coincides well with the data presented here [43]. For example, the 1-2 week old lenses analyzed in this manuscript reveal a distinguishable decrease in Cx43 expression (Fig. 6), as well as an obvious reduction of biochemical coupling from that of the wild-type mice, a difference not seen when the wild-type and Cx50 knockout lenses were compared (Fig. 8). Taken together, these data indicate that Cx43-mediated coupling of lens epithelial cells is not dispensable, and may play an important role in postnatal lens function and development.
Many studies have focused on the relevance of preserving lens connexin diversity in order to maintain proper organ function [10;11;25;39;41]. However, the recent analysis of heterozygous mutant mice, Cx50 (S50P/+), revealed that the mixing of wild-type and mutant Cx50 subunits causes aberrations in embryonic fiber cell elongation without altering connexin diversity; implicating that Cx50 may play a role in embryonic lens formation [17;49]. This study also reported the formation of a large cystic lumen beneath the anterior epithelial cells in embryonic mutant lenses. In light of the data presented in this manuscript, the phenotypic anomalies reported herein (Fig. 7) may be attributed, to some extent, to the inhibitory affects of Cx50-S50P on endogenous Cx43 in lens fiber elongation.
Acknowledgments
We thank Daniel A. Goodenough (Harvard Medical School) and Michael L. Robinson (Miami University of Ohio) for providing mice. This work was supported by a NYSTEM Institutional Support Grant (P.RB.) and NIH grants; EY13849 (X.G.) and EY13163 (T.W.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arora A, Minogue PJ, Liu X, Addison PK, Russel-Eggitt I, Webster AR, Hunt DM, Ebihara L, Beyer EC, Berthoud VM, Moore AT. A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J Med Genet. 2008;45:155–160. doi: 10.1136/jmg.2007.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, Bhattacharya SS, Webster AR, Hunt DM, Ebihara L, Moore AT, Beyer EC, Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 2006;43:e2. doi: 10.1136/jmg.2005.034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruzzone R, Veronesi V, Gomes D, Bicego M, Duval N, Marlin S, Petit C, D'Andrea P, White TW. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett. 2003;533:79–88. doi: 10.1016/s0014-5793(02)03755-9. [DOI] [PubMed] [Google Scholar]
- 5.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 6.Calera MR, Topley HL, Liao Y, Duling BR, Paul DL, Goodenough DA. Connexin43 is required for production of the aqueous humor in the murine eye. J Cell Sci. 2006;119:4510–4519. doi: 10.1242/jcs.03202. [DOI] [PubMed] [Google Scholar]
- 7.Chang B, Wang X, Hawes NL, Ojakian R, Davisson MT, Lo WK, Gong X. A Gja8 (Cx50) point mutation causes an alteration of alpha 3 connexin (Cx46) in semi-dominant cataracts of Lop10 mice. Hum Mol Genet. 2002;11:507–513. doi: 10.1093/hmg/11.5.507. [DOI] [PubMed] [Google Scholar]
- 8.DeRosa AM, Xia CH, Gong X, White TW. The cataract-inducing S50P mutation in Cx50 dominantly alters the channel gating of wild-type lens connexins. J Cell Sci. 2007 doi: 10.1242/jcs.012237. [DOI] [PubMed] [Google Scholar]
- 9.DeRosa AM, Xia CH, Gong X, White TW. The Loss-of-Function Cx50-R205G Mutation Dominantly Alters Wild-Type Lens Connexin Function. Invest Ophthalmol Vis Sci. 2008;49:1522. E-Abstract. [Google Scholar]
- 10.Donaldson PJ, Dong Y, Roos M, Green C, Goodenough DA, Kistler J. Changes in lens connexin expression lead to increased gap junctional voltage dependence and conductance. Am J Physiol. 1995;269:C590–C600. doi: 10.1152/ajpcell.1995.269.3.C590. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson PJ, Roos M, Evans C, Beyer E, Kistler J. Electrical properties of mammalian lens epithelial gap junction channels. Invest Ophthalmol Vis Sci. 1994;35:3422–3428. [PubMed] [Google Scholar]
- 12.Du X, Tabeta K, Hoebe K, Liu H, Mann N, Mudd S, Crozat K, Sovath S, Gong X, Beutler B. Velvet, a dominant Egfr mutation that causes wavy hair and defective eyelid development in mice. Genetics. 2004;166:331–340. doi: 10.1534/genetics.166.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans WH, Martin PE. Gap junctions: structure and function (Review) Mol Membr Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Spray DC. Structural changes in lenses of mice lacking the gap junction protein connexin43. Invest Ophthalmol Vis Sci. 1998;39:1198–1209. [PubMed] [Google Scholar]
- 15.Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159–170. doi: 10.1016/j.bbamem.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Gong X, Cheng C, Xia CH. Connexins in Lens Development and Cataractogenesis. J Membr Biol. 2007 doi: 10.1007/s00232-007-9033-0. [DOI] [PubMed] [Google Scholar]
- 18.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 19.Goodenough DA. The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol. 1992;3:49–58. doi: 10.1016/s1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- 20.Graw J, Loster J, Soewarto D, Fuchs H, Meyer B, Reis A, Wolf E, Balling R, dA Hrabe. Characterization of a mutation in the lens-specific MP70 encoding gene of the mouse leading to a dominant cataract. Exp Eye Res. 2001;73:867–876. doi: 10.1006/exer.2001.1096. [DOI] [PubMed] [Google Scholar]
- 21.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 22.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol. 2008;131:293–305. doi: 10.1085/jgp.200709934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–1364. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Wittinghan FJ, Sellitto C, Li L, Gong X, Brink PR, Mathias RT, White TW. Dominant cataracts result from incongruous mixing of wild-type lens connexins. J Cell Biol. 2003;161:969–978. doi: 10.1083/jcb.200303068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 27.Moreno AP. Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res. 2004;62:276–286. doi: 10.1016/j.cardiores.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- 29.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 32.Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–174. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- 33.Rouan F, White TW, Brown N, Taylor AM, Lucke TW, Paul DL, Munro CS, Uitto J, Hodgins MB, Richard G. trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J Cell Sci. 2001;114:2105–2113. doi: 10.1242/jcs.114.11.2105. [DOI] [PubMed] [Google Scholar]
- 34.Runge PE, Hawes NL, Heckenlively JR, Langley SH, Roderick TH. Autosomal dominant mouse cataract (Lop-10). Consistent differences of expression in heterozygotes. Invest Ophthalmol Vis Sci. 1992;33:3202–3208. [PubMed] [Google Scholar]
- 35.Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet. 1998;62:526–532. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77:77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steele EC, Jr, Lyon MF, Favor J, Guillot PV, Boyd Y, Church RL. A mutation in the connexin 50 (Cx50) gene is a candidate for the No2 mouse cataract. Curr Eye Res. 1998;17:883–889. doi: 10.1076/ceyr.17.9.883.5144. [DOI] [PubMed] [Google Scholar]
- 38.Vanita V, Singh JR, Singh D, Varon R, Sperling K. A novel mutation in GJA8 associated with jellyfish-like cataract in a family of Indian origin. Mol Vis. 2008;14:323–326. [PMC free article] [PubMed] [Google Scholar]
- 39.White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–320. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- 40.White TW. Nonredundant gap junction functions. News Physiol Sci. 2003;18:95–99. doi: 10.1152/nips.01430.2002. [DOI] [PubMed] [Google Scholar]
- 41.White TW, Bruzzone R. Intercellular communication in the eye: clarifying the need for connexin diversity. Brain Res Brain Res Rev. 2000;32:130–137. doi: 10.1016/s0165-0173(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 42.White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White TW, Gao Y, Li L, Sellitto C, Srinivas M. Optimal lens epithelial cell proliferation is dependent on the connexin isoform providing gap junctional coupling. Invest Ophthalmol Vis Sci. 2007;48:5630–5637. doi: 10.1167/iovs.06-1540. [DOI] [PubMed] [Google Scholar]
- 44.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- 46.White TW, Sellitto C, Paul DL, Goodenough DA. Prenatal lens development in connexin43 and connexin50 double knockout mice. Invest Ophthalmol Vis Sci. 2001;42:2916–2923. [PubMed] [Google Scholar]
- 47.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 48.Xia CH, Cheng C, Huang Q, Cheung D, Li L, Dunia I, Benedetti LE, Horwitz J, Gong X. Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Exp Eye Res. 2006;83:688–696. doi: 10.1016/j.exer.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Xia CH, Liu H, Cheung D, Cheng C, Wang E, Du X, Beutler B, Lo WK, Gong X. Diverse gap junctions modulate distinct mechanisms for fiber cell formation during lens development and cataractogenesis. Development. 2006;133:2033–2040. doi: 10.1242/dev.02361. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Ebihara L. Characterization of a mouse Cx50 mutation associated with the No2 mouse cataract. Invest Ophthalmol Vis Sci. 1999;40:1844–1850. [PubMed] [Google Scholar]
- 51.Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:1930–1939. doi: 10.1167/iovs.03-0856. [DOI] [PubMed] [Google Scholar]


