Abstract
Metabotropic serotonin receptors such as 5-HT1A and 5-HT1B receptors shape the level, selectivity, and timing of auditory responses in the inferior colliculus (IC). Less is known about the effects of ionotropic 5-HT3 receptors, which are cation channels that depolarize neurons. In the current study, the influence of the 5-HT3 receptor on auditory responses in vivo was explored by locally iontophoresing a 5-HT3 receptor agonist and antagonists onto single neurons recorded extracellularly in mice. Three main findings emerge from these experiments. First, activation of the 5-HT3 receptor can either facilitate or suppress auditory responses, but response suppressions are not consistent with 5-HT3 effects on presynaptic GABAergic neurons. Both response facilitations and suppressions are less pronounced in neurons with high precision in response latency, suggesting functional differences in the role of receptor activation for different classes of neuron. Finally, the effects of 5-HT3 activation vary across repetition rate within a subset of single neurons, suggesting that the influence of receptor activation sometimes varies with the level of activity. These findings contribute to the view of the 5-HT3 receptor as an important component of the serotonergic infrastructure in the IC, with effects that are complex and neuron- selective.
Keywords: serotonin, neuromodulation, 5-HT3 receptor, inferior colliculus
INTRODUCTION
The neural processing of auditory stimuli incorporates information from many non-auditory sources. One of these is the serotonergic neuromodulatory system of the brain, which sends projections into many auditory structures along the neuraxis, including the IC (Campbell et al. 1987; DeFelipe et al. 1991; Klepper and Herbert 1991; Gil-Loyzaga et al. 1997, 2000; Thompson et al. 1994; Thompson and Schofield 2000; Thompson and Thompson 2001, Hurley and Thompson 2001). Serotonin influences ongoing sensory processing across different sensory modalities in a variety of ways, including changing receptive fields and altering the balance of inputs from different sources (Waterhouse et al. 1990; Hurley and Pollak 2001, 2005, Xiang and Prince 2003). These effects are tied to increasing serotonergic activity during elevated levels of behavioral arousal, including waking versus sleeping states and during stressful events (Trulson and Jacobs 1979, 1981; Boutelle et al. 1990; Mas et al. 1995; Clement et al. 1998). Serotonin also contributes to sensory plasticity, including the developmental establishment or adult refinement of sensory topographic maps (Bennett-Clarke et al. 1993; Roerig and Katz 1997; Butt et al. 2002; Maya-Vetencourt et al. 2008).
The inferior colliculus (IC) is densely innervated by serotonergic projections that originate mainly in the dorsal and median raphe nuclei (Klepper and Herbert 1991; Kaiser and Covey 1997; Hurley and Thompson 2001). Exogenously applied and endogenously released serotonin both have a range of effects on IC auditory responses including the contraction or expansion of frequency receptive fields and the alteration of temporal response properties (Hurley and Pollak 1999, 2001; Hall and Hurley 2007). Similar to other regions of the brain, variation in the expression of different types of serotonin receptors contributes to these effects. Members of at least 5 of the 7 major families of 5-HT receptor have been identified within the IC (Chalmers and Watson 1991; Pompeiano et al. 1992; Thompson et al. 1994; Waeber et al. 1994; To et al. 1995; Wright et al. 1995; Morales et al. 1998; Harlan et al. 2000; Peruzzi and Dut 2004). The most well-studied serotonin receptors in the IC are several metabotropic receptors in the 5-HT1A family, the 5-HT1A and 5-HT1B receptors. 5-HT1A receptors, which hyperpolarize neurons, decrease the general auditory responsiveness of many IC neurons, particularly those with long response latencies (Hurley 2006, 2007). In contrast, 5-HT1B receptors increase the auditory responses of many neurons, likely by decreasing the release of GABA (Hurley et al. 2008).
In contrast to these metabotropic receptors, which activate second messenger cascades, 5-HT3 receptors are ligand-gated cation channels structurally related to nicotinic acetylcholine receptors (Chameau and van Hooft 2006). Little is known about the role of ionotropic 5-HT3 receptors in the neural circuitry of the IC. In other regions of the brain, activation of 5-HT3 receptors results in rapid depolarization (Akasu et al. 1987). In addition to depolarizing neurons, 5-HT3 receptors also cause increases in intracellular calcium, either by passing Ca2+ ions directly, or by activating voltage-dependent calcium channels (Nichols and Mollard 1996; Rondé and Nichols 1998; Turner et al. 2004). As a result, 5-HT3 receptors may trigger or modulate events that outlast activation of the receptors themselves (Maeda et al. 1994; Alkadhi et al. 1996). 5-HT3 receptors also may selectively target GABAergic neurons or subtypes of GABAergic neurons, leading to the suppression rather than enhancement of neuron responses due to enhanced GABAergic transmission (Morales and Bloom 1997; Morales et al. 1998; Xiang and Prince 2003). Thus, activation of the 5-HT3 receptor could potentially have long-term effects in the IC or reduce evoked activity, despite this receptor acting as a cation channel.
The companion paper within this issue (Miko and Sanes) demonstrates that a train of pulses to the lateral lemniscal tract induces potentiation of the responses to direct current injection and synaptic stimulation in vitro in the IC. The 5-HT3 receptor can facilitate this potentiation, indicating that the receptor plays a role in short-term synaptic plasticity. Whether and how the 5-HT3 receptor influences auditory response properties in vivo, however, has not been examined. The goal of the current study was therefore to measure the effects of the 5-HT3 receptor on IC auditory responses through the iontophoretic application of a selective 5-HT3 agonist and complementary antagonists. We tested whether the 5-HT3 receptor affects the level of auditory responses, targets the inhibitory circuitry of the IC as it does in other brain regions, or differentially affects neurons with particular auditory response properties like the 5-HT1A receptor does. We find that the effects of 5-HT3 activation are complex, are less pronounced in neurons with high precision in latency and spike number, and are correlated with the stimulus repetition rate in a subset of neurons.
METHODS
Animals and surgery
158 single neurons were recorded from 47 male CBA/J mice ranging from 5–10 weeks of age. 5-HT3 receptors increase steeply before P21 in some regions of the brain and spinal cord but are close to adult levels at birth in others (Rosenberg et al. 1997; Choi et al. 2007). Prior to surgery, mice were briefly anesthetized by exposure to isoflurane fumes for the intraperitoneal injection of a mixture of 120 mg/kg of ketamine and 5 mg/kg of xylazine. After the achievement of surgical anesthesia as assessed by lack of response to tail and toe pinch and the removal of hair on the top of the head with a depilatory cream, an incision of approximately 1.5 cm was made in the skin along the midline of the head. The skin was reflected to each side and the surface of the skull cleared of adherent tissue. Bilateral holes of approximately 1 mm in diameter were drilled in the skull above the IC, the dura was incised with a sharpened tungsten probe, and the holes were covered with a surgical-grade silicon gel to prevent drying. A layer of glass beads and cyanoacrylate glue was applied to the skull anterior to lambda, and the mouse transferred to a sound attenuated chamber and placed in a custom stereotaxic device (Schuller et al. 1986), where a post was affixed to the skull between bregma and lambda with dental cement. Body temperature was maintained between 36 and 37 °C with a temperature regulation system (FHC; Bowdoinham, ME). During the experiment, the level of anesthesia was maintained with supplemental doses of 1/5 of the pre-surgical doses of the anesthetic mixture.
Electrodes and recording procedures
Extracellular recordings of single neurons were made and drugs iontophoretically administered through combination electrodes in ‘piggyback’ configuration (Havey and Caspary 1980). Briefly, tribarreled pipettes were pulled (Stoelting 51210; Wood Dale, IL) and broken back to a tip diameter of 10–15 μm, then attached to single-barreled recording pipettes (Flaming-Brown P-97; Sutter Instrument Co., Novato, CA) so that the tip of the recording pipette protruded 10–20 μm from the tip of the tribarreled pipette (single electrode blanks: 6010, tribarreled blanks: 6090; A-M systems, Carlsborg, WA). The three barrels of the tribarreled iontophoresis pipette allowed the administration of two drugs during each experiment, with one barrel reserved to eject balancing current. Pipettes were connected by a silver-silver chloride wire to a Dagan 2400 amplifier (Minneapolis, MN). These combination electrodes were positioned above the IC under visual control through a dissecting microscope and lowered with a piezoelectric microdrive (Burleigh/EXFO inchworm, Mississauga, Ontario) until action potentials were observed. The resistance of the recording electrode (8–20 MΩ when filled with 1M NaCl) allowed single neurons to be recorded. Recorded spikes had signal: noise ratios of 10 or more and could be ‘killed’ by the injection of small amounts of current at the end of recording. Neurons that did not meet these criteria were rejected from the database. Spikes were fed through a spike signal enhancer (FHC; Bowdoinham, ME) before being digitized through a data acquisition processor board (Microstar; Bellevue, WA). Data was collected and stored for later analysis by the software package Batlab (Dr. Donald Gans, Kent State University).
Auditory stimuli
Batlab was also used to generate auditory stimuli. Stimuli consisted of tones and frequency modulated (FM) sweeps. Tones were presented at the characteristic frequency (CF) of each neuron, and were 20 ms in duration with .5 ms rise-fall time. FM sweeps were centered at CF, extended from 5 kHz above to 5 kHz below CF, and were 20 ms in duration. For a given neuron, the stimulus evoking the strongest response was used to test drug effects. To generate rate-level functions, stimuli were varied in intensity from 10 dB below to 30–50 dB above threshold. Stimuli were routinely presented at a rate of 4 Hz, but the repetition rate for the presentation rate of tones was varied from .25 to 7 Hz in a subset of neurons. Stimuli were fed through a PA5 attenuator and FT-6 antialias filter (TDT; Alachua, FL). Stimuli were played through either an earphone biased with 200 V DC (Schuller 1997), positioned in the ear contralateral to the recording electrode, or a midline freefield speaker (Infinity Emit B, Harman International Industries; Woodbury, NY). The frequency response of the custom-made earphone was flat ± 6 dB from 10 to 120 kHz, with harmonic distortions at least 34 dB below the fundamental frequency. Calibration of the freefield speaker was accomplished by placing a measuring microphone (ACO Pacific PS9200 kit; Belmont, CA) in the position occupied by the mouse’s head during experiments. The response of the freefield speaker was flat within ± 6 dB from 13-–40 kHz, but produced a higher intensity of sound at lower frequencies.
Drug application
The responses of neurons to auditory stimuli were collected before, during, and after the iontophoresis of different drugs. Drugs included the selective 5-HT3 receptor agonist m-chlorophenylbiguanide hydrochloride (mCPBG, Kilpatrick et al. 1990; Sepúlveda et al. 1991, Tocris Bioscience, Ellisville, MO), the highly selective and potent 5-HT3 receptor antagonists ondansetron and Y- 25130 (Gyermek et al. 1995; Fukuda et al. 1992, Tocris Bioscience, Ellisville, MO), and the GABAA receptor antagonists bicuculline methiodide (Sigma-Aldrich Corp., St. Louis, MO) and gabazine (Tocris Bioscience, Ellisville, MO). All drugs were dissolved at 10 mM in 200 mM NaCl, pH 4.5. This vehicle solution does not alter the responses of IC neurons when applied alone (Hurley and Pollak 1999, 2001). Drugs were retained in the tribarreled pipette with a negative current of 10–20 nA, and ejected with positive currents of 10–90 nA (Dagan ION-100; Minneapolis, MN). After the collection of control data, drugs were ejected until a stable response was achieved or for 10 minutes, and a comparable dataset was then collected. Drug effects were monitored over time by the rapid repetition of a single stimulus in sets of 32 presentations, in order to closely monitor the timecourse of drug effects. The same procedure was repeated to measure the recovery of neurons from the drugs.
Analysis
Spike counts were extracted in Batlab and exported in ascii format to Excel (Microsoft Corp; Redmond, WA). Response magnitudes were expressed as either total spike counts in response to 32 stimulus repetitions, or as the number of spikes per stimulus ± the standard error of the mean (s.e.m.), as indicated in the text. Neurons that varied in spike count by 20% or more before drug application were rejected from the dataset. The onset time of the effects of mCPBG were measured as the first measured time point exceeding half of the peak effect of mCPBG. One-way ANOVAs with Fisher’s LSD posthoc tests were used to analyze the effects of different drug treatments on the spike counts of single neurons, and 2-tailed unpaired t-tests were used to assess the effect of single drugs on spike counts and to compare drug-evoked changes in different categories of neurons. Pearson’s product-moment correlations were used to assess correlations between spike rates and the effects of mCPBG.
All procedures were approved by the Bloomington Institutional Animal Care and Use Committee.
RESULTS
mCPBG increases or decreases responses to auditory stimuli in different neurons
To determine the effects of activation of the 5-HT3 receptor on the auditory responses of IC neurons in vivo, 158 single neurons in male CBA/J mice of 5–10 weeks of age were recorded before, during, and after iontophoretic application of the selective 5-HT3 receptor agonist, mCPBG. Neurons were recorded at depths ranging from 157 to 1569 μm (average = 825 μm), and demonstrated characteristic frequencies of 6 to 42 kHz (average = 17 kHz) and mean first spike latencies of 6.7 to 42.1 ms (population average = 19.5 ms). These values are comparable to those previously recorded from mouse IC (Portfors and Felix 2005; Ehret et al. 2003). Seventy-two neurons responded preferentially to CF tones, and 86 preferred FM sweeps of 20 ms in duration with a 10 kHz bandwidth centered at CF. Consistent with its role as a ligand-gated cation channel, mCPBG increased the evoked responses of some neurons. An example is presented in the upper plot of figure 1A, the response of a single neuron to an FM sweep centered at 23 kHz before, during, and after the application of mCPBG. Values are the average number of spikes per stimulus ± the s.e.m. calculated from 32 repeated presentations of the tone. Soon after the onset of drug application, the response of the neuron increased. Peristimulus time histograms (PSTHs) at representative time points in the control, during iontophoresis, and after the recovery illustrate that this neuron added spikes to the latter half of its spike train in response to mCPBG. Activation of the 5-HT3 receptor also decreased evoked responses in many neurons. An example of such a neuron is shown in the lower plot of figure 1A. Soon after mCPBG application, this neuron decreased its average response to an FM sweep centered at 21 kHz by approximately two-thirds and its response also recovered rapidly after drug application stopped.
Figure 1.
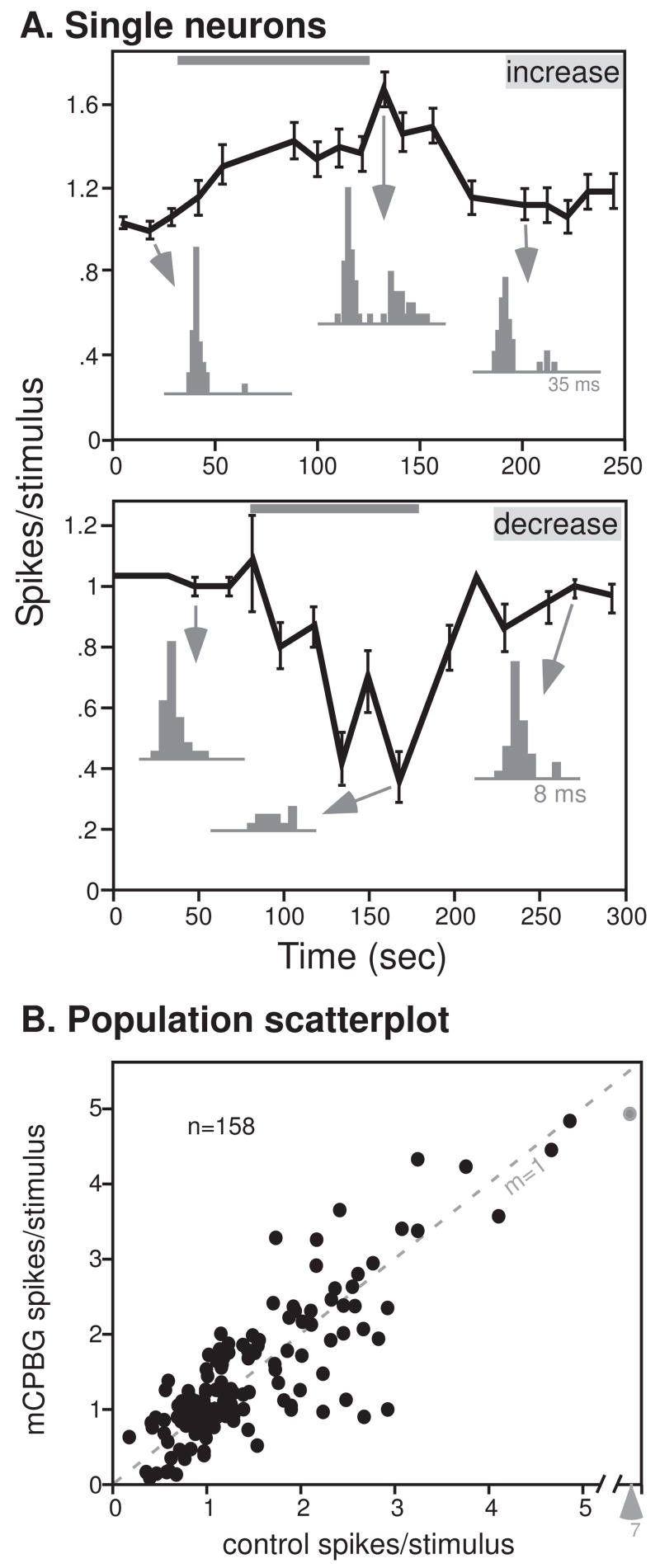
5-HT3 activation has mixed effects. A. 5-HT3 application increases evoked responses for some neurons. Plot of the average spikes/stimulus (± s.e.m.) for a single neuron before, during, and after the application of mCPBG (gray bar). PSTHs are of the time points indicated by arrows. The stimulus evoking spikes consisted of an FM sweep with a center frequency of 23 kHz. B. 5-HT3 application decreases evoked responses for some neurons. The stimulus evoking spikes consisted of an FM sweep with a center frequency of 21 kHz. Conventions are as in Fig. 1A. C. Effects of 5-HT3 activation on 158 neurons, plotted as the total spike count in response to 32 stimulus presentations of FM sweeps (n = 86) or CF tones (n = 72) in the control versus during mCPBG application. The dashed line with a slope of 1 marks no change in spike count.
Figure 1B plots the number of spikes per stimulus during the control versus mCPBG application in all 158 neurons recorded. Although neurons with high average spike counts in the control also tended to have high average spike counts during mCPBG iontophoresis, many neurons deviated from the dashed line with a slope of one, indicating that mCPBG changed their average spike counts. Of 82 neurons showing changes in spike count of 20% or more, 37 responded to mCPBG with an increase in spike count, and 45 with a decrease in spike count. Spike count decreases were no larger than spike count increases on average (53.4% increase versus 42.9% change for neurons with spike count changes of 20% or more, p =.20, 2-tailed unpaired t-test). Whether FM sweeps or CF tones were used as stimuli did not significantly alter the effects of mCPBG, either when decreases in spike count were represented by negative values (p =.36, 2-tailed unpaired t-test), or when the absolute values of changes in spike count were used to compare the effect sizes (p =.25, 2-tailed unpaired t-test).
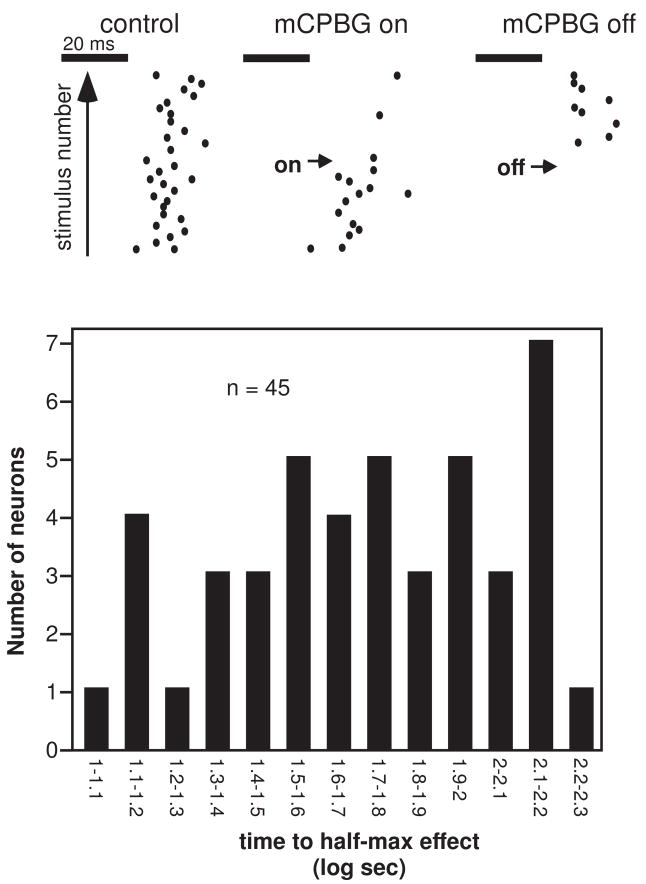
The effects of mCPBG were relatively rapid in some neurons. An example is presented in Figure 2A, a set of 3 dot raster plots depicting a neuron’s response to 32 presentations of a 10 kHz FM sweep centered at 28 kHz in the control, during a time period in which mCPBG application was initiated (‘mCPBG on’), and during a time period in which mCPBG application was halted (‘mCPBG off’). The neuron responded rapidly to mCPBG, dramatically decreasing its firing and recovering soon after drug application was stopped. Both the response and recovery occurred within 500 ms (two stimulus repetitions at a 4 Hz repetition rate). For the majority of neurons that responded with smaller changes in spike count to mCPBG application than the neuron in figure 2A, responses were summed over all repetitions in a 32-stimulus test in order to determine whether mCPBG had any effect. As a consequence, the limit of resolution of the timecourse of mCPBG-evoked changes was 8 sec (32 repetitions/4Hz per repetition) plus the time needed to trigger the collection of additional data. The resulting histogram of the time to the half-maximum effect (Fig. 2B) shows that both rapid and slower changes in spike count occurred, ranging from 10 to over 150 seconds. The time courses for neurons responding to mCPBG with spike count increases versus decreases were not different from each other, however (68.05 ± 8.94 sec for spike count increases versus 56.57 ± 8.45 sec for spike count decreases, p =.36, 2-tailed unpaired t-test).
Figure 2.
Timecourse of effects of mCPBG. A. A single neuron exhibiting a rapid effect of mCPBG. Raster dots indicate individual spikes in response to single stimulus presentations, with sequential responses presented above each other. The stimulus consisted of a 10 kHz FM sweep with a center frequency of 28 kHz, presented at a rate of 4 Hz. B. Histogram of the time to the half-maximum effect of mCPBG on spike count for 45 neurons responding to mCPBG application with a change of 25% or greater in spike count.
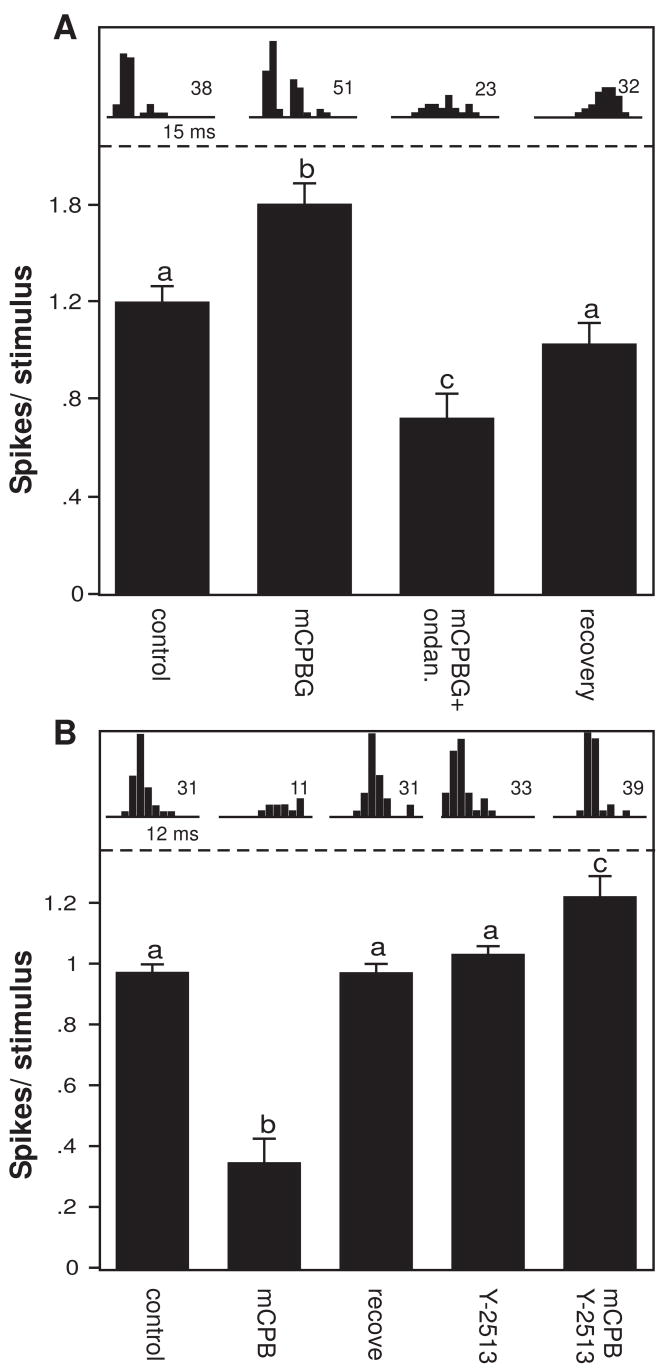
Both the increases and decreases in spike count evoked by mCPBG were blockable by 5-HT3 receptor antagonists in some neurons. We tested the effects of two antagonists, ondansetron and Y-25130, in a population of 29 neurons (n = 17 for ondansetron and n = 12 for Y-25130). For 24 of these, the antagonists were applied by themselves as well as in combination with mCPBG; in 22, the antagonist alone had no significant effect on the average response per stimulus, although ondansetron did significantly change the response in two neurons. A neuron for which ondansetron blocked an mCPBG-evoked increase in spikes is shown in figure 3A. mCPBG alone significantly increased the average number of spikes fired per stimulus, and the addition of ondansetron not only abolished this increase, but reduced the spike number to a lower level than the control (one-way ANOVA, p <.001 for the overall model, Fisher’s LSD posthoc tests as indicated by letters). After the application of all drugs were stopped, the neuron partially recovered. This pattern of drug effects is consistent with the endogenous activation of the 5-HT3 receptor, because the addition of the antagonist decreased spikes below even the control level. For a second neuron (Fig. 3B), mCPBG decreased the average spike count relative to the control, and the spike count recovered after drug application was stopped. The 5-HT3 antagonist Y-25130 did not alter the spike count alone, but prevented the mCPBG-evoked decrease when the agonist and antagonist were presented together (one-way ANOVA, p <.001 for the overall model, Fisher’s LSD posthoc tests as indicated by letters). For this neuron, the combination of the agonist and antagonist resulted in a small but significant increase in the spike count. Within the entire group of 29 neurons, 13 responded with significant changes in spike count to mCPBG, and of these, the antagonists significantly decreased the effect of mCPBG in 7 (53.8%, 2-tailed unpaired t-tests), did not significantly change the effect of mCPBG in 4 (30.8%), and significantly facilitated the effect of mCPBG in 2 (15.4%). The iontophoretic application of these selective 5-HT3 receptor ligands makes an assessment of their concentration, and thus their pharmacological selectivity, difficult in our in vivo preparation. However, the blockade of the effects of the agonist in at least some of the neurons supports the 5-HT3 receptor as a target for these agents.
Figure 3.
Effects of 2 selective 5-HT3 receptor antagonists. A. Average spike counts (± s.e.m.) for a single neuron in the control and during mCPBG application, application of both mCPBG and ondansetron, and recovery. Letters indicate the results of a Fisher’s LSD posthoc test for an ANOVA. mCPBG significantly increased the response relative to the control, and ondansetron significantly reduced the effect of mCPBG. The stimulus consisted of an FM sweep with a center frequency of 30 kHz. B. Average spike counts (± s.e.m.) for a single neuron during the control, mCPBG application, a recovery, application of Y-25130 alone, and application of both mCPBG and Y-25130. Conventions for posthoc tests are as in Fig. 3A. The stimulus consisted of an FM sweep with a center frequency of 21 kHz.
Do mCPBG-evoked decreases in response occur through a GABAergic mechanism?
GABAergic neurons are targets of 5-HT3 receptor activation in hippocampus, amygdala, and some sensory cortices (Ropert and Guy 1991; Morales and Bloom 1997; Morales et al. 1998; Koyama et al. 2000, 2002; Xiang and Prince 2003; Choi et al. 2007). The IC also contains numerous intrinsic GABAergic neurons that could inhibit the evoked responses of neurons we recorded (Oliver et al. 1994). To test whether the presynaptic depolarization of these intrinsic GABAergic neurons could account for mCPBG-evoked response suppressions in the IC, we applied both mCPBG and the GABAA antagonists bicuculline (n = 14) or gabazine (n = 3) to a set of 17 neurons. If 5-HT3 receptors depolarize presynaptic GABAergic neurons in the IC, then blockade of GABAA receptors should reduce the suppressive effects of mCPBG. Two of the neurons tested in this way are illustrated in figure 4, in which bars represent the average spike count ± s.e.m. in the control, the presence of mCPBG, and during the recovery, either in the control baseline (black bars), or in a baseline of bicuculline (gray bars). PSTHs above each bar depict the total spike count and the pattern of spikes in each condition. For one neuron (Fig. 4A), mCPBG significantly reduced the average spike count, which recovered partially after drug application stopped (ANOVA, df = 2, 31; p <.001 for the overall model, Fisher’s LSD posthoc tests as indicated by letters). Although bicuculline significantly increased the baseline response of the neuron, mCPBG had a similar pattern of effects in the control and during bicuculline application, significantly decreasing the average spike count relative to the bicuculline baseline and recovering partially after mCPBG iontophoresis. A second neuron (Fig. 4B) also showed a small but significant decrease in average spike count in the presence of mCPBG in the control (ANOVA, df = 2, 31; p <.001 for the overall model, Fisher’s LSD posthoc tests as indicated by letters). Interestingly, not only did bicuculline fail to block this decrease, but the effect of mCPBG was also proportionally greater in the presence of bicuculline.
Figure 4.
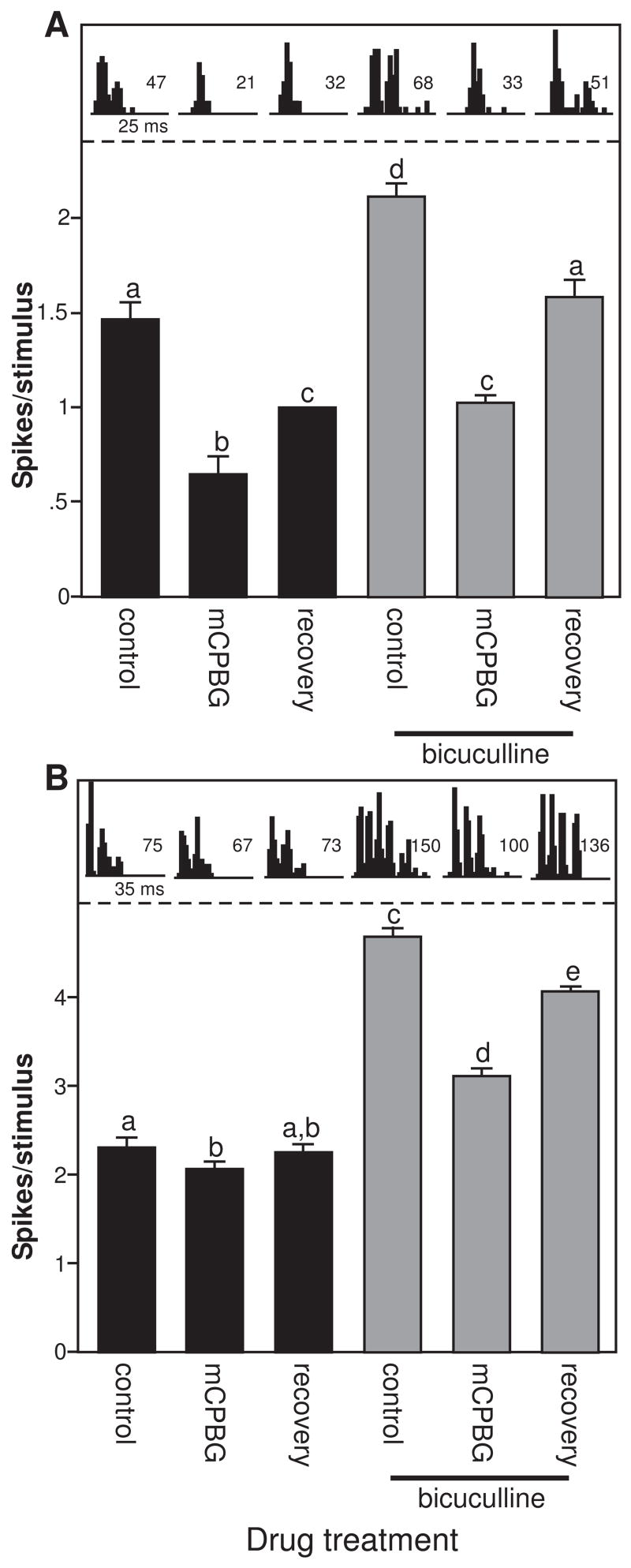
Bicuculline does not block the effects of mCPBG. A. Average spike counts (± s.e.m.) for a single neuron in the control and during mCPBG application and recovery (black bars), and during these same 3 conditions during bicuculline application (gray bars). Parallel PSTHs are taken from the same conditions. Conventions for posthoc tests are as in Fig. 3A, so that mCPBG significantly reduces spike counts in the control and during bicuculline application. The stimulus consisted of an FM sweep with a center frequency of 25 kHz. B. Responses of a second neuron during the same drug treatments as in Fig. 5A. mCPBG significantly decreased spike counts in the control and during bicuculline application, but ha a larger proportional effect during bicuculline application. The stimulus consisted of an FM sweep with a center frequency of 18 kHz.
Within the entire group of 17 neurons, 10 were recorded for long enough that mCPBG and GABAA antagonists were applied alone as well as in combination. GABAA blockade did not significantly change the effects of mCPBG in 7 of these neurons. GABAA blockade proportionally increased the mCPBG-evoked suppressions in average spike count in two neurons, and significantly reduced the effect of mCPBG in only one neuron. These experiments suggest that blockade of GABAA receptors does not consistently reduce the effects of mCPBG application in this subset of neurons.
mCPBG disproportionately affects a subpopulation of neurons with high response variance and high CF
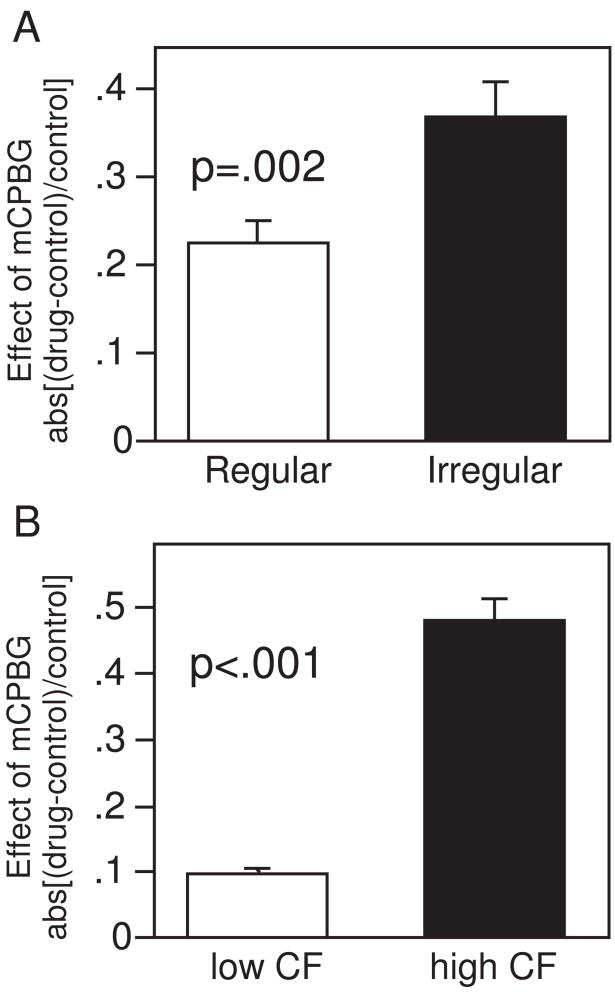
Because serotonin receptors, including the 5-HT1A receptor within the IC, often selectively affect particular subpopulations of neurons (Xiang and Prince 2003; Choi et al. 2007; Hurley 2007), we explored whether the same was true of the 5-HT3 receptor. Specifically, because neurons with regular latencies in response to tones or FM sweeps seemed to show less of an effect of mCPBG, we assessed whether mCPBG discriminated among neurons with regular versus irregular firing patterns. To do this, we divided the neuron population in half based on the variability in the latencies of the initial spikes in response to 32 repetitions of the same stimulus. ‘Regular’ neurons, with low variability in the first-spike latency, had an average variance of .50 ms and ‘irregular’ neurons, with high variability in the first-spike latency, had an average variance of 12.94 ms (n = 79 each). This division did not correspond to whether tones versus FM sweeps were used as stimuli, since responses to tones and FM sweeps showed no significant difference in the variance of their first-spike latencies (p =.77, 2-tailed unpaired t-test). Regular and irregular neurons also differed in a number of other ways. Although both groups of neurons fired a similar number of spikes per stimulus, regular neurons showed greater consistency in the spike number per stimulus, with an average variance in spike count of .19 ±.02 versus .35 ±.03 for irregular neurons (p <.001, 2-tailed unpaired t-test). Most interestingly in terms of the 5-HT3 receptor, when mCPBG-evoked changes in spike counts were compared between the two groups, regular neurons exhibited significantly less of a response to mCPBG (Fig. 5; p =.002, 2-tailed unpaired t-test; Fig. 5A). Regular neurons showed proportional changes in spike count (absval[(drug-control)/control]) of .23 ±.02 on average, compared to changes of .37 ±.04 for irregular neurons. Thus, neurons with firing patterns that are relatively regular in latency and number are significantly less responsive to mCPBG than are neurons with less regular firing patterns.
Figure 5.
Average effects of mCPBG differ for different populations of neurons. Effects of mCPBG are measured as the proportional change in spike count relative to the control abs([drug-control]/control). Absolute values of these proportional changes are compared, so that increases and decreases in response do not cancel each other. P values refer to the outcome of two-tailed unpaired t-tests. A. ‘Regular’ neurons, with relatively low variance in spike latency and number, are less affected than ‘irregular’ neurons. B. Neurons with relatively low CFs are less affected than neurons with higher CFs.
To determine whether the 5-HT3 receptor has effects that vary regionally or tonotopically within the IC, we also tested whether the effects of mCPBG corresponded to either the depth at which neurons were recorded or their CF. The effect of mCPBG on evoked response was not correlated with depth (r =.018, p =.827), but was weakly correlated with CF (r =.200, p =.012), so that neurons with higher CFs also had larger effects of mCPBG on spike count [absval(drug-control)/control]. The correlation between the effect of mCPBG and CF only existed for the size and not the direction of the effects of mCPBG. When the population of neurons was simply divided in half around the median CF of 16 kHz (range = 6 to 42 kHz), then the mCPBG-evoked change in spike count was much greater in the neurons with higher CFs than with lower CFs (.48 ±.03 for above-median CFs and .10 ±.01 for below-median CFs; p <.001, 2-tailed unpaired t-test; Fig. 5B). This difference did not exist for neurons above versus below the median values of depth (p =.999, 2-tailed unpaired t-test).
Effects of mCPBG are activity-dependent in some neurons
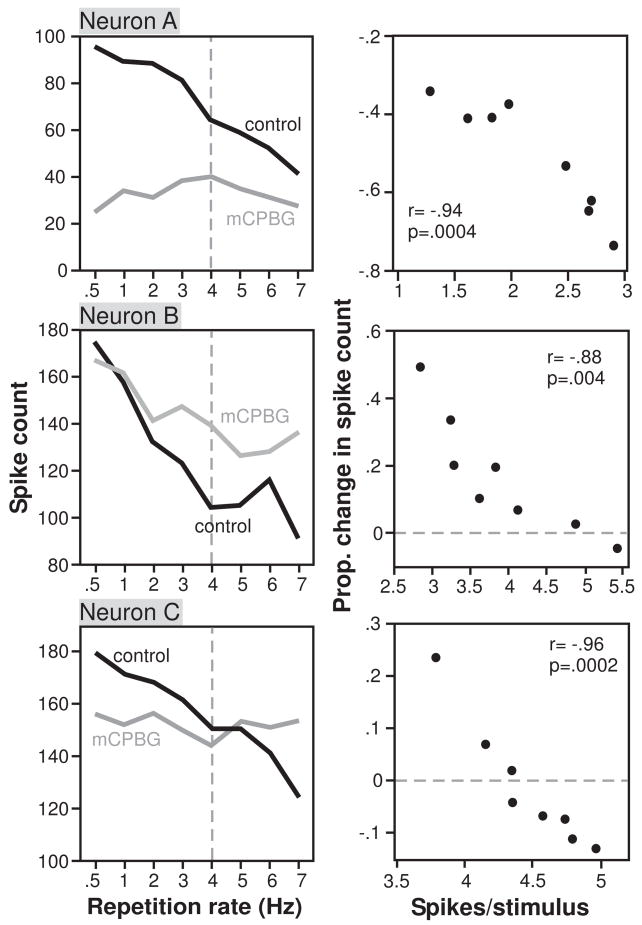
Most stimuli were presented at a standard repetition rate of 4 Hz. In a subset of 22 neurons, however, the repetition rate was varied from 0.5 to 7 Hz. Similar to IC neurons recorded in previous studies (Willott and Demuth 1986; Chen and Jen 1994; Jen et al. 2002; Bibikov et al. 2008), neurons often decreased their firing rates as repetition rates increased. We therefore compared the proportional effects of mCPBG to the average spike count per stimulus (‘spike rate’; Fig 6, right column) in addition to the repetition rate itself (Fig 6, left column). For each of the three neurons of Figure 6, mCPBG had different effects at different stimulus repetition rates and spike rates. For Neuron A, mCPBG suppressed the response most strongly at low repetition rates, where spike rates were highest. For Neuron B, mCPBG facilitated the response most strongly at high repetition rates, where spike rates were lowest. For Neuron C, mCPBG crossed from suppressing the response at low repetition rates/high spike rates, to facilitating the response at the highest repetition rates/lowest spike rates. Despite this difference in the specific effects among neurons, however, mCPBG had the same qualitative effects on the neurons. Across different repetition rates (left column), the net effect of mCPBG was to flatten the response functions of all 3 neurons, so that the responses were more equal at different repetition rates during mCPBG application than in the control. With increasing spike rates (right column), mCPBG either increased the suppression of responses (Neuron A), decreased the facilitation of responses (Neuron B), or both (Neuron C), so that the relationship between the proportional effect of mCPBG relative to the control and the spike rate was significantly correlated (Pearson’s correlations, p <.01 for all neurons) and had a negative slope in all 3 neurons. For 10 of the sample of 22 neurons tested with different repetition rates, the size of the effect of mCPBG was significantly correlated with the spike rate (Pearson’s product moment correlations, p <.05). Of these 10 neurons, 8 had the same type of inverse relationship between spike count and the effects of mCPBG as Neurons AC.
Figure 6.
Examples of 3 neurons for which the effects of mCPBG varied depending on the stimulus repetition rate. A. Left plot shows the total spike count for 32 stimulus repetitions, during the control and the application of mCPBG as a function of the stimulus repetition rate, in Hz. Right plot shows the proportional change in spike count evoked by mCPBG versus the average number of spikes per stimulus. The stimulus consisted of a 20 ms tone at 16 kHz. For this neuron, mCPBG decreased the response less at higher repetition rates, corresponding to lower spike rates. B. Similar plots for a second neuron using a stimulus consisting of a 20 ms tone at 12 kHz. For this neuron, mCPBG facilitated the response more at high repetition rates, corresponding to lower spike rates. C. Similar plots for a third neuron using a stimulus consisting of a 20 ms tone at 13 kHz. For this neuron, mCPBG suppressed the response at low repetition rates (high spike rates), and facilitated the response at high repetition rates (low spike rates).
DISCUSSION
5-HT3 receptors are the only ionotropic serotonin receptors, and their activation results in the rapid depolarization of neurons (Akasu et al. 1987; Chameau and van Hooft 2006). Despite this, locally activating the 5-HT3 receptor in the IC in vivo not only facilitated but sometimes also suppressed auditory responses. In the following discussion we address several different mechanisms that could account for this dual effect of 5-HT3 activation, and the potential consequences of receptor activation for auditory responses in behaving animals.
Mechanism of 5-HT3 receptor effects in vivo
Using in vitro recordings, the companion study established that activation of the 5-HT3 receptor depolarizes baseline membrane potentials of IC neurons, as it does for neurons in other brain regions (Miko and Sanes). The companion study also demonstrated that the 5-HT3 receptor contributes to enhanced responses to synaptic stimulation or direct current injection. These findings are consistent with our in vivo observations of facilitated auditory responses during 5-HT3 receptor activation. However, the suppression of responses that we also often observed when we activated the 5-HT3 receptor requires additional explanation. One way for activation of the 5-HT3 receptor to evoke suppression rather than facilitation of auditory responses would be to depolarize GABAergic neurons presynaptic to the neurons being recorded. In regions such as the hippocampus and amygdala, GABAergic neurons are indeed major targets of the 5-HT3 receptor (Ropert and Guy 1991; Morales and Bloom 1997; Morales et al. 1998; Koyama et al. 2000, 2002; Choi et al. 2007). To assess whether this was the case for intrinsic GABAergic neurons in the IC, we tested whether blocking the GABAA receptor with bicuculline reduced the 5-HT3-evoked suppression of spikes in our experiments, as would be expected if the 5-HT3 receptor depolarizes presynaptic GABAergic neurons. The lack of effect of bicuculline on the mCPBG-induced decrease in spikes suggests that mCPBG was not acting on presynaptic GABAergic inputs for these neurons, although an increase in the overall response level by bicuculline methiodide could result from a direct blockade of calcium-activated potassium channels (Johnson and Seutin 1997; Debarbieux et al. 1998). The number of neurons tested in this experiment was relatively small, but if this finding can be extrapolated to the larger neuron population, it makes the depolarization of GABAergic neurons an unlikely pathway for the suppressive effects of the 5-HT3 receptor in the IC.
The lack of a strong association between the 5-HT3 receptor and GABAergic neurons means that our data do not support a particular mechanism for 5-HT3 receptor-induced suppressions in the response to auditory stimuli. However, two features of this receptor make multiple other mechanisms compatible with our findings. These are that the 5-HT3 receptor admits calcium into neurons, and that the receptor can increase calcium in presynaptic terminals as well as acting postsynaptically (Kidd et al. 1993; Nichols and Mollard 1996; van Hooft and Vijverberg 2000; Chameau and van Hooft 2006; Choi et al. 2007). For example, a presynaptic increase in calcium entry either directly through the 5-HT3 receptor or via voltage-dependent calcium channels could result in synaptic depression (Xu et al. 2007). Postsynaptically, calcium entry could trigger mechanisms leading to hyperpolarization and a smaller auditory response, such as the activation of calcium-dependent potassium channels, several types of which are expressed by IC neurons (Sivaramakrishnan and Oliver 2001). Thus, the mechanisms of the ionotropic 5-HT3 receptor could include indirect effects on voltage-gated ion channels or even a form of short-term plasticity.
Consequences of 5-HT3 receptor activation
The functional consequences of 5-HT3 activation are suggested by effects that are more pronounced in subpopulations of IC neurons possessing particular auditory response characteristics. The effects of 5-HT3 activation were more prevalent in neurons with higher variance in their first-spike latencies and spike numbers. Effects of the 5-HT3 agonist were also more common in neurons with above-median CFs. These patterns suggest that temporally precise neural responses to auditory stimuli, as well as responses to lower-frequency auditory signals, would be less affected by receptor activation.
One of the most pronounced type of variation in the effects of mCPBG was whether it evoked increases or decreases in auditory responses. Interestingly, this difference did not correspond to other distinctions among neuron groups, such as their location in the IC or response characteristics such as CF or latency. In fact, in a subset of neurons, receptor activation resulted in changes in spike count that were different in magnitude and even in direction within the same individual neurons, depending on the stimulus repetition rate. In the neurons that exhibited it, this stimulus dependence usually resulted in a decreased selectivity for repetition rate, with neurons firing more equally across a range of repetition rates during activation of the 5-HT3 receptor. Because we only tested a limited range of stimulus rates comparable to those used in previous mouse studies (0.5 to 10 Hz, Willott and Demuth 1986; Bibikov et al. 2008, it is unclear whether a larger range of repetition rates would reveal this type of effect in more neurons.
Although the direct activation of the 5-HT3 receptor with mCPBG bypasses its activation by the endogenous serotonergic system, these effects of 5-HT3 activation in a behaving animal should be tied to the release of serotonin in the IC. The IC receives much of its serotonergic innervation from neurons in the dorsal and median raphe nuclei, where rates of activity are generally tied to the level of behavioral arousal. Dorsal raphe neurons fire at a higher tonic level in awake than in asleep animals, and may also respond transiently to sensory stimuli (Trulson and Jacobs 1979, 1981; Rasmussen et al. 1986). When levels of serotonin have been measured in regions that are the targets of the dorsal raphe nucleus, they increase during waking and in response to sensory stimuli as well as in response to social stimuli or stressors such as physical restraint (Boutelle et al. 1990; Mas et al. 1995; Clement et al. 1993, 1998). Pilot studies have confirmed that serotonin levels increase in the IC during waking from anesthesia (Hall and Hurley 2007). Serotonin levels are therefore expected to be relatively low in anesthetized preparations such as the one in our study. This could potentially account for the failure of 5-HT3 antagonists to affect the responses of the majority of a subset of tested neurons when applied alone, despite exceptions such as the neuron in figure 3A.
A logical inference of these observations is that the 5-HT3 receptor would be increasingly activated in vivo during heightened levels of behavioral arousal. This type of change in behavioral state would therefore be associated with a complex set of changes in different subsets of neurons due to activation of the 5-HT3 receptor. Specifically, our data suggests that the role of the 5-HT3 receptor in vivo is to regulate activity most heavily in a subpopulation of neurons characterized by relatively low temporal precision and relatively high response frequency. Within this subpopulation, the effects of receptor activation would vary further between an increase and a decrease in gain. In some neurons, the rate-dependence of receptor activation would result in an equalization of activity levels for different repetition rates, compatible with a general arousal hypothesis of serotonin function. How this diversity of effects during behavioral arousal would influence the responses to the different vocalizations of mice, which incorporate both audible and ultrasonic frequencies and elements with different rates of repetition (Liu et al. 2003; Gourbal et al. 2004; Holy and Guo 2005; Portfors 2007), is unknown.
Comparison to companion study
Although both our study and the companion study within this issue (Miko and Sanes) present findings related to the role of the 5-HT3 receptor in the responses of IC neurons, the two studies differ in a number of methods. These include the use of in vitro versus in vivo preparations, of different species and individuals of different ages, of different types of stimuli to evoke responses, and of different pharmacological agents to activate or block the 5-HT3 receptor. Despite such methodological differences, there are a number of broad similarities in the findings of the two studies. Both studies conclude that the 5-HT3 receptor regulates the number of spikes, consistent with the 5-HT3 receptor controlling the gain of responses in the IC. Many of the effects of 5-HT3 activation in both studies are also consistent with the receptor acting as a depolarizing cation channel.
One of the most striking differences between the studies is the greater diversity of effects of receptor activation on auditory responses in vivo. The 5-HT3 receptor has unidirectional effects in vitro, causing only potentiation of the response to injected current, but can decrease as well as increase auditory responses in vivo. The mechanisms of this diversity of effects in vivo are not yet understood. However, it is clear that the 5-HT3 receptor does not simply drive activity in the IC in vivo, but performs a genuinely neuromodulatory role in regulating activity in the IC, despite being the only ionotropic class of serotonin receptor.
Acknowledgments
The authors would like to thank Kristin Harris for assistance in data analysis. These experiments were funded in part by National Institute of Deafness and Other Communication Disorders Grant DC-006608 plus supplement 02S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akasu T, Hasuo H, Tokimasa T. Activation of 5-HT3 receptor subtypes causes rapid excitation of rabbit parasympathetic neurones. Br J Pharmacol. 1987;91:453–455. doi: 10.1111/j.1476-5381.1987.tb11236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkadhi KA, Salgado-Commissariat D, Hogan YH, Akpaudo SB. Induction and maintenance of ganglionic long-term potentiation require activation of 5-hydroxytryptamine (5-HT3) receptors. J Physiol. 1996;496:479–789. doi: 10.1113/jphysiol.1996.sp021700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proc Natl Acad Sci U S A. 1993;90:153–7. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov NG, Chen QC, Wu FJ. Responses of inferior colliculus neurons to sounds presented at different rates in anesthetized albino mouse. Hear Res. 2008;241:43–51. doi: 10.1016/j.heares.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Boutelle MG, Zetterstrom T, Pei Q, Svensson L, Fillenz M. In vivo neurochemical effects of tail pinch. J Neurosci Methods. 1990;34:151–7. doi: 10.1016/0165-0270(90)90053-i. [DOI] [PubMed] [Google Scholar]
- Butt CM, Zhao B, Duncan MJ, Debski EA. Sculpting the visual map: the distribution and function of serotonin-1A and serotonin-1B receptors in the optic tectum of the frog. Brain Res. 2002;931:21–31. doi: 10.1016/s0006-8993(01)03370-4. [DOI] [PubMed] [Google Scholar]
- Campbell M, Lewis D, Foote S, Morrison J. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta-hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987;261:209–220. doi: 10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- Chalmers D, Watson S. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain--a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- Chameau P, van Hooft JA. Serotonin 5-HT (3) receptors in the central nervous system. Cell Tissue Res. 2006;326:573–581. doi: 10.1007/s00441-006-0255-8. [DOI] [PubMed] [Google Scholar]
- Chen QC, Jen PH. Pulse repetition rate increases the minimum threshold and latency of auditory neurons. Brain Res. 1994;654:155–158. doi: 10.1016/0006-8993(94)91582-2. [DOI] [PubMed] [Google Scholar]
- Choi IS, Cho JH, Kim JT, Park EJ, Lee MG, Shin HI, Choi BJ, Jang IS. Serotoninergic modulation of GABAergic synaptic transmission in developing rat CA3 pyramidal neurons. J Neurochem. 2007;103:2342–2353. doi: 10.1111/j.1471-4159.2007.04945.x. [DOI] [PubMed] [Google Scholar]
- Clement HW, Schafer F, Ruwe C, Gemsa D, Wesemann W. Stress-induced changes of extracellular 5-hydroxyindoleacetic acid concentrations followed in the nucleus raphe dorsalis and the frontal cortex of the rat. Brain Res. 1993;614:117–24. doi: 10.1016/0006-8993(93)91024-m. [DOI] [PubMed] [Google Scholar]
- Clement HW, Kirsch M, Hasse C, Opper C, Gemsa D, Wesemann W. Effect of repeated immobilization on serotonin metabolism in different rat brain areas and on serum corticosterone. J Neural Transm. 1998;105:1155–70. doi: 10.1007/s007020050119. [DOI] [PubMed] [Google Scholar]
- Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of IAHP in reticularis neurons. J Neurophysiol. 1998;79:2911–2918. doi: 10.1152/jn.1998.79.6.2911. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry HSC, Hashikawa T, Jones EG. Synaptic relationships of serotonin-immunoreactive terminal baskets on GABA neurons in the cat auditory cortex. Cerebral Cortex. 1991;1:117–133. doi: 10.1093/cercor/1.2.117. [DOI] [PubMed] [Google Scholar]
- Ehret G, Egorova M, Hage SR, Muller BA. Spatial map of frequency tuning-curve shapes in the mouse inferior colliculus. NeuroReport. 2003;14:1365–1369. doi: 10.1097/01.wnr.0000078545.07662.85. [DOI] [PubMed] [Google Scholar]
- Gil-Loyzaga P, Bartolome M, Vicente-Torres M. Serotonergic innervation of the organ of Corti of the cat cochlea. Neuroreport. 1997;8:3519–3522. doi: 10.1097/00001756-199711100-00020. [DOI] [PubMed] [Google Scholar]
- Gil-Loyzaga P, Bartolomé V, Vicente-Torres A, Carricondo F. Serotonergic innervation of the organ of Corti. Acta Otolaryngol. 2000;120:128–132. doi: 10.1080/000164800750000757. [DOI] [PubMed] [Google Scholar]
- Gourbal BEF, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalizations of adult male and female BALB/c mice. Naturwissenschaften. 2004;91:381–385. doi: 10.1007/s00114-004-0543-7. [DOI] [PubMed] [Google Scholar]
- Gyermek L. 5-HT3 receptors: pharmacologic and therapeutic aspects. J Clin Pharmacol. 1995;35:845–855. doi: 10.1002/j.1552-4604.1995.tb04129.x. [DOI] [PubMed] [Google Scholar]
- Hall IC, Hurley LM. The serotonin releaser fenfluramine alters the auditory responses of inferior colliculus neurons. Hearing Research. 2007;228:82–94. doi: 10.1016/j.heares.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan R, Yuan Y, Garcia M. Serotonin 5-HT2C receptors in central auditory pathways. ARO abstr. 2000;23:113. [Google Scholar]
- Havey DC, Caspary DM. A simple technique for constructing ‘piggy-back’ multibarrel microelectrodes. Electroencephalography & Clinical Neurophysiology. 1980;48:249–251. doi: 10.1016/0013-4694(80)90313-2. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3(12):2177–2186. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J Neurophysiol. 2006 doi: 10.1152/jn.00046.2006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res. 2007;1181:21–29. doi: 10.1016/j.brainres.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. Journal of Neuroscience. 1999;19:8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed bat, Tadarida brasiliensis. J Comp Neurol. 2001;435:77–88. doi: 10.1002/cne.1194. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin effects on frequency tuning of inferior colliculus neurons. J Neurophysiol. 2001;85:828–842. doi: 10.1152/jn.2001.85.2.828. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:535–546. doi: 10.1007/s00359-005-0623-y. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Tracy JA, Bohorquez A. Serotonin 1B Receptor Modulates Frequency Response Curves and Spectral Integration in the Inferior Colliculus by Reducing GABAergic Inhibition. J Neurophysiol. 2008;100:1656–67. doi: 10.1152/jn.90536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen PH, Wu CH, Luan RH, Zhou X. GABAergic inhibition contributes to pulse repetition rate-dependent frequency selectivity in the inferior colliculus of the big brown bat, Eptesicus fuscus. Brain Res. 2002;948:159–164. doi: 10.1016/s0006-8993(02)03056-1. [DOI] [PubMed] [Google Scholar]
- Johnson S, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neuroscience Letters. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- Kaiser A, Covey E, editors. 5-HT innervation of the auditory pathway in birds and bats. Plenum; New York: 1997. pp. 71–78. [Google Scholar]
- Kidd EJ, Laporte AM, Langlois X, Fattaccini CM, Doyen C, Lombard MC, Gozlan H, Hamon M. 5-HT3 receptors in the rat central nervous system are mainly located on nerve fibers and terminals. Brain Res. 1993;612:289–298. doi: 10.1016/0006-8993(93)91674-h. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Butler A, Burridge J, Oxford AW. 1-(m-chlorophenyl)-biguanide, a potent high affinity 5-HT3 receptor agonist. European Journal of Pharmacology. 1990;182:193–197. doi: 10.1016/0014-2999(90)90513-6. [DOI] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Research. 1991;557:190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Kubo C, Akaike N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J Physiol. 2000;529:373–383. doi: 10.1111/j.1469-7793.2000.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Murakami N, Kubo C, Nabekura J, Akaike N. Role of presynaptic 5-HT1A and 5-HT3 receptors in modulation of synaptic GABA transmission in dissociated rat basolateral amygdala neurons. Life Sci. 2002;72:375–87. doi: 10.1016/s0024-3205(02)02280-4. [DOI] [PubMed] [Google Scholar]
- Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;144(6 Pt 1):3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kaneko S, Satoh M. Inhibitory influence via 5-HT3 receptors on the induction of LTP in mossy fiber-CA3 system of guinea-pig hippocampal slices. Neurosci Res. 1994;18:277–282. doi: 10.1016/0168-0102(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Mas M, Fumero B, Gonzalez-Mora JL. Voltammetric and microdialysis monitoring of brain monoamine neurotransmitter release during sociosexual interactions. Behav Brain Res. 1995;71:69–79. doi: 10.1016/0166-4328(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Sale A, Vieg iA, Baroncelli L, De Pasquale R, O’Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Battenberg E, Bloom F. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- Nichols RA, Mollard P. Direct observation of serotonin 5-HT3 receptor-induced increases in calcium levels in individual brain nerve terminals. J Neurochem. 1996;67:581–92. doi: 10.1046/j.1471-4159.1996.67020581.x. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Winer JA, Beckius GE, Saint Marie RL. Morphology of GABAergic neurons in the inferior colliculus of the cat. J Comp Neurol. 1994;340(1):27–42. doi: 10.1002/cne.903400104. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Dut A. GABA, serotonin and serotonin receptors in the rat inferior colliculus. Brain Res. 2004;998:247–250. doi: 10.1016/j.brainres.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios J, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46(1):28–34. [PubMed] [Google Scholar]
- Portfors CV, Felix RA., II Spectral integration in the inferior colliculus of the CBA/CaJ mouse. Neuroscience. 2005;136:1159–1170. doi: 10.1016/j.neuroscience.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Strecker RE, Jacobs BL. Single unit responses of noradrenergic, serotonergic, and dopaminergic neurons in freely moving cats to simple sensory stimuli. Brain Research. 1986;369:336–340. doi: 10.1016/0006-8993(86)90546-9. [DOI] [PubMed] [Google Scholar]
- Roerig B, Katz L. Modulation of intrinsic circuits by serotonin 5-HT3 receptors in developing ferret visual cortex. Journal of Neuroscience. 1997;17:8324–8338. doi: 10.1523/JNEUROSCI.17-21-08324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronde P, Nichols RA. High calcium permeability of serotonin 5-HT3 receptors on presynaptic nerve terminals from rat striatum. J Neurochem. 1998;70:1094–103. doi: 10.1046/j.1471-4159.1998.70031094.x. [DOI] [PubMed] [Google Scholar]
- Ropert N, Guy N. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol. 1991 Sep;441:121–36. doi: 10.1113/jphysiol.1991.sp018742. 1991. 441:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, Pie B, Cooper E. Developing neonatal rat sympathetic and sensory neurons differ in their regulation of 5-HT3 receptor expression. J Neurosci. 1997;17:6629–6638. doi: 10.1523/JNEUROSCI.17-17-06629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Sakamori M, Haga K, Shuzo T, Setoguchi M. Antagonistic activity of Y-25130 on 5-HT3 receptors. Japan J Pharmacol. 1992;59:443–448. doi: 10.1254/jjp.59.443. [DOI] [PubMed] [Google Scholar]
- Schuller G. A cheap earphone for small animals with good frequency response in the ultrasonic frequency range. J Neurosci Methods. 1997;71:187–190. doi: 10.1016/s0165-0270(96)00142-2. [DOI] [PubMed] [Google Scholar]
- Schuller G, Radtke-Schuller S, Betz M. A stereotaxic method for small animals using experimentally determined reference profiles. Journal of Neuroscience Methods. 1986;18:339–350. doi: 10.1016/0165-0270(86)90022-1. [DOI] [PubMed] [Google Scholar]
- Sepulveda MI, Lummis SCR, Martin IL. The agonist properties of m-chlorophenylbiguanide and 2-methyl-5-hydroxytryptamine on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br J Pharmacol. 1991;104:536–540. doi: 10.1111/j.1476-5381.1991.tb12464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Oliver DL. Distinct K currents result in physiologically distinct cell types in the inferior colliculus of the rat. J Neurosci. 2001;21:2861–77. doi: 10.1523/JNEUROSCI.21-08-02861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Thompson G. Serotonin projection patterns to the cochlear nucleus. Brain Res. 2001;907:195–207. doi: 10.1016/s0006-8993(01)02483-0. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Schofield BR. Afferent projections of the superior olivary complex. Microsc Res Tech. 2000;51:330–354. doi: 10.1002/1097-0029(20001115)51:4<330::AID-JEMT4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Thompson GC, Thompson AM, Garrett KM, Britton BH. Serotonin and serotonin receptors in the central auditory system. Otolaryngology- Head and Neck Surgery. 1994;110:93–102. doi: 10.1177/019459989411000111. [DOI] [PubMed] [Google Scholar]
- To Z, Bonhaus D, Eglen R, Jakeman L. Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br J Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Research. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Activity of serotonin-containing neurons in freely moving cats. In: Jacobs BL, Gelperin A, editors. Serotonin Neurotransmission and Behavior. The MIT Press; Cambridge, Massachusetts: 1981. pp. 339–365. [Google Scholar]
- Turner TJ, Mokler DJ, Luebke JI. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience. 2004;129:703–718. doi: 10.1016/j.neuroscience.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology. 1994;33:527–541. doi: 10.1016/0028-3908(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Waterhouse B, Azizi S, Burne R, Woodward D. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Research. 1990;514:276–292. doi: 10.1016/0006-8993(90)91422-d. [DOI] [PubMed] [Google Scholar]
- Willott JF, Demuth RM. The influence of stimulus repetition on the acoustic startle response and on responses of inferior colliculus neurons of mice. Brain Res. 1986;386:105–112. doi: 10.1016/0006-8993(86)90146-0. [DOI] [PubMed] [Google Scholar]
- Wright D, Seroogy K, Lundgren K, Davis B, Jennes L. Comparative localization of serotonin 1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Prince DA. Heterogeneous actions of serotonin on interneurons in rat visual cortex. J Neurophysiol. 2003;89:1278–87. doi: 10.1152/jn.00533.2002. [DOI] [PubMed] [Google Scholar]
- Xu J, He L, Wu LG. Role of Ca(2+) channels in short-term synaptic plasticity. Curr Opin Neurobiol. 2007;17:352–359. doi: 10.1016/j.conb.2007.04.005. [DOI] [PubMed] [Google Scholar]