Abstract
There may be a role of age-related decline in androgen production and/or its metabolism for late-onset depression disorders of men and women. Thus, the antidepressant-like effects of testosterone (T) and its metabolites are of interest. Given that these androgens have disparate mechanisms of action, it is important to begin to characterize and compare their effects in an aged animal model. We hypothesized that there would be sex differences in depression behavior of aged mice and that androgens would reduce depression-like behaviors in the forced swim test. To investigate this, male and female mice (~24 months old) were subcutaneously administered T, or one of its 5α-reduced metabolites (dihydrotesterone- DHT, 5α-androstane,17β-diol-3α-diol), or aromatized metabolite (estradiol- E2), or oil vehicle. Mice were administered androgens (1 mg/kg) 1 hour before being tested in the forced swim test, an animal model of depression. We found that males spent more time immobile, and less time swimming, than females. Administration of T, DHT, or 3α-diol similarly reduced time spent immobile, and increased time spent struggling, of male and female mice. E2, compared to vehicle-administration, decreased time spent immobile of males and females, but increased time spent swimming of females and time spent struggling of male mice. Together, these data suggest that T and its 5α-reduced and aromatized metabolites have anti-depressant-like effects in aged male and female mice.
Keywords: neurosteroids, dihydrotestosterone, 3α-androstanediol, 5α-androstane, 17β-diol, estradiol, estrogen, anxiety
1. Introduction
The prevalence of depression disorders in the aged population is a major public health concern. It is estimated that in the next 10–15 years, depression will be second, after heart disease, as a major contributor to global disease burden [1]. As such, factors, such as steroid hormones, that change with aging and may contribute to the etiology and/or treatment of depression are of great interest.
There are several endocrine changes with aging in men and women, with the net effect of reducing gonadal steroids, such as androgens and estrogens. The menopause of women typically involves a reduction in menstrual cyclicity and an abrupt decline in circulating levels of estrogens with aging. In aged men, there is a more progressive decline in androgen levels until acyclicity in circadian rhythms of androgen production, termed andropause, occurs. Unlike menopause, which typically occurs in women in their late 40s and early 50s, andropause occurs much later. In fact, in 75 year old men, plasma levels of testosterone (T), the main androgen produced by the testes, are 35% lower than those in younger men and only about 25% of this cohort could be considered T-deficient [2]. Despite differences in the timeframes in which these endocrine changes occur, it has been suggested that changes in cognitive performance and increases in depression disorders with aging may be related to reductions in androgens and estrogens in men and women [3], [4], [5], and [6]. For example, in men aged 50–89, depression increased with age and this was related to reductions in bioavailable T [7]. Another way to consider the role of androgens in depression is by examining hypogonadal populations. IIn hypogondal men (30–65 years old) with depression that is refractory to antidepressant treatments, administration of T for 8 weeks improved mood and other symptoms of depression, such as reductions in sleep, appetite, and libido [8]. Thus, androgen-replacement to individuals with hypogonadism and/or aging individuals as an antidepressive intervention is of interest.
A recent study investigated T levels and depression scores in men and women 70–79 years old and found that, in men and women, low total or free T levels, respectively, were associated with higher depression scores of men and women [10]. However, measuring the effects of T is complicated by the fact that its metabolites also have clear functional effects. E2 is produced by aromatization of T in males and females (albeit levels of ovarian E2 in females fluctuate and reach greater asymptotic levels than are observed in males) and changes in endogenous E2, or E2 extirpation and replacement, in young and aged rodents enhance affective behaviors (i.e. anxiety, fear, depression, etc) [9]. T can also be metabolized to dihydrotestosterone (DHT) and 5α-androstane, 17β-diol (3α-diol) by actions of 5α-reductase and 3α-hydroxysteroid dehydrogenase (3αHSD) and are then non-aromatizable to E2. DHT and/or 3α-diol administration reduce anxiety and depressive behaviors of gonadectomized young or aged mice [11], [12], [13], [14] and [15]. Thus, it is not entirely clear whether age-related increases in depression are due to actions of T as a prohormone in both males and females. Clearly, the role of T and its metabolites for depression needs further investigation.
Whether there are sex differences in response to T and its metabolites for depressive behavior of aged individuals is not known. Using immobility in the forced swim test as a measure of depression-like behavior, we have found that there are sex differences in responses to progesterone in young and aged male mice. Administration of progesterone decreases immobility in the forced swim test of young, ovariectomized and aged intact female mice [16]. In contrast, progesterone did not alter behavior of young male mice but had robust effects to reduce immobility of aged male mice. Given the potential role of androgens in depression disorders, it was important to characterize the effects T and its metabolites would have on immobility in the forced swim test of aged mice. In the present study, behavior of aged male and female mice in the forced swim test was compared following administration of vehicle, T, DHT, 3α-diol, or E2. We hypothesized that there would be sex differences in depression behavior and that androgens would reduce time spent immobile in the forced swim test.
2. Methods
All methods were approved by the Institutional Animal Care and Use Committee at University at Albany- SUNY, and were done in accordance with established standards of humane animal use.
2.1. Subjects and housing
Subjects were aged congenic C57/BL6 mice (average age 24 months, range 20–28 months; N=46; n=26 males, n=20 females) bred in the Social Sciences Laboratory Animal Care Facility at the University at Albany, SUNY. Mice were group-housed (2–5 per cage) with unlimited access to Purina Rodent Chow and tap water in their home cages. Mice were housed in a temperature-controlled room that had a reversed 12/12 hour light/dark cycle (lights on 0800 hr; off 2000 hr).
2.2. Natural hormonal milieu
Although a typical approach to investigate steroid-behavior interactions is to use extirpation of the gonads and replacement of steroids, the advanced age of the subjects in this study precluded this approach due to a likely risk of attrition following undergoing a surgical procedure. As such, experimental mice were gonadally-intact in the present study. Intact, aged mice have been reported to have significantly lower levels of gonadal hormones than do their younger same-sex counterparts [17] and [18]. In mice of a similar background and age as those in the present study and bred in our facility, we have shown that levels of androgens and E2 are comparable to young male and female mice that are gonadectomized or ovariectomized, respectively [19] and [20].
2.3. Androgen administration
Male and female experimental mice (4–6/group) were randomly-assigned to receive a 1 mg/kg subcutaneous injection of T, DHT, 3α-diol, E2, or sesame oil vehicle 1 hour before testing in the forced swim test. This 1 hour dosing of these androgens produce levels of androgens in aged, intact male mice that are similar to those observed in intact, young male mice [19]. Similarly, this timing of E2 dosing produces E2 levels in the hippocampus of aged mice that are similar to those in young mice [20].
2.4. Behavioral testing- forced swim test
The forced swim test is typically utilized in examining depression-like behavior of rodents. It has been validated in mice as a screening tool for potential pharmacological interventions in depression [21] and [22]. The time spent by mice immobile, defined as floating or the absence of active behaviors, such as swimming or struggling to escape chamber, is utilized as an index of depression-like behavior. The protocol in our lab for the forced swim test was modified from that previously described in mice [23] to take into account the advanced age of the subjects and potential for difficulties due to the physical demands associated with performing this task. In brief, mice were placed in a glass cylinder (diameter of 20.5 cm and depth of 21.5 cm) that was filled with 18 cm of 25°C water for five minutes. The duration that mice spent immobile, swimming, and struggling was recorded during the five minute task. Mice were removed from the cylinder, gently dried with paper towels, and then placed in a transport cage without bedding until they were dry.
2.5. Statistical Analyses
Two-way analyses of variance tests (ANOVAs) were utilized to determined effects of sex and androgen administration for behavior in the forced swim test. Fisher’s LSD post hoc tests were utilized to determine group differences when main effects of these variables were revealed. A p-value of ≤0.05 was considered significant.
3. Results
There were significant main effects of sex F (1, 36) = 6.37, p<0.02, and androgen administration F (4,36) = 6.95, p<0.01, but not an interaction between these main effects, for time spent immobile in the forced swim test of aged mice (Figure 1). Overall, aged male mice spent more time immobile than did aged female mice Moreover, regardless of sex, androgen administration decreased the amount of time spent immobile. Compared to vehicle, administration of T or its metabolites, DHT, 3α-diol, or E2, significantly reduced time spent immobile.
Figure 1.
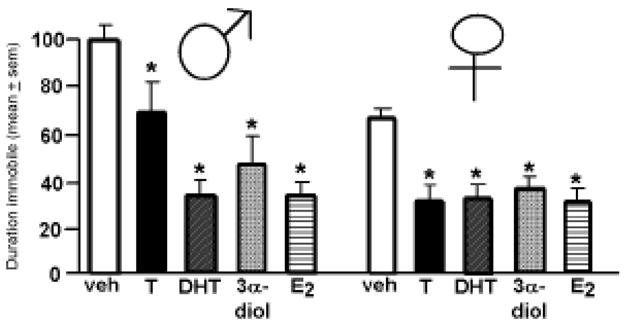
Time spent immobile (mean in secs ± sem) in the forced swim test of male (left) and female (right) mice administered oil vehicle (veh), testosterone (T), dihydrotestosterone (DHT), 3α-androstanediol (3α-diol), or estradiol (E2). * above bar indicates a significant (P ≤ 0.05) effect of androgen vs. vehicle condition.
There were significant main effects of sex F (1, 36) = 18.76, p<0.01, and androgen condition F (4,36) = 7.44, p<0.01, as well as an interaction between these main effects F (4, 36) = 13.14, p<0.01, for time spent swimming in the forced swim test (Table 1). Aged female mice spent more time swimming than did aged male mice. Female, compared to male mice, had significantly increased time spent swimming following administration of vehicle or E2, but not T, DHT, or 3α-diol.
Table 1.
Time spent struggling or swimming in the forced swim test of in the forced swim test of male (left) and female (right) mice administered oil vehicle (veh), testosterone (T), dihydrotestosterone (DHT), 3α-androstanediol (3α-diol), or estradiol (E2). Data are expressed as means in seconds ± s.e.m.
| Measure | Condition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||||
| veh | T | DHT | 3α-diol | E2 | veh | T | DHT | 3α-diol | E2 | |
| n | 6 | 5 | 4 | 5 | 6 | 4 | 4 | 4 | 4 | 4 |
| Time spent struggling | 36.5 ± 17.8 | 57.0 ± 24.1 | 107.8 ± 5.9 | 83.4 ± 19.5 | 114.5 ± 6.4 | 14.3 ±5.3 | 101.5 ± 3.6 | 107.8 ± 10.8 | 104.0 ± 5.7 | 31.5 ± 8.6* |
| Time spent swimming | 43.0 ± 8.2 | 54.4 ± 13.8 | 35.8 ± 9.5 | 48.4 ± 11.2 | 29.2 ± 3.2 | 103.5 ± 5.5* | 44.5 ± 9.4 | 35.5 ± 9.9 | 35.5 ± 4.8 | 114.3 ± 5.1* |
indicates a significant interaction between main effects.
There was a significant main effect of androgen condition F (4,36) = 9.54, p<0.01, an interaction between these main effects F (4, 36) = 5.92, p<0.01, but not a significant main effects of sex, for time spent struggling in the forced swim test (Table 1). Compared to vehicle administration, T, DHT, or 3α-diol significantly increased time spent struggling of male and female mice, but E2 only increased time spent struggling in male mice.
4. Discussion
The results of the present study supported our hypothesis that there are sex differences in the forced swim test behavior of aged mice and that androgen administration has anti-depressant-like effects in aged male mice in this task. Overall, aged male mice spent more time immobile than did age-matched female mice. Administration of T or its metabolites, DHT, 3α-diol, or E2, significantly reduced the time spent immobile by both aged male and female mice. T, DHT, or 3α-diol increased time spent struggling, of male and female mice. E2, compared to vehicle-administration, increased time spent swimming of females and time spent struggling of male mice. Thus, androgens can have anti-depressant-like effects in aged individuals.
The present data confirm and extend previous studies that reported effects of T and its 5α-reduced metabolites for anxiety and depression behavior in animal models. Young, gonadally-intact male rats have decreased anxiety and depression-like behavior compared to gonadectomized counterparts, and administration of T or its metabolites decrease anxiety and depression behavior [11], [12], [13], [14], and [24]. By using pharmacological means to reduce the metabolism of T to DHT and, subsequent conversion to 3α-diol, these studies supported the notion that T’s functional effects are through actions of 3α-diol [13]. Indeed, in our recent study in aged mice, the most consistent anti-anxiety and cognitive-enhancing effects were observed with administration of 3α-diol, compared to T [19]. Given that DHT and T, which are both prohormones for 3α-diol, had similar effects as did 3α-diol itself in males and females in the present study, the possibility remains that 3α-diol is essential for some of the beneficial effects of T for mood in males and females.
The present findings also suggest that T’s aromatized metabolite, E2, can have anti-depressant-like effects in aged male and female mice. Other studies have demonstrated that changes in endogenous E2 levels can alter depression-like behavior. Female aromatase knockout mice have a life-long deficiency in E2 levels and increased immobility in the forced swim test compared to their wildtype littermates [25]. There are estrous cycle differences in depressive behavior, such that higher endogenous E2 levels during proestrus are associated with decreased immobility in the forced swim test of rats and mice [26] and [27]. Administration of E2 to young, ovariectomized rodents has effects to reduce anxiety and depressive-like behavior in a regimen-dependent manner [28] and [29]. Furthermore, ovariectomy can produce decrements in, and estrogen can enhance, the effects of some therapeutics for anxiety or depression measures in rodents [28]. Decline in cognitive and affective performance observed in aged female rats and mice can be reversed with systemic E2 administration [9] and [20]. Thus, these studies, and the present findings, suggest that E2 can have functional effects in young and aged male and female mice.
Although the present data contribute to the growing literature on androgens’ role for depression and affective responding, there are some issues that need to be addressed. First the sex difference that we observed in aged mice was somewhat unexpected given that young male mice typically have less depression-like behavior than do females in the forced swim test [16] and [27]. However, gender differences in depression, in which more women are diagnosed with depression than are men, are most evident in younger adults [30], and needs further investigation in aging populations. Second, although direct comparisons of young and aged mice were not done in the present study, future studies should directly investigate this further. Age-related differences in metabolism of androgens, as well as their receptor targets, may have contributed to the present findings. As well, an important question for further investigation is whether some of the subtle differences that we observed in the responses of males and females in the present study to androgen administration may have also been modulated by differences in length of hypogonadism. Similar to what occurs in people, hypogonadism with aging typically occurs earlier in life of female compared to male rodents. Third, the mechanisms of androgens’ anti-depressant-like effects remain to be determined. Unlike T and DHT, which bind cognate androgen receptors (ARs) with high avidity, 3α-diol is an allsoteric modulator of γ-aminobutyric acid (GABA) receptors, and may bind estrogen receptors (ERs), as does E2 [31], [32], and [33]. In the present study, we compared the effects of different androgens to begin to address the mechanisms for the anti-anxiety and anti-depressive effects. Interestingly, we found similar effects for all androgens suggesting that a likely target is ERs, specifically the β form of ER, which 3α-diol and E2 bind. Administration of ERβ ligands to young, gonadectomized rats and mice enhance object recognition and decrease anxiety in the elevated plus maze; however, these effects are not observed in ERβ knockout mice [14]. Furthermore, in young rats, the effects of E2 and 3α-diol for affective and cognitive behavior are attenuated with knock down of ERβ in the hippocampus [34] and [35]. Systematic studies in young and aged individuals are necessary to more thoroughly investigate the mechanisms of these effects.
Supplementation of gonadal steroids that are reduced with aging may be a potential therapeutic tool in the treatment of depression and other mood disorders. Steroid hormones can clearly have trophic effects, which may underlie their effects to increase neuroplasticity, changes of which have been associated with depression and its treatment [36], [37], and [38]. However, long-term studies of androgen administration would more clearly address this. As well, there are serious limitations to existing androgen- or E2-based therapies related to their potential to increase hormone-sensitive cancers. A strategy that can be utilized in this regard is to use androgen-based therapies with more specific mechanisms of action, such as ERβ, which may be protective in hormone-sensitive cancers [39] and [40] and is involved in steroids’ anti-anxiety and anti-depressant-like effects [41]. Thus, it is important to further investigate the effects of ERβ ligands, such as 3α-diol and E2, for functional processes in aging.
Acknowledgments
This research was supported, in part, by grants from the National Science Foundation and National Institute of Mental Health and intramural funding to CAF. Technical assistance, provided by Dr. Madeline Rhodes and Kanako Sumida, is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chapman DP, Perry GS. Depression as a major component of public health for older adults. Prev Chronic Dis. 2008;5:A22. [PMC free article] [PubMed] [Google Scholar]
- 2.Seidman SN. Testosterone deficiency and mood in aging men: pathogenic and therapeutic interactions. World J Biol Psychiatry. 2003;4:14–20. doi: 10.3109/15622970309167905. [DOI] [PubMed] [Google Scholar]
- 3.Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–20. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Rehman HU, Masson EA. Neuroendocrinology of female aging. Gend Med. 2005;2:41–56. doi: 10.1016/s1550-8579(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt PJ, Rubinow DR. Reproductive ageing, sex steroids and depression. J Br Menopause Soc. 2006;12:178–85. doi: 10.1258/136218006779160454. [DOI] [PubMed] [Google Scholar]
- 6.Zitzmann M. Testosterone and the brain. Aging Male. 2006;9:195–9. doi: 10.1080/13685530601040679. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–7. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 8.Pope HG, Jr, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160:105–11. doi: 10.1176/appi.ajp.160.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Walf AA, Frye CA. A review and update of: Mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morsink LF, Vogelzangs N, Nicklas BJ, Beekman AT, Satterfield S, Rubin SM, Yaffe K, Simonsick E, Newman AB, Kritchevsky SB, Penninx BW. Health ABC study, Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: results from the Health ABC study. Psychoneuroendocrinology. 2007;32:874–83. doi: 10.1016/j.psyneuen.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–60. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 12.Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–64. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- 13.Edinger KL, Frye CA. Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–30. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Edinger KL. Testosterone’s metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78:473–81. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm Behav. 2008;54:726–34. doi: 10.1016/j.yhbeh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumida K, Frye CA. Progesterone attenuates depressive behavior of young, old and progesterone receptor knock out mice. Journal of Psychopharmacology. 2008 in revision. [Google Scholar]
- 17.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 18.Perrot-Sinal TS, Kavaliers M, Ossenkopp KP. Spatial learning and hippocampal volume in male deer mice: relations to age, testosterone and adrenal gland weight. Neuroscience. 1998;86:1089–1099. doi: 10.1016/s0306-4522(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 19.Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008;33:1049–61. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res. 2005;1036:101–8. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–86. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 22.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177:245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 23.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–24. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–26. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 25.Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. Eur J Neurosci. 2004;20:217–28. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- 26.Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–15. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- 27.Walf AA, Koonce CJ, Frye CA. Adult female wildtype, but not ERβ knockout, mice have decreased depression-like behavior during proestrus and following administration of estradiol or diarylpropionitrile. J Psychopharmacol. 2008 doi: 10.1177/0269881108089598. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estrada-Camarena E, Fernández-Guasti A, López-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology. 2004;173:139–45. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- 29.Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- 30.Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham GR, Tindall DJ, Means AR. Differences in steroid specificity for rat androgen binding protein and the cytoplasmic receptor. Steroids. 1979;33:261–76. doi: 10.1016/0039-128x(79)90003-5. [DOI] [PubMed] [Google Scholar]
- 32.Gee KW. Steroid modulation of the GABA/benzodiazepine receptor-linked chloride ionophore. Mol Neurobiol. 1988;2:291–317. doi: 10.1007/BF02935636. [DOI] [PubMed] [Google Scholar]
- 33.Pak TR, Chung WCJ, Hinds LR, Handa RJ. Estrogen receptor-β mediates dihydrotestosterone-induced stimulation of arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148:3371–82. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- 34.Edinger KL, Frye CA. Androgens’ effects to enhance learning and memory may be mediated in part by actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walf AA, Ciriza I, Garcia-Segura LM, Frye CA. Antisense oligodeoxynucleotides for estrogen receptor-beta and alpha attenuate estradiol’s modulation of affective and sexual behavior, respectively. Neuropsychopharmacology. 2008;33:431–40. doi: 10.1038/sj.npp.1301416. [DOI] [PubMed] [Google Scholar]
- 36.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 37.Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–41. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 38.D’Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–94. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 39.Shupnik MA. Estrogen receptor-β: why may it influence clinical outcome in estrogen receptor-α positive breast cancer? Breast Cancer Res. 2007;9:107. doi: 10.1186/bcr1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugiura H, Toyama T, Hara Y, Zhang Z, Kobayashi S, Fujii Y, Iwase H, Yamashita H. Expression of estrogen receptor β wild-type and its variant ERβcs/β2 is correlated with better prognosis in breast cancer. Jpn J Clin Oncol. 2007;37:820–8. doi: 10.1093/jjco/hym114. [DOI] [PubMed] [Google Scholar]
- 41.Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


