Summary
Background
The CD4 cell count at which combination antiretroviral therapy should be started is a central, unresolved issue in the care of HIV-1-infected patients. In the absence of randomised trials, we examined this question in prospective cohort studies.
Methods
We analysed data from 18 cohort studies of patients with HIV. Antiretroviral-naive patients from 15 of these studies were eligible for inclusion if they had started combination antiretroviral therapy (while AIDS-free, with a CD4 cell count less than 550 cells per μL, and with no history of injecting drug use) on or after Jan 1, 1998. We used data from patients followed up in seven of the cohorts in the era before the introduction of combination therapy (1989–95) to estimate distributions of lead times (from the first CD4 cell count measurement in an upper range to the upper threshold of a lower range) and unseen AIDS and death events (occurring before the upper threshold of a lower CD4 cell count range is reached) in the absence of treatment. These estimations were used to impute completed datasets in which lead times and unseen AIDS and death events were added to data for treated patients in deferred therapy groups. We compared the effect of deferred initiation of combination therapy with immediate initiation on rates of AIDS and death, and on death alone, in adjacent CD4 cell count ranges of width 100 cells per μL.
Findings
Data were obtained for 21 247 patients who were followed up during the era before the introduction of combination therapy and 24 444 patients who were followed up from the start of treatment. Deferring combination therapy until a CD4 cell count of 251–350 cells per μL was associated with higher rates of AIDS and death than starting therapy in the range 351–450 cells per μL (hazard ratio [HR] 1·28, 95% CI 1·04–1·57). The adverse effect of deferring treatment increased with decreasing CD4 cell count threshold. Deferred initiation of combination therapy was also associated with higher mortality rates, although effects on mortality were less marked than effects on AIDS and death (HR 1·13, 0·80–1·60, for deferred initiation of treatment at CD4 cell count 251–350 cells per μL compared with initiation at 351–450 cells per μL).
Interpretation
Our results suggest that 350 cells per μL should be the minimum threshold for initiation of antiretroviral therapy, and should help to guide physicians and patients in deciding when to start treatment.
Funding
UK Medical Research Council.
Introduction
Combination antiretroviral therapy has substantially reduced morbidity and mortality in HIV-1-infected individuals since its introduction in 1996.1,2 Short-term randomised controlled trials in immunodeficient patients showed that rates of AIDS or death were halved after approximately 1 year of combination therapy compared with rates in patients treated with drugs from only one antiretroviral drug class.3 The clinical effect of combination therapy has not been examined in a long-term trial, but observational data suggest that this treatment reduces rates of AIDS or death over several years, both in immunodeficient patients and in those with high CD4 cell counts.4,5
A central, unresolved issue is the CD4 cell count at which combination antiretroviral therapy should be started in patients who have not yet had an AIDS-defining event. The best way to address this question is to randomise AIDS-free HIV-1-infected patients to treatment with combination therapy that is either started when the CD4 cell count is in an upper range or deferred until the upper threshold of a lower CD4 cell count range is reached. So far, no such randomised controlled trial has been done: the evidence is limited to a sub-study in the Strategies for Management of Antiretroviral Therapy (SMART) trial,6 which suggested that compared with initiation of treatment at a CD4 cell count of more than 350 cells per μL, delayed initiation until the CD4 cell count was less than 250 cells per μL more than tripled the rate of AIDS or death and, unexpectedly, increased the rate of other serious adverse events.7
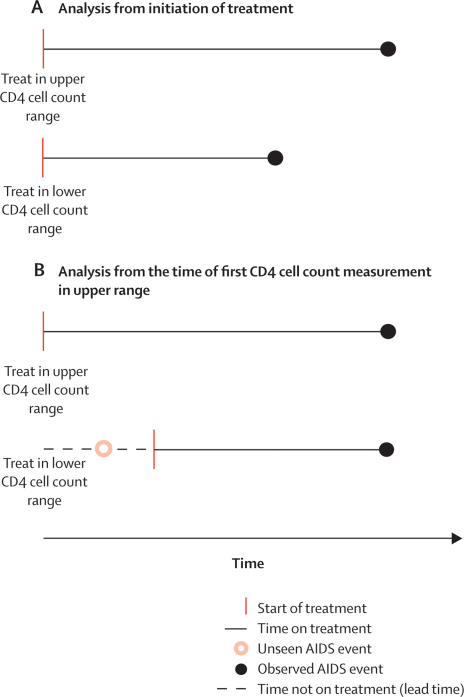
In the absence of evidence from randomised trials, the question of when to start combination therapy is best addressed in prospective observational studies of HIV-1-infected individuals. Most analyses of such data have compared rates of AIDS and death from the time that patients started treatment8–10 (figure 1A). However, such comparisons are problematic because they do not account for AIDS events or deaths that occur during the so-called lead time, before the upper threshold of the lower CD4 cell count range is reached (figure 1B). These unseen events, as well as lead times, will be ignored in analyses where patients' follow-up time is measured from the start of treatment, which introduces lead-time bias.11,12
Figure 1.
Comparison of analyses from (A) initiation of treatment and (B) time of first CD4 cell count measurement in the upper range
We undertook a collaborative analysis of data from cohort studies to estimate the effect of initiation of combination antiretroviral therapy in different CD4 cell count ranges.
Methods
Patients and procedures
We used data from seven cohort studies with patients followed up during the era before the introduction of combination antiretroviral therapy, and data from the Antiretroviral Therapy (ART) Cohort Collaboration of patients followed up from the start of treatment, to estimate rates of AIDS or death in patients starting treatment in different CD4 cell count ranges, taking into account the probability of progression to AIDS or death before the upper threshold of the lower CD4 cell count range is reached. Patients whose presumed HIV transmission was by injecting drug use were analysed separately, because they have a high prevalence of comorbidities such as chronic hepatitis C13 and worse prognosis on combination therapy.14,15
Analyses of progression before starting combination therapy included patients followed before the introduction of this treatment (July 1, 1989, to Dec 31, 1995). We included patients with a CD4 cell count in the range 0 cells per μL to 550 cells per μL from the following seven cohort studies: the Multicenter AIDS Cohort Study (MACS),16 the Swiss HIV Cohort Study (SHCS),17 the ANRS CO4 French Hospital Database on HIV (FHDH),18 the ANRS CO3 Aquitaine Cohort,19 the Amsterdam Cohort Studies,20 the South Alberta Clinic,21 and the Concerted Action on Seroconversion to AIDS and Death in Europe (CASCADE) collaboration (excluding patients who were also included in the other cohorts).22 Patients had CD4 cell count measurements taken at scheduled clinics and were followed up for clinical AIDS events and death. A small number of patients who started combination therapy before Jan 1, 1996, were excluded.
Analyses of progression after the start of combination therapy included all patients enrolled in one of 15 cohorts participating in the ART Cohort Collaboration who started treatment on or after Jan 1, 1998, with a CD4 cell count between 0 cells per μL and 550 cells per μL. We excluded patients if they had an AIDS diagnosis before starting combination therapy. The ART Cohort Collaboration includes cohort studies from Europe and North America, and was established with the aim of describing the prognosis of antiretroviral-naive patients starting combination therapy. The study design has been described in detail elsewhere.10,23,24 Prospective cohort studies were eligible for inclusion in the ART Cohort Collaboration if they had enrolled at least 100 HIV-1-infected patients aged 16 years or older who had not previously received antiretroviral therapy and who had started treatment with a combination of at least three drugs, including nucleoside reverse transcriptase inhibitors, protease inhibitors, or non-nucleoside reverse transcriptase inhibitors, with a median duration of follow-up of at least 1 year. All cohorts provided anonymised data on a predefined set of demographic, laboratory, and clinical variables.
The 15 cohorts from the ART Cohort Collaboration that contributed data for this analysis included four previously mentioned cohorts17–19,21 as well as the AIDS Therapy Evaluation Netherlands project (ATHENA),25 the Italian Cohort of Antiretroviral-Naive Patients (ICONA),26 the Frankfurt HIV Cohort,27 the Köln-Bonn Cohort,28 the Collaborations in HIV Outcomes Research United States (CHORUS),29 the 1917 Clinic Cohort University of Birmingham, Alabama,30 the Veterans Aging Cohort Study (VACS),31 the London Royal Free Hospital Cohort,32 the British Columbia Centre for Excellence in HIV/AIDS,33 the Proyecto para la Informatizacion del Seguimiento Clinico epidemiologico de los pacientes con Infección por VIH/SIDA (PISCIS),34 and the EuroSIDA study, which obtains data from 20 countries in Europe and Argentina (excluding patients who were also included in other ART Cohort Collaboration cohorts).35 Contributors to each cohort are listed in the webappendix (pp 1–8).
Statistical analysis
Data for patients who remained alive were censored at the patient's last visit, plus 50% of the mean time between visits for each cohort. For example, if a cohort had a mean of 6 months between follow-up visits, data were censored at the patient's last visit plus 3 months. Patients with a gap of more than 1 year between clinic visits were deemed lost to follow-up and their data were censored at the beginning of the gap plus 50% of the mean time between visits. Follow-up of patients in the era before the introduction of combination antiretroviral therapy was administratively censored on Dec 31, 1995, and, in patients starting treatment, at 6 years after initiation of treatment or at the (cohort-specific) date of the close of the database.
We used data for patients receiving combination therapy to derive Kaplan-Meier estimates of cumulative probabilities of progression to AIDS and death from the time of treatment initiation, according to CD4 cell count at initiation. We used Cox regression to estimate naive hazard ratios (HRs) for AIDS or death (ie, HRs based on analyses that ignored lead time and unseen AIDS and death events) that compared individuals in different CD4 cell count categories at the start of treatment. In sensitivity analyses, we examined whether adjustment for patient characteristics at the time of treatment initiation altered these naive HRs.
To account for lead time and unseen AIDS and death events, we used a method described by Cole and colleagues,12 in which missing data on lead time and unseen events in the deferred initiation group are recovered by use of multiple imputation.36 Full details are given in the webappendix (p 9). We used data from before the introduction of combination therapy to model the distribution of times from the first CD4 cell count measurement in the upper CD4 cell count range to the upper threshold of the lower CD4 cell count range (ie, lead time) and the probability of progression to AIDS or death before reaching the upper threshold of the lower CD4 cell count range (ie, unseen events; webappendix p 10). We repeated all comparisons with death alone as the endpoint, assuming that combination therapy has no effect on deaths for 2 weeks and that an AIDS diagnosis will lead to immediate initiation of treatment. Therefore, in patients receiving combination therapy, deaths within 2 weeks of initiation were excluded, whereas in data from the era before combination therapy, deaths included in analyses were those before the upper threshold of the lower CD4 cell count range was reached, or within 2 weeks of an AIDS diagnosis. We examined whether progression rates differed between the earlier (1989–91) and later (1992–95) years of the era before combination therapy, by separating follow-up time and including interaction terms in Cox regression models. We used random-effects regression models for log-transformed CD4 cell counts to estimate the median decline in CD4 cell counts during the era before combination therapy.
On the basis of the fitted distributions, imputation was used to create completed datasets, in which lead times and unseen AIDS and death events were added to the data for combination therapy, for patients in the deferred initiation group. We used Cox regression to estimate HRs that compared deferred with immediate initiation of treatment for each completed dataset, then combined these by use of Rubin's formula.36 We used generalised gamma distributions for the lead times and unseen events, after redistributing patients with censored data according to the proportions of patients that progressed to AIDS or death and that reached the upper threshold of the lower CD4 cell count range to estimate the probability of progression before reaching the threshold. We examined the proportional hazards assumption by comparing progression rates in the first 2 years with rates from 2 years to the end of follow-up (6 years).
We compared deferred with immediate initiation of combination therapy in adjacent ranges of width 100 cells per μL. We started with a comparison of initiation at 101–200 cells per μL compared with deferred initiation at 0–100 cells per μL, then compared initiation at 126–225 cells per μL with deferred initiation at 26–125 cells per μL and, by use of successive increments of 25 cells per μL, made similar comparisons up to initiation at 451–550 cells per μL with deferred initiation at 351–450 cells per μL. We undertook sensitivity analyses restricted to patients included in four cohorts that provided both data for patients in the era before combination therapy and data for patients receiving combination therapy (ANRS CO4 FHDH,18 ANRS CO3 Aquitaine Cohort,19 Swiss HIV Cohort Study,17 and the South Alberta Clinic Cohort21). We also undertook further sensitivity analyses in which distributions of lead times and unseen events were estimated on the assumption that treatment in the deferred initiation group was started at the first CD4 cell count measurement in the lower range or, if there was no such measurement, at the midpoint of the range. We also plotted HRs for the cumulative effects of delayed initiation compared with initiation in the range 351–450 cells per μL, by multiplying HRs for successive non-overlapping CD4 cell count ranges (251–350, 151–250, and 51–150 cells per μL). 95% CIs for the plotted cumulative effects were obtained by use of a Poisson approximation after decomposition of the variance into contributions from each of the CD4 cell count groups. All analyses were done with SAS version 9 and Stata version 10.
Role of the funding source
The sponsor of the study had no role in the design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
21 247 AIDS-free patients with presumed transmission not by injecting drug use who were followed up during the era before the introduction of combination antiretroviral therapy (ie, between 1989 and 1995), and 24 444 such patients from the ART Cohort Collaboration who were followed up from the start of combination therapy were included in the main analyses. 4159 (17%) patients were followed up for more than 6 years after the start of treatment (at which time follow-up data were censored because fewer than 20% of patients on combination therapy were followed for more than this length of time). Patient characteristics are shown in table 1. Compared with patients starting treatment, those followed up in 1989–95 were younger, more likely to be men who have sex with men, and more likely to develop AIDS or die during follow-up. We found little evidence that progression rates differed between the earlier (1989–91) and later (1992–95) years of the era before combination therapy (webappendix p 11). The median annual decline in CD4 cell count during 1989–95 was 60 cells per μL per year (95% CI 58–61). Among patients starting combination therapy, 9103 (37%) started treatment with a CD4 cell count in the range 201–350 cells per μL, 5513 (23%) started in the range 101–200 cells per μL, and 5053 (21%) in the range 351–550 cells per μL.
Table 1.
Characteristics at baseline and events recorded during follow-up for patients in the era before the introduction of combination antiretroviral therapy and for patients receiving combination therapy
| Patients followed up in the era before combination antiretroviral therapy (n=21 247) | Patients receiving combination antiretroviral therapy (n=24 444) | ||
|---|---|---|---|
| Age (years) | 34 (28–41) | 37 (31–45) | |
| Female | 4813 (23%) | 7154 (29%) | |
| CD4 cell count (cells per μL) | 354 (264–448) | 230 (130–330) | |
| Log10 HIV-1 RNA | NA | 4·9 (4·4–5·3) | |
| Transmission group* | |||
| Heterosexual sex | 6961 (33%) | 11 382 (51%) | |
| Men who have sex with men | 11 874 (56%) | 8483 (38%) | |
| Other/unknown | 2412 (11%) | 2485 (11%) | |
| Year of enrolment | |||
| 1989–90 | 5784 (27%) | .. | |
| 1991–92 | 6586 (31%) | .. | |
| 1993–95 | 8877 (42%) | .. | |
| 1998–99 | .. | 7000 (29%) | |
| 2000–02 | .. | 9490 (39%) | |
| 2003–06 | .. | 7954 (33%) | |
| Initial combination antiretroviral therapy regimen | |||
| Protease inhibitor-based triple regimen | .. | 116 44 (48%) | |
| NNRTI-based triple regimen | .. | 8696 (36%) | |
| NRTI only | .. | 2347 (10%) | |
| Other† | .. | 1757 (7%) | |
| AIDS and death during follow-up | |||
| Total follow-up (years) | 68 253 | 81 071 | |
| Length of follow-up (years) | 3·1 (1·9–4·5) | 3·2 (1·5–5·3) | |
| Development of AIDS | 5356 (25%) | 1860 (8%) | |
| Deaths | 3630 (17%) | 808 (3%) | |
| AIDS or death | 5893 (28%) | 2366 (10%) | |
NA=not available. NNRTI=non-nucleoside reverse transcriptase inhibitor. NRTI=nucleoside reverse transcriptase inhibitor. Data are n (%) or median (IQR). Baseline is date of start of follow-up in the era before combination antiretroviral therapy, and date of start of treatment for patients receiving combination therapy.
Excluding 2064 patients from the Veterans Aging Cohort Study,31 in whom transmission group was classified only as injecting drug use or other.
Non-standard regimen consisting of more than one protease inhibitor and/or NNRTI, or more than three drugs (excluding ritonavir-boosting of protease inhibitors).
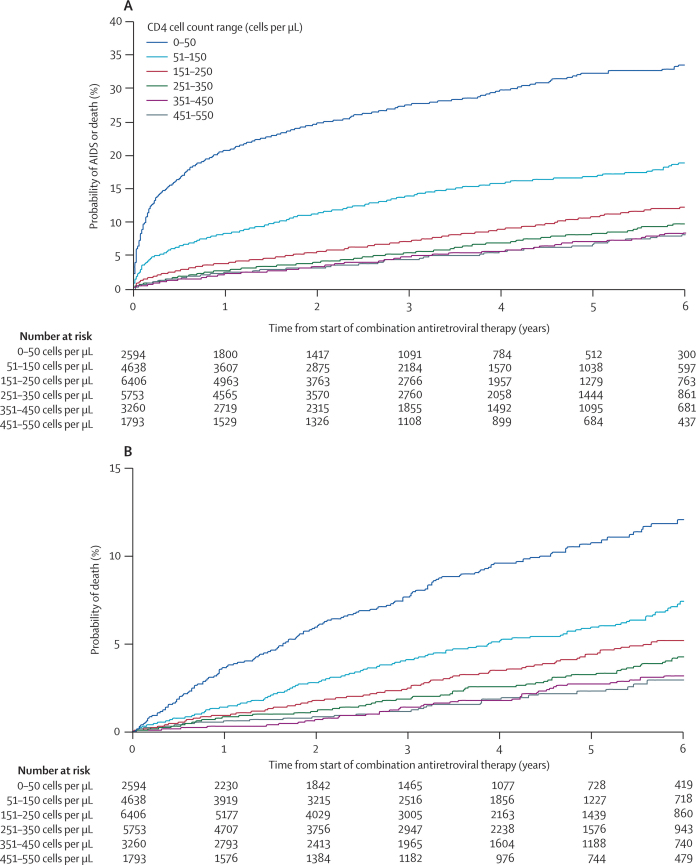
As reported previously,10,37 the cumulative probability of AIDS and death increased substantially with decreasing CD4 cell count at the time of treatment initiation (figure 2). However, this finding does not necessarily imply that treatment should be started before CD4 cell counts decline to the lower ranges, because this comparison does not account for lead time or unseen AIDS and death events (figure 1).
Figure 2.
Cumulative probability of (A) AIDS or death or (B) death alone after initiation of combination antiretroviral therapy, according to range of CD4 cell count at the time of treatment initiation
Table 2 compares rates of progression to AIDS or death in adjacent CD4 cell count ranges of width 100 cells per μL. The median decline in CD4 cell count from the first measurement in the upper range to the upper threshold of the lower range varied from 48 cells per μL for upper range 451–550 cells per μL to 61 cells per μL for upper range 101–200 cells per μL. Estimated lead times increase with decreasing CD4 cell count. This finding occurs, in part, because the variability of CD4 cell counts is greater at higher ranges than at lower ranges; therefore, observed declines are more rapid. As expected, the estimated proportion of patients progressing to AIDS or death before reaching the upper threshold of the lower CD4 cell count range (the percentage of patients with unseen AIDS and death events) increases with decreasing CD4 cell count.
Table 2.
Hazard ratios for AIDS or death for deferral of combination antiretroviral therapy to a lower CD4 cell count range versus initiation at a higher CD4 cell count range
| Higher CD4 cell count range (cells per μL) | Lower CD4 cell count range (cells per μL) |
Data for 1989–95* |
Data for patients on combination antiretroviral therapy |
Hazard ratio (95% CI) for AIDS or death |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients in higher CD4 cell count range | Estimated median lead time (years)† | Estimated proportion of patients progressing to AIDS/death before reaching upper threshold of lower CD4 cell count range (% [95% CI]) | Number of patients | Number of AIDS/death events | Estimated number of unseen events | Naive‡ | Adjusted for lead times and unseen events | ||
| 451–550 | 351–450 | 5015 | 0·67 | 1·6% (1·1–2·1) | 5047 | 260 | 53 | 1·04 (0·81–1·34) | 0·99 (0·76–1·29) |
| 426–525 | 326–425 | 5792 | 0·77 | 2·3% (1·7–2·9) | 5898 | 314 | 91 | 1·12 (0·89–1·42) | 1·12 (0·87–1·43) |
| 401–500 | 301–400 | 6536 | 0·80 | 2·7% (2·0–3·4) | 6874 | 366 | 126 | 1·04 (0·84–1·29) | 1·09 (0·85–1·38) |
| 376–475 | 276–375 | 7029 | 0·84 | 2·8% (2·2–3·5) | 7926 | 400 | 151 | 1·11 (0·91–1·37) | 1·19 (0·96–1·47) |
| 351–450 | 251–350 | 7433 | 0·84 | 3·2% (2·5–3·9) | 8989 | 472 | 189 | 1·17 (0·97–1·41) | 1·28 (1·04–1·57) |
| 326–425 | 226–325 | 7775 | 0·86 | 3·3% (2·7–3·8) | 10067 | 530 | 208 | 1·08 (0·90–1·28) | 1·21 (1·01–1·46) |
| 301–400 | 201–300 | 8226 | 0·89 | 3·8% (3·1–4·5) | 10980 | 584 | 258 | 1·15 (0·98–1·36) | 1·34 (1·12–1·61) |
| 276–375 | 176–275 | 8519 | 0·91 | 5·3% (4·3–6·3) | 11 775 | 640 | 366 | 1·23 (1·05–1·44) | 1·59 (1·30–1·95) |
| 251–350 | 151–250 | 8748 | 0·92 | 6·1% (5·2–7·0) | 12 104 | 719 | 412 | 1·30 (1·12–1·51) | 1·71 (1·43–2·04) |
| 226–325 | 126–225 | 8788 | 0·91 | 7·0% (6·2–7·8) | 12 206 | 763 | 452 | 1·47 (1·27–1·70) | 2·01 (1·73–2·35) |
| 201–300 | 101–200 | 8878 | 0·92 | 8·1% (7·2–9·1) | 11 976 | 822 | 485 | 1·59 (1·38–1·82) | 2·21 (1·91–2·56) |
| 176–275 | 76–175 | 8282 | 0·90 | 9·5% (8·6–10·4) | 11 534 | 908 | 523 | 1·82 (1·60–2·08) | 2·61 (2·27–3·00) |
| 151–250 | 51–150 | 7484 | 0·95 | 10·8% (9·9–11·7) | 10 926 | 957 | 549 | 1·76 (1·55–2·01) | 2·59 (2·29–2·92) |
| 126–225 | 26–125 | 6742 | 0·95 | 13·1% (12·1–14·1) | 10 276 | 1088 | 642 | 2·01 (1·78–2·27) | 2·88 (2·56–3·25) |
| 101–200 | 0–100 | 5871 | 0·92 | 17·6% (16·3–18·9) | 10 014 | 1332 | 969 | 2·25 (2·01–2·51) | 3·35 (2·99–3·75) |
CD4 cell count ranges have widths of 100 cells per μL, in increments of 25 cells per μL. Note that results in overlapping ranges are not statistically independent of each other.
Data for patients followed up in the era before the introduction of combination antiretroviral therapy.
Time from first CD4 cell count measurement in upper range to upper threshold of lower CD4 cell count range, AIDS, or death.
Hazard ratio based on analyses that ignored lead time and unseen AIDS and death events.
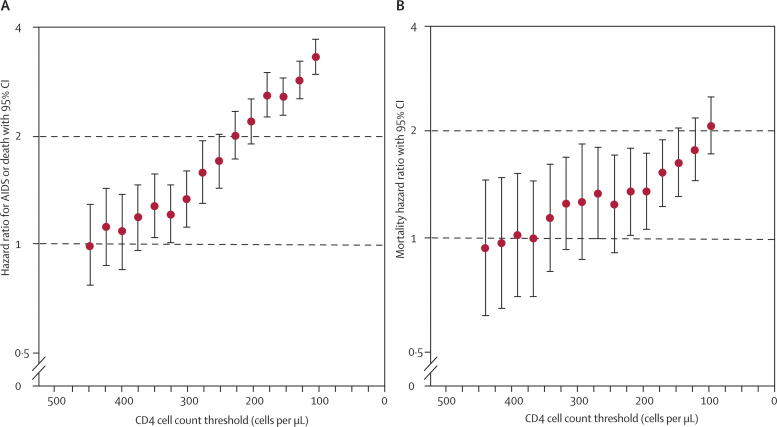
Table 2 also shows naive HRs and HRs adjusted for lead time and unseen events for AIDS and death for initiation of combination therapy that is deferred until a lower CD4 cell count range, compared with initiation of treatment at a higher CD4 cell count range. Adjustment for age at initiation, sex, and risk group (men who have sex with men versus other) did not substantially affect naive HRs (webappendix p 11). Compared with initiation of treatment when CD4 cell count is in the range 351–450 cells per μL, deferring treatment to 251–350 cells per μL leads to increased rates of AIDS or death (adjusted HR 1·28, 95% CI 1·04–1·57). At the higher CD4 cell count ranges, there was little evidence to suggest that deferred initiation of treatment was associated with higher rates of AIDS and death. The effect of accounting for unseen AIDS and death events outweighs the effect of lead time in comparisons of lower CD4 cell count ranges, so that adjusted HRs exceed naive HRs. By contrast, at higher CD4 cell counts, rates of unseen events are lower, and approximately balance the effect of lead time, so that the naive and adjusted HRs are similar. Figure 3 shows the successive increase in rates of AIDS or death as combination therapy is deferred to lower CD4 cell count thresholds.
Figure 3.
Adjusted hazard ratios for (A) AIDS or death and (B) death alone for initiation of combination antiretroviral therapy at a lower CD4 cell count threshold (ie, deferred initiation) versus initiation in a range up to 100 cells per μL higher
The horizontal axis shows the threshold values (upper limits of the CD4 cell count ranges in the deferred initiation groups [from 351–450 cells per μL, in steps of 25 cells per μL, to 0–100 cells per μL]). See table 2 and table 3 for lists of hazard ratios and 95% CIs.
Table 3 shows that, as expected, mortality rates increase with declining CD4 cell count. Compared with HRs for the combined endpoint of progression to AIDS and death, the HRs for mortality alone have wider 95% CIs, because the number of deaths is smaller than the number of combined AIDS and death events. Deferred initiation of combination therapy was associated with higher mortality rates, although effects on mortality were less marked than effects on AIDS and death. The mortality HR for deferred initiation of treatment at CD4 cell count 251–350 cells per μL compared with initiation at 351–450 cells per μL was 1·13 (0·80–1·60): there was little evidence that deferred initiation of treatment was associated with increased mortality rates in higher CD4 cell count ranges. The beneficial effects of earlier initiation of treatment were greater in the first 2 years of follow-up than in the period from 2 to 6 years' follow-up, apart from in the highest CD4 cell count ranges (webappendix p 12).
Table 3.
Hazard ratios for death for deferral of combination antiretroviral therapy to a lower CD4 cell count range versus initiation at a higher CD4 cell count range
| Higher CD4 cell count range (cells per μL) | Lower CD4 cell count range (cells per μL) |
Data for 1989–95* |
Data for patients on combination antiretroviral therapy |
Mortality hazard ratio (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients in higher CD4 cell count range | Estimated median lead time (years)† | Estimated proportion of patients progressing to death before reaching upper threshold of lower CD4 cell count range (% [95% CI]) | Number of patients | Number of deaths | Estimated number of unseen deaths | Naive‡ | Adjusted for lead times and unseen deaths | ||
| 451–550 | 351–450 | 5015 | 0·66 | 0·5% (0·3–0·7) | 5053 | 92 | 15 | 1·06 (0·69–1·62) | 0·93 (0·60–1·44) |
| 426–525 | 326–425 | 5792 | 0·77 | 0·6% (0·3–1·0) | 5910 | 108 | 26 | 1·03 (0·69–1·52) | 0·96 (0·63–1·46) |
| 401–500 | 301–400 | 6536 | 0·81 | 0·7% (0·4–0·9) | 6887 | 129 | 29 | 1·15 (0·80–1·65) | 1·01 (0·68–1·50) |
| 376–475 | 276–375 | 7029 | 0·84 | 0·6% (0·4–0·9) | 7943 | 149 | 30 | 1·20 (0·86–1·69) | 0·99 (0·68–1·43) |
| 351–450 | 251–350 | 7433 | 0·84 | 0·7% (0·4–1·1) | 9013 | 183 | 41 | 1·38 (1·01–1·88) | 1·13 (0·80–1·60) |
| 326–425 | 226–325 | 7775 | 0·88 | 0·8% (0·5–1·1) | 10 099 | 208 | 48 | 1·43 (1·07–1·91) | 1·24 (0·92–1·67) |
| 301–400 | 201–300 | 8226 | 0·87 | 1·0% (0·2–1·8) | 11 021 | 239 | 67 | 1·43 (1·10–1·86) | 1·25 (0·86–1·82) |
| 276–375 | 176–275 | 8519 | 0·89 | 1·1% (0·8–1·5) | 11 825 | 264 | 72 | 1·43 (1·12–1·84) | 1·32 (0·98–1·78) |
| 251–350 | 151–250 | 8748 | 0·92 | 1·4% (0·8–2·0) | 12 159 | 294 | 90 | 1·32 (1·04–1·66) | 1·23 (0·90–1·69) |
| 226–325 | 126–225 | 8788 | 0·90 | 1·5% (0·9–2·1) | 12 269 | 324 | 93 | 1·41 (1·13–1·76) | 1·34 (1·01–1·77) |
| 201–300 | 101–200 | 8878 | 0·92 | 1·6% (1·1–2·0) | 12 051 | 348 | 84 | 1·44 (1·16–1·78) | 1·34 (1·05–1·71) |
| 176–275 | 76–175 | 8282 | 0·91 | 1·8% (1·6–2·1) | 11 626 | 362 | 91 | 1·48 (1·21–1·82) | 1·51 (1·21–1·87) |
| 151–250 | 51–150 | 7484 | 0·93 | 2·2% (1·8–2·6) | 11 044 | 369 | 104 | 1·48 (1·21–1·82) | 1·61 (1·29–2·01) |
| 126–225 | 26–125 | 6742 | 0·92 | 2·7% (2·2–3·1) | 10 432 | 417 | 125 | 1·58 (1·30–1·91) | 1·75 (1·43–2·15) |
| 101–200 | 0–100 | 5871 | 0·89 | 3·7% (3·0–4·4) | 10 248 | 500 | 184 | 1·73 (1·45–2·08) | 2·04 (1·70–2·46) |
CD4 cell count ranges have widths of 100 cells per μL, in increments of 25 cells per μL.
Data for patients followed up in the era before the introduction of combination antiretroviral therapy.
Time from first CD4 cell count measurement in upper range to upper threshold of lower CD4 cell count range, AIDS, or death.
Hazard ratio based on analyses that ignored lead time and unseen AIDS and death events.
We repeated analyses in patients with presumed transmission by injecting drug use. In 4605 such patients receiving combination therapy, there were 653 AIDS or death events (334 deaths) during 15 141 years of follow-up. In 9860 patients followed during 1989–95, there were 905 AIDS or death events (823 deaths) during 27 182 years of follow-up. Estimated HRs for deferring start of treatment to lower CD4 cell count ranges compared with starting at higher ranges are shown in the webappendix (p 12). For comparisons in which the threshold CD4 cell count was low, the estimated benefits of earlier initiation were lower for patients whose presumed transmission was by injecting drug use than for patients in the main analyses. At higher CD4 cell count thresholds, the estimated benefits of earlier initiation were generally consistent with those found in the main analyses, although as expected (given that the numbers of patients with transmission by injecting drug use were smaller than in the main analyses), 95% CIs were wide.
Four cohorts contributed data for patients receiving combination therapy (13 084 [54%] patients) as well as data for patients followed up during 1989–95 (17 993 [85%] patients). The results of sensitivity analyses for the combined AIDS and death endpoint, restricted to patients from these cohorts, were consistent with the main analyses (webappendix p 13). Most HRs for the adverse effect of deferred treatment were larger than those for patients in the main analyses, particularly for low CD4 cell count ranges.
The webappendix (p 13) shows the results of sensitivity analyses in which distributions of lead times and unseen events were estimated on the assumption that treatment in the deferred initiation group was started at the first CD4 cell measurement in the lower range or, if there was no such measurement, at the midpoint of the range. The effect of the resulting additional unseen events outweighed the effect of the additional lead time, so that hazard ratios for the adverse effect of deferring treatment on rates of AIDS and death generally increased.
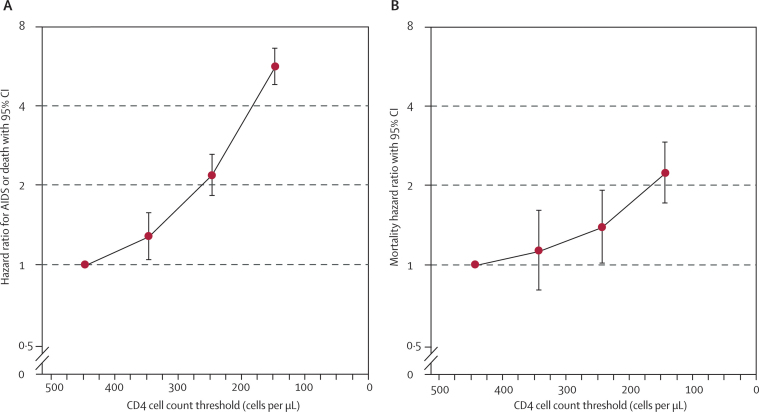
Compared with start of treatment in the range 351–450 cells per μL, deferred initiation of treatment at a CD4 cell count between 51 cells per μL and 150 cells per μL was associated with an HR of 5·67 (4·83–6·65) for the combined endpoint of AIDS and death, and an HR of 2·24 (1·72–2·92) for mortality (figure 4). In the ART Cohort Collaboration, 37% of patients started combination therapy with a CD4 cell count below 150 cells per μL.
Figure 4.
Hazard ratios for the cumulative effect of deferred initiation of combination antiretroviral therapy for (A) AIDS or death and (B) death alone, compared with starting treatment at CD4 cell count range 351–450 cells per μL
The horizontal axis shows the upper limits of the lower CD4 cell count range (251–350 cells per μL, 151–250 cells per μL, and 51–150 cells per μL).
Discussion
This collaborative analysis of data from over 45 000 patients who were followed up in cohort studies in Europe and North America suggests that in AIDS-free HIV-1-infected individuals, deferring the start of combination antiretroviral therapy until CD4 cell counts are in the range 251–350 cells per μL leads to increased rates of the combined endpoint of AIDS or death compared with starting in the range 351–450 cells per μL. As expected, the excess of AIDS or death associated with deferred initiation of combination therapy became more pronounced as the CD4 cell count threshold for starting treatment decreased. Effects of deferring treatment on mortality alone were less pronounced, but patterns were consistent with those for rates of the combined endpoint of AIDS or death. Beneficial effects of early initiation tended to be greater during the first 2 years of follow-up than in the period from 2 to 6 years' follow-up.
By contrast with previous studies that have compared rates of progression to AIDS or death from the time of initiation of combination antiretroviral therapy,8–10 we accounted for the treatment-free time spent by patients when treatment is delayed, and for the events that occur before initiation of treatment in these patients. Thus, our analyses aimed to estimate the intervention effects that would be seen in studies in which patients were allocated to either initiation of treatment in a higher CD4 cell count range or delayed initiation until reaching a lower CD4 cell count range. Because of the large number of patients included in this collaborative study, our analyses had reasonable power to detect differences in progression rates. By contrast, previous studies that compared mortality rates in patients with immediate initiation of treatment with rates in patients in whom treatment was deferred were limited by a small number of endpoints.38,39 The analysis of the HIV Outpatient Study showed that mortality was reduced by 39% in patients who started treatment with CD4 cell count 350–500 cells per μL compared with patients who deferred treatment until after the CD4 cell count had fallen to below 350 per μL; however, this result did not reach conventional levels of statistical significance (p=0·17).38 Previous analyses that used the method described here to account for lead time and unseen AIDS and death events were also based on much smaller numbers of patients.12,40
Since we combined data for large numbers of patients in this analysis, we were able to compare narrow CD4 cell count strata, of width 100 cells per μL, with the aim of identifying CD4 cell count ranges within which earlier initiation has beneficial effects on rates of AIDS and death. Had we compared wider CD4 cell count ranges, HRs might have increased, at the cost of reduced clinical relevance. For example, a comparison of initiation of treatment in the range 301–500 cells per μL with deferral to the range 101–300 cells per μL would compare some patients who started at 490 cells per μL with some patients who started at 110 cells per μL.
Patients who had an AIDS event before the start of combination therapy were excluded because they have worse prognosis and are likely to start treatment irrespective of their CD4 cell count.41 Nevertheless, our analyses included patients from many countries from Europe and North America who were treated in different settings. The range of patients was broad: men and women, patients aged from 16 years to 90 years, and patients presumed to have been infected through heterosexual sex as well as men who have sex with men. We analysed data from patients infected by injecting drug use separately, to avoid possible confounding because of comorbidities, deferred treatment, and non-adherence in these patients. HIV-infected intravenous drug users have a high prevalence of comorbidities13 and worse prognosis after combination antiretroviral therapy.14,15 Because our conclusions for this subgroup of patients were similar to those for all patients, our results should be applicable to many patients starting or considering combination therapy in developed countries. The clear disadvantages of delaying initiation of treatment until CD4 count is below 200 cells per μL might also have implications for resource-limited settings, where eligibility criteria for initiation of combination artiretroviral therapy are often advanced immunodeficiency or clinical disease.42
An important assumption made in these analyses is that progression rates and mortality in the era before the introduction of combination antiretroviral therapy (ie, 1989–95), are an appropriate reflection of what they would have been in the absence of this treatment in recent years. During the 1990s, the introduction of chemoprophylaxis, immunisation, and better strategies for the management of acute opportunistic infections contributed to prevention of clinical progression and improvement of survival rates in HIV-1-infected patients. In particular, the introduction of prophylaxis against Pneumocystis jirovecii pneumonia43 and against Mycobacterium avium complex disease in 199344 were important developments. Although substantially less effective than combination antiretroviral therapy, monotherapy (mainly with zidovudine)45 became available in the late 1980s, and dual therapy with two nucleoside reverse transcriptase inhibitors46 became available during the mid-1990s. These factors will have acted in opposite directions: rates of AIDS and death in 1989–91 might have been higher than contemporary rates in the absence of treatment because of lower rates of prophylaxis, whereas rates in 1992–95 might have been reduced because some patients were treated with monotherapy or dual therapy. We suggest that biases introduced by these factors will have been limited. First, both prophylaxis and treatment were used mainly in patients with CD4 cell counts less than 200 cells per μL, in whom the adverse consequences of delayed initiation of treatment are clear. Second, monotherapy was of only limited, transient benefit,45 and dual therapy became widely available only during late 1994 and early 1995. Third, we found little evidence that rates of AIDS and death differed between the earlier (1989–91) and later (1992–95) years of the era before combination antiretroviral therapy within the different CD4 cell count ranges. Some patients included in the dataset of patients receiving combination therapy had an initial regimen that included an unboosted rather than boosted protease inhibitor, which might have attenuated the beneficial effect of treatment in the early portion of follow-up.47
As is the case for any observational study, our results might have been affected by confounding and selection biases, if patient characteristics associated with deferred initiation of treatment are also predictive of progression rates on or off therapy. We aimed to deal with such biases by excluding patient groups known to have higher progression rates. As well as excluding patients infected by injecting drug use and those who had an AIDS event before the start of combination therapy, we excluded patients who started treatment before 1998, when regimens were less effective than those now available.48,49 Nonetheless, our results might still be affected by unmeasured confounding factors. A randomised controlled trial would overcome such concerns; moreover, it could take into account factors outside the scope of our analysis, such as non-AIDS-defining events, severe and non-severe AIDS events, and drug-related toxic effects. Therefore, a more definitive answer to the question of when to start combination therapy will only be given when results become available from randomised controlled trials, such as the Strategic Timing of Antiretroviral Treatment (START) trial (registered with ClinicalTrials.gov, number NCT00821171). This study aims to establish whether immediate initiation of combination treatment is superior (in terms of morbidity and mortality) to deferral of treatment until the CD4 cell count falls below 350 cells per μL in HIV-1-infected people who are antiretroviral naive with a CD4 cell count greater than 500 cells per μL.
In the absence of definitive evidence from randomised controlled trials, it is necessary to rely on observational evidence when formulating guidelines on the CD4 cell count at which combination therapy should be started. When patients and their physicians consider starting antiretroviral treatment, they must balance its beneficial effects on rates of progression to AIDS and death with several other issues.50,51 Eradication of HIV from an individual is not currently possible;52 therefore, treatment is expected to be lifelong. Antiretroviral drugs can be inconvenient to take, and have side-effects that include nausea, diarrhoea, and headache. Combination antiretroviral therapy is associated with serious toxic effects including lipodystrophy and lipoatrophy syndromes,53,54 hepatitis, renal failure and mitochondrial toxicity,27 and an increased risk of cardiovascular disease.55 However, these toxic effects are to an extent avoidable through choice of drug regimen: for example, increases in cardiovascular risk seem greater for protease inhibitors than for non-nucleoside reverse transcriptase inhibitors,56 and lipoatrophy is associated with thymidine analogues.57 Further, the HR of 1·28 for the comparison of deferring initiation of treatment to the CD4 cell count range 251–350 cells per μL with initiation at 351–450 cells per μL represents only a small absolute difference in the risk of AIDS or death over the follow-up period considered here.
Recent results from the SMART trial of structured treatment interruptions have also brought new perspectives to our understanding of the benefits and risks of combination antiretroviral therapy.6 In that trial, patients who had started treatment at high CD4 cell counts and subsequently had treatment interrupted had higher rates not only of AIDS and death, but also of serious non-AIDS events such as myocardial infarction, stroke, liver cirrhosis, and renal failure. The analyses presented in this report do not account for non-fatal serious non-AIDS events, which might be the major causes of morbidity and subsequent mortality at higher CD4 cell counts. Non-AIDS deaths in untreated individuals might account for the early high mortality HRs for deferred initiation compared with immediate initiation during the first 2 years of follow-up (webappendix p 12). Data from the EuroSIDA study show that rates of death from non-AIDS causes declined substantially at the start of the era of combination antiretroviral therapy.58,59
Thus, our findings should help to guide physicians and patients in deciding when to start antiretroviral treatment. The evolution of guidelines has been compared to the swings of a pendulum,60,61 from initial enthusiasm for early treatment,62 through to caution because of concern about toxic effects and the risk of resistance and loss of treatment options,63 to more recent calls for earlier treatment.64 The International AIDS Society USA panel recommended in August, 2008, that antiretroviral therapy is started in individuals with CD4 cell counts less than 350 cells per μL, and that this decision should be individualised when the CD4 cell count is greater than 350 cells per μL.65 Recent US66 and European guidelines make similar recommendations.67 Because we found evidence that deferral of treatment until the patient's CD4 cell count is less than 350 cells per μL was associated with increased progression rates, and in view of diminished concerns about toxic effects and resistance,51,68 our results suggest that 350 cells per μL should be the minimum threshold at which antiretroviral therapy is started.
Acknowledgments
Acknowledgments
We are grateful to all patients, doctors, nurses, and other people who were involved with the participating cohort studies. We would like to thank participating cohort members, in particular Caroline Sabin and Sarah Walker, who provided thoughtful review and feedback on the content of this manuscript. The ART Cohort Collaboration is supported by the UK Medical Research Council grant G0700820. Sources of funding of individual cohorts include the Agence Nationale de Recherche contre le SIDA (ANRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the French, Italian, Spanish and Swiss Ministries of Health, the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant 33CSC0-108787), the Stichting HIV Monitoring, the European Commission, the British Columbia and Alberta Governments, the Michael Smith Foundation for Health Research, the Canadian Institutes of Health Research, the VHA Office of Research and Development and unrestricted grants from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Janssen-Cilag, Pfizer, and Roche. Supported in part by the Spanish Network for AIDS Research (RIS; ISCIII-RETIC RD06/006). CASCADE has been funded through grants BMH4-CT97-2550, QLK2-2000-01431, QLRT-2001-01708, and LSHP-CT-2006-018949 from the European Union.
Contributors
JACS, ME, DC, ANP, MM, and SRC participated in study conception and design. MM, RHa, JACS, and SRC participated in data analysis. DC, FdW, BL, ME, ANP, MJF, RG, JG, FD, JMM, ACJ, GF, RHo, AD'AM, MS, CS, SS, MM, RHa, and JACS contributed to acquisition of data or interpretation of data. JACS, SRC, MM, and ME drafted the manuscript. DC, FdW, ANP, RHa, MJF, RG, JG, FD, JMM, ACJ, BL, GF, RHo, AD'AM, MS, CS, and SS participated in critical revision of the manuscript. All authors saw and approved the final version of the manuscript.
When To Start Consortium Writing Committee
Jonathan A C Sterne (Department of Social Medicine, University of Bristol, Bristol, UK); Margaret May (Department of Social Medicine, University of Bristol, Bristol, UK); Dominique Costagliola (INSERM, U 943; UPMC Univ Paris 06, UMR S 943; AP-HP, Groupe Hospitalier Pitié Salpétrière, Service de Maladies Infectieuses et Tropicales, Paris, France); Frank de Wolf (HIV Monitoring Foundation, Amsterdam, Netherlands); Andrew N Phillips (Department of Infection and Population Health, University College London Medical School, London, UK); Ross Harris (Department of Social Medicine, University of Bristol, Bristol, UK); Michele Jönsson Funk (Department of Epidemiology, University of North Carolina, Chapel Hill, NC, USA); Ronald B Geskus (Infectious Diseases Cluster, Amsterdam Health Service and Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Academic Medical Center, Amsterdam, Netherlands); John Gill (Department of Medicine, University of Calgary, Calgary, AB, Canada); François Dabis (INSERM U897 and ISPED, Université Victor Segalen, Bordeaux, France); Jose M Miró (Infectious Diseases Service, Hospital Clinic i Provincial, Institut d'Investigacions Biomèdiques August Pi i Sunyer, University of Barcelona, Barcelona, Spain); Amy C Justice (Department of Medicine, Schools of Medicine and Public Health, Yale University and Section of General Internal Medicine, VA Connecticut Healthcare System, West Haven, CT, USA); Bruno Ledergerber (Division of Infectious Diseases and Hospital Epidemiology, University of Zurich, Zurich, Switzerland); Gerd Fätkenheuer (Department of Internal Medicine, University of Cologne, Köln, Germany); Robert S Hogg (BC Centre for Excellence in HIV/AIDS and Simon Fraser University, Vancouver, BC, Canada); Antonella D'Arminio Monforte (San Paolo University Hospital, Milan, Italy); Michael Saag (Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA); Colette Smith (Department of Infection and Population Health, University College London Medical School, London, UK); Schlomo Staszewski (HIVCENTER, HIV Treatment and Clinical Research Unit, Department of Internal Medicine II, Johann Wolfgang Goethe University, Frankfurt am Main, Germany); Matthias Egger (Institute of Social and Preventive Medicine [ISPM], University of Bern, Switzerland); Stephen R Cole (Epidemiology, UNC School of Global Public Health, Chapel Hill, NC, USA).
Antiretroviral Therapy Cohort Collaboration Steering Committee
Hans Reinhard Brodt (Frankfurt), Jordi Casabona (PISCIS), Geneviève Chêne (ANRS CO3 Aquitaine), Dominique Costagliola (ANRS CO4 FHDH), François Dabis (ANRS CO3 Aquitaine), Antonella D'Arminio Monforte (ICONA), Julia del Amo (CoRIS-MD), Frank de Wolf (ATHENA), Matthias Egger (SHCS), Gerd Fätkenheuer (Köln/Bonn), John Gill (South Alberta Clinic), Jodie Guest (HAVACS), Robert Hogg (BCCfE-HIV), Amy Justice (VACS), Mari Kitahata (Washington), Fiona Lampe (Royal Free), Bruno Ledergerber (SHCS), Amanda Mocroft (EuroSIDA), Peter Reiss (ATHENA), Michael Saag (Alabama).
Conflicts of interest
JACS has received travel grants from GlaxoSmithKline and honoraria from Gilead Sciences. MM has received travel grants from GlaxoSmithKline. DC has received travel grants, consultancy fees, and honoraria from various pharmaceutical companies including Abbott, GlaxoSmithKline, Bristol-Myers Squibb, Gilead, Roche, and Boehringer-Ingelheim. AP has received reimbursement for either attending a symposium; a fee for speaking; a fee for organising education; funds for research; funds for a member of staff; or fees for consulting from various pharmaceutical companies including Roche, BMS, GSK, Abbott, Boehringer-Ingelheim, Gilead, Tibotec, and Oxxon Therapeutics. RHa has received travel grants from GlaxoSmithKline. MF has received travel grants from GlaxoSmithKline and salary support through an unrestricted educational grant to the University of North Carolina from GlaxoSmithKline. JG has served on advisory boards or received research grants through the University of Calgary from Abbott, Bristol-Myers Squibb, Boehringer-Ingelheim, Gilead Sciences, GlaxoSmithKline, Merck, Pfizer, Roche, and Tibotec. FD has served on advisory boards and or received research grants from Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Roche, and Tibotec. BL has received travel grants, grants, or honoraria from Abbott, Aventis, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck Sharp & Dohme, Roche, and Tibotec. GF has received travel grants, grants, or honoraria from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck Sharp and Dohme, Pfizer, Roche, Schering-Plough, and Tibotec. RHo has received travel grants and grant support from Abbott, Boehringer-Ingelheim, GlaxoSmithKline, and Merck. MS has received grants or research support from, or acted as a consultant to Achillion Pharmaceutica, Boehringer-Ingelheim, Gilead Sciences, GlaxoSmithKline, Merck, Panacos, Pfizer, Progenics, Roche Laboratories, Serono, Tibotec, Avexa, Bristol-Myers Squibb, Monogram Biosciences, Progenics, and Virco. SS has received honoraria for either attending a symposium; a fee for speaking; funds for research; or for participation in the advisory boards and for lectures at satellite symposia from various pharmaceutical companies including GlaxoSmithKline, Abbott, Gilead Sciences, Boehringer-Ingelheim, Bristol-Myers Squibb, Tibotec, and Roche. All other authors declare that they have no conflicts of interest.
Correspondence to: Prof Jonathan Sterne, Department of Social Medicine, University of Bristol, Canynge Hall, Whatley Road, Bristol BS8 2PS, UK jonathan.sterne@bristol.ac.uk
Web Extra Material
References
- 1.Egger M, Hirschel B, Francioli P. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palella FJ, Delaney KM, Moorman AC. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–908. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, Squires KE, Hughes MD. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 4.Cole SR, Hernan MA, Robins JM. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–694. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 5.Sterne JAC, Hernan MA, Ledergerber B. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 6.El-Sadr WM, Lundgren JD, Neaton JD. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 7.Emery S, Neuhaus JA, Phillips AN. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 8.Hogg RS, Yip B, Chan KJ. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 9.Phillips AN, Staszewski S, Weber R. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001;286:2560–2567. doi: 10.1001/jama.286.20.2560. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, May M, Chêne G. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 11.Pomerantz RJ. Initiating antiretroviral therapy during HIV infection: confusion and clarity. JAMA. 2001;286:2597–2599. doi: 10.1001/jama.286.20.2597. [DOI] [PubMed] [Google Scholar]
- 12.Cole SR, Li R, Anastos K. Accounting for leadtime in cohort studies: evaluating when to initiate HIV therapies. Stat Med. 2004;23:3351–3363. doi: 10.1002/sim.1579. [DOI] [PubMed] [Google Scholar]
- 13.Prins M, Hernandez Aquado IH, Brettle RP. Pre-AIDS mortality from natural causes associated with HIV disease progression: evidence from the European Seroconverter Study among injecting drug users. AIDS. 1997;11:1747–1756. doi: 10.1097/00002030-199714000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Wood E, Montaner JS, Yip B. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169:656–661. [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JA, May M, Sabin C. Importance of baseline prognostic factors with increasing time since initiation of highly active antiretroviral therapy: collaborative analysis of cohorts of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2007;46:607–615. doi: 10.1097/QAI.0b013e31815b7dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 17.Ledergerber B, von Overbeck J, Egger M, Luethy R. The Swiss HIV cohort study: rationale, organization and selected baseline characteristics. Soz Praventivmed. 1994;39:387–394. doi: 10.1007/BF01299670. [DOI] [PubMed] [Google Scholar]
- 18.Grabar S, Pradier C, Le Corfec E. Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS. 2000;14:141–149. doi: 10.1097/00002030-200001280-00009. [DOI] [PubMed] [Google Scholar]
- 19.Binquet C, Chene G, Jacqmin-Gadda H. Modeling changes in CD4-positive T-lymphocyte counts after the start of highly active antiretroviral therapy and the relation with risk of opportunistic infections: the Aquitaine Cohort, 1996–1997. Am J Epidemiol. 2001;153:386–393. doi: 10.1093/aje/153.4.386. [DOI] [PubMed] [Google Scholar]
- 20.de Wolf F, Lange JM, Houweling JT. Numbers of CD4+ cells and the levels of core antigens of and antibodies to the human immunodeficiency virus as predictors of AIDS among seropositive homosexual men. J Infect Dis. 1988;158:615–622. doi: 10.1093/infdis/158.3.615. [DOI] [PubMed] [Google Scholar]
- 21.Mocroft A, Gill MJ, Davidson W, Phillips AN. Predictors of a viral response and subsequent virological treatment failure in patients with HIV starting a protease inhibitor. AIDS. 1998;12:2161–2167. doi: 10.1097/00002030-199816000-00011. [DOI] [PubMed] [Google Scholar]
- 22.CASCADE Collaboration Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 23.May M, Royston P, Egger M, Justice AC, Sterne JA. Development and validation of a prognostic model for survival time data: application to prognosis of HIV positive patients treated with antiretroviral therapy. Stat Med. 2003;23:2375–2398. doi: 10.1002/sim.1825. [DOI] [PubMed] [Google Scholar]
- 24.May M, Porter K, Sterne J, Royston P, Egger M. Prognostic model for HIV-1 disease progression in patients starting antiretroviral therapy was validated using independent data. J Clin Epidemiol. 2005;58:1033–1041. doi: 10.1016/j.jclinepi.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Gras L, Kesselring AM, Griffin JT. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007;45:183–192. doi: 10.1097/QAI.0b013e31804d685b. [DOI] [PubMed] [Google Scholar]
- 26.D'Arminio MA, Lepri AC, Rezza G. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 27.Brodt HR, Kamps BS, Gute P, Knupp B, Staszewski S, Helm EB. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS. 1997;11:1731–1738. doi: 10.1097/00002030-199714000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Fatkenheuer G, Theisen A, Rockstroh J. Virological treatment failure of protease inhibitor therapy in an unselected cohort of HIV-infected patients. AIDS. 1997;11:F113–F116. doi: 10.1097/00002030-199714000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Becker SL, Raffanti SR, Hansen NI. Zidovudine and stavudine sequencing in HIV treatment planning: findings from the CHORUS HIV cohort. J Acquir Immune Defic Syndr. 2001;26:72–81. doi: 10.1097/00126334-200101010-00011. [DOI] [PubMed] [Google Scholar]
- 30.Chen RY, Accortt NA, Westfall AO. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 31.Fultz SL, Skanderson M, Mole LA. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 suppl 2):S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 32.Mocroft A, Barry S, Sabin CA. The changing pattern of admissions to a London hospital of patients with HIV: 1988–1997. Royal Free Centre for HIV Medicine. AIDS. 1999;13:1255–1261. doi: 10.1097/00002030-199907090-00016. [DOI] [PubMed] [Google Scholar]
- 33.Hogg RS, Yip B, Kully C. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ. 1999;160:659–665. [PMC free article] [PubMed] [Google Scholar]
- 34.Jaen A, Casabona J, Esteve A. Clinical-epidemiological characteristics and antiretroviral treatment trends in a cohort of HIV infected patients. The PISCIS Project. Med Clin (Barc) 2005;124:525–531. doi: 10.1157/13073938. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 35.Lundgren JD, Phillips AN, Vella S. Regional differences in use of antiretroviral agents and primary prophylaxis in 3122 European HIV-infected patients. EuroSIDA Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:153–160. doi: 10.1097/00042560-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 36.Rubin D. Multiple imputation for nonresponse in surveys. Wiley; New York: 1987. [Google Scholar]
- 37.May M, Sterne JA, Sabin C. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palella FJ, Deloria-Knoll M, Chmiel JS. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–626. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 39.Sterling TR, Chaisson RE, Moore RD. Initiation of highly active antiretroviral therapy at CD4+ T lymphocyte counts of >350 cells/mm3: disease progression, treatment durability, and drug toxicity. Clin Infect Dis. 2003;36:812–815. doi: 10.1086/367934. [DOI] [PubMed] [Google Scholar]
- 40.Jaen A, Esteve A, Miro JM. Determinants of HIV progression and assessment of the optimal time to initiate highly active antiretroviral therapy: PISCIS Cohort (Spain) J Acquir Immune Defic Syndr. 2008;47:212–220. doi: 10.1097/qai.0b013e31815ee282. [DOI] [PubMed] [Google Scholar]
- 41.Hammer SM, Saag MS, Schechter M. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 42.Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration, ART Cohort Collaboration (ART-CC) Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 43.CDC Recommendations for prophylaxis against Pneumocystis carinii pneumonia for adults and adolescents infected with HIV. JAMA. 1992;267:2294–2299. [PubMed] [Google Scholar]
- 44.Masur H. Recommendations on prophylaxis and therapy for disseminated Mycobacterium avium complex disease in patients infected with the human immunodeficiency virus. Public Health Service Task Force on Prophylaxis and Therapy for Mycobacterium avium Complex. N Engl J Med. 1993;329:898–904. doi: 10.1056/NEJM199309163291228. [DOI] [PubMed] [Google Scholar]
- 45.Concorde Coordinating Committee Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Lancet. 1994;343:871–881. [PubMed] [Google Scholar]
- 46.Darbyshire JH, Delta Coordinating Committee Delta: a randomised double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Lancet. 1996;348:283–291. [PubMed] [Google Scholar]
- 47.Walmsley S, Bernstein B, King M. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 48.Moore RD, Keruly JC, Gebo KA, Lucas GM. An improvement in virologic response to highly active antiretroviral therapy in clinical practice from 1996 through 2002. J Acquir Immune Defic Syndr. 2005;39:195–198. [PubMed] [Google Scholar]
- 49.Lampe FC, Gatell JM, Staszewski S. Changes over time in risk of initial virological failure of combination antiretroviral therapy: a multicohort analysis, 1996 to 2002. Arch Intern Med. 2006;166:521–528. doi: 10.1001/archinte.166.5.521. [DOI] [PubMed] [Google Scholar]
- 50.Wood E, Hogg RS, Harrigan PR, Montaner JSG. When to initiate antiretroviral therapy in HIV-1-infected adults: a review for clinicians and patients. Lancet Infect Dis. 2005;5:407–414. doi: 10.1016/S1473-3099(05)70162-6. [DOI] [PubMed] [Google Scholar]
- 51.Phillips AN, Gazzard BG, Clumeck N, Losso MH, Lundgren JD. When should antiretroviral therapy for HIV be started? BMJ. 2007;334:76–78. doi: 10.1136/bmj.39064.406389.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chun TW, Fauci AS. Latent reservoirs of HIV: obstacles to the eradication of virus. Proc Natl Acad Sci USA. 1999;96:10958–10961. doi: 10.1073/pnas.96.20.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 54.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 55.Friis-Moller N, Sabin CA, Weber R. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 56.Friis-Moller N, Reiss P, Sabin CA. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 57.Nolan D, Mallal S. Antiretroviral-therapy-associated lipoatrophy: current status and future directions. Sex Health. 2005;2:153–163. doi: 10.1071/sh04058. [DOI] [PubMed] [Google Scholar]
- 58.Mocroft A, Brettle R, Kirk O. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16:1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 59.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lane HC, Neaton JD. When to start therapy for HIV infection: a swinging pendulum in search of data. Ann Intern Med. 2003;138:680–681. doi: 10.7326/0003-4819-138-8-200304150-00018. [DOI] [PubMed] [Google Scholar]
- 61.Schechter M. Therapy for early HIV infection: how far back should the pendulum swing? J Infect Dis. 2004;190:1043–1045. doi: 10.1086/422852. [DOI] [PubMed] [Google Scholar]
- 62.Ho DD. Time to hit HIV, early and hard. N Engl J Med. 1995;333:450–451. doi: 10.1056/NEJM199508173330710. [DOI] [PubMed] [Google Scholar]
- 63.Burman WJ, Reves RR, Cohn DL. The case for conservative management of early HIV disease. JAMA. 1998;280:93–95. doi: 10.1001/jama.280.1.93. [DOI] [PubMed] [Google Scholar]
- 64.Holmberg SD, Palella FJ, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection. Clin Infect Dis. 2004;39:1699–1704. doi: 10.1086/425743. [DOI] [PubMed] [Google Scholar]
- 65.Hammer SM, Eron JJ, Reiss P. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society—USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 66.Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. November 3, 2008; 1–139. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (accessed Jan 15, 2009).
- 67.Clumeck N, Pozniak A, Raffi F. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of HIV-infected adults. HIV Med. 2008;9:65–71. doi: 10.1111/j.1468-1293.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 68.Phillips AN, Dunn D, Sabin C. Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS. 2005;19:487–494. doi: 10.1097/01.aids.0000162337.58557.3d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.