Abstract
Objectives
Neuropsychological studies in subjects with bipolar disorder (BD) have reported deficits on a variety of cognitive measures. However, because the majority of subjects were medicated at the time of testing in previous studies, it is currently unclear whether the pattern of deficits reported is related to BD itself or to psychotropic medication. We addressed this issue by examining cognitive performance in a group of unmedicated, currently depressed subjects with BD.
Methods
Forty-nine unmedicated subjects who met DSM-IV criteria for BD, depressed phase, and 55 control subjects participated in this study. Most patients were diagnosed with bipolar II disorder. Performance on emotion-dependent, or ‘hot’, and emotion-independent, or ‘cold’, cognitive tasks was assessed using tests from the Cambridge Neuropsychological Test Automated Battery.
Results
The groups were well matched with respect to general intelligence and demographic variables. Deficits in the unmedicated depressed BD group were apparent on tests tapping ‘hot’ cognitive processing, for example the Cambridge Gamble task and the Probabilistic Reversal Learning task. However, other than a deficit on the Spatial Span test in the depressed BD subjects, the groups performed equivalently on most measures of ‘cold’ cognitive processing, for example visual memory, attention, and working memory.
Conclusions
These data suggest that deficits on tests involving reward processing, short-term spatial memory storage, and sensitivity to negative feedback in depressed BD subjects represent an effect of the illness itself and not mood-stabilizing medication.
Keywords: bipolar disorder, depression, hot cognition, neuropsychology, unmedicated
Most of the published neuropsychological investigations of cognitive function in bipolar disorder (BD) have been confounded by the effects of medication. While some studies reported that medicated subjects with BD performed worse than matched controls on a number of neuropsychological tests (1–5, see 6 for a review), it remains unclear whether these deficits are present in unmedicated subjects. Furthermore, while recent studies have reported less pronounced cognitive impairment in patients with bipolar II disorder (BD-II) than bipolar I disorder (BD-I) (3, 7–10), in some studies BD-II patients had a different profile of medication than those with BD-I (7, 8). The aim of this study was to determine whether cognitive impairment is observed in unmedicated subjects with BD in a group primarily comprising patients with a diagnosis of BD-II.
There is some evidence that mood-stabilizing medications, such as lithium, might themselves result in cognitive impairment. While a recent review concluded that the net effect of lithium administration was probably protective (11), it has long been known that patients using such medications report a dulling of intellectual capacity and concentration difficulties (12), and a meta-analysis concluded that lithium administration may negatively affect motor speed and verbal memory (13). Furthermore, studies administering lithium to experimental animals reported changes in intracellular signaling processes related to synaptic plasticity, providing a plausible mechanism for changes to cognitive performance following treatment in BD patients (14). Consistent with this hypothesis, recent studies reported that the degree of neuropsychological impairment in BD subjects was related to medication status, particularly the use of antipsychotics and mood-stabilizing agents (15, 16), and that medicated, but not unmedicated, BD-II subjects were impaired in several domains of cognitive function (10).
The most consistently reported deficits in BD are in the domains of sustained attention, memory, and executive function (6). Sustained attention is commonly assessed using variants of the continuous performance test (CPT), such as the Rapid Visual Information Processing Test (17). There have been numerous reports of a deficit on such tests in subjects with BD, almost exclusively in BD-I samples, typically expressed as a reduction in the percentage of correct responses in both the manic (18, 19) and euthymic (1, 20) states. This reduction in target sensitivity is not attributable to working-memory dysfunction (21) or poor inhibitory control (22). It has been hypothesized that an attentional impairment might be a trait deficit in BD (1). However, in the vast majority of studies, most BD subjects were medicated at the time of testing, and studies have so far failed to demonstrate an attentional deficit in first-degree relatives of patients with BD (2), raising the possibility that attentional deficits may be related to mood-stabilizing medication.
Some studies that have examined the performance of unmedicated subjects with BD-I on measures of attention found an attentional impairment relative to controls, with no difference relative to medicated subjects. However, the interpretation of these studies is complicated by methodological issues. Two of the studies tested pediatric samples (23, 24), in which comorbidity with attention-deficit hyperactivity disorder is an important confounding factor. Pavuluri and colleagues (24) reported no differences on a composite measure of attention, while DelBello and colleagues (23) reported that medicated BD-I subjects responded significantly more slowly than unmedicated subjects on a CPT, without any differences in accuracy. A third study also reported no difference between unmedicated BD-I subjects and controls (19), but included only five unmedicated subjects, making this negative result difficult to interpret. Moreover, a recent large study reported an attentional deficit in medicated but not unmedicated depressed BD subjects, the majority of whom were diagnosed with BD-II (10).
Deficits in the integrity of both verbal and nonverbal memory, indexed using tests such as the California Verbal Learning Test and the Pattern and Spatial Recognition Memory tests (25), have also commonly been reported in BD subjects, mainly in BD-I groups. Impairments have been reported in the manic (4, 26, 27), euthymic (5, 20), and depressed (4, 28, 29) phases in BD-I subjects. Furthermore, several studies reported that BD-II subjects’ performance was intermediate between BD-I subjects and controls on episodic memory measures (3, 7, 8). However, again subjects in these studies were medicated at the time of testing. Deficits have been reported for both recall and recognition memory in both BD-I and BD-II subjects, suggesting an impairment at encoding or consolidation (3, 4). The one study assessing memory in unmedicated pediatric BD subjects reported an impairment on measures of verbal but not visual memory (24). A recent study that directly compared groups of medicated and unmedicated depressed patients, primarily diagnosed with BD-II, reported no visual recognition memory deficit in either group (10). Notably, it has also been reported that first-degree relatives of subjects with BD are impaired on tests of verbal recall memory (30, 31), suggesting that this deficit in BD may represent a core feature of the illness unrelated to medication.
Performance on tests of ‘executive function’, including cognitive flexibility and planning, has been assessed using tests such as the Spatial Working Memory test (32), the Wisconsin Card Sort Test, and the Tower of London test (32) in BD subjects. Impaired executive function is another consistent finding in BD-I, and has been reported in mixed/manic (4, 26, 27) and euthymic (20) subjects relative to controls. Studies of BD-II subjects have also reported deficits in executive function, but less consistently (3, 7–10). The only study investigating executive function in unmedicated pediatric BD-I subjects found equivalent deficits to those seen in medicated subjects (24). There is some evidence for executive impairments in first-degree relatives of subjects with BD, though studies have produced mixed results (30, 33).
Although attention, memory, and traditional ‘cold’ measures of executive function have been investigated in a number of studies in subjects with BD, there is a dearth of studies investigating measures of function that rely on emotional or ‘hot’ processing, for example decision-making or response to negative feedback, especially in depressed BD subjects. Subjects with unipolar depression have been reported to show attentional biases towards negative stimuli, for example on the Affective Go/No-go test (26, 34), perform poorly on decision-making tasks, for example the Cambridge Gamble task (35), and show an exaggerated response to negative feedback (36, 37) that may impact on cognitive performance (38). While some studies have reported deficits in medicated manic BD subjects on such measures (26, 35, 39), few studies exist investigating performance in depressed BD subjects. One recent study reported no differences between unmedicated depressed BD-II subjects and controls on the Cambridge Gamble task (9), while another reported a deficit in decision-making on the same test in medicated depressed BD-I subjects (29). Another study reported a negative bias exclusively in medicated depressed BD-II subjects on the Affective Go/No-go test (10), though another reported no such bias in medicated depressed BD-I subjects (29).
In summary, a variety of cognitive processes have previously been shown to be impaired in subjects with BD, with BD-II subjects often scoring intermediate between BD-I subjects and controls. However, the majority of these studies included medicated BD subjects, which represents an important confound. A vital advance in our understanding of the profile of cognitive deficits associated with BD is to distinguish the effects of medication from those of illness. Hence, we examined a group of BD subjects, primarily meeting the BD-II diagnosis, who were unmedicated at the time of testing. Since deficits in memory and executive function (30, 31, 33), but not attention (2), have also been found in groups of close relatives of BD subjects (40), we hypothesized that the deficits in memory and executive function represent a core feature of the illness, and therefore predicted that they would also be apparent in this group of unmedicated subjects, while an attentional deficit would not. We also predicted that unmedicated depressed BD subjects would resemble unmedicated unipolar depressives on ‘hot’ processing tests, insofar as they would display impaired risk-adjustment on the Cambridge Gamble task and demonstrate a mood-congruent processing bias towards negative words on the Affective Go/No-go test. We also predicted that BD subjects would exhibit an exaggerated response to negative feedback, since in our previous study BD subjects scored intermediate between subjects with unipolar depression and controls on the Probabilistic Reversal Learning test (41).
Materials and methods
Participants
Forty-nine unmedicated depressed subjects with BD (36 female, mean ± SD = 33.6 ± 8.9 years) and 55 healthy controls (36 female, mean ± SD = 34.9 ± 8.1 years), matched for IQ (see Table 1), were recruited from the community by advertisement, or from the outpatient psychiatric services of the National Institute of Mental Health (NIMH) and the Howard University School of Medicine. The BD subjects met DSM-IV criteria for BD-I or BD-II and for a current major depressive episode; no patient met criteria for a mixed episode. Eleven BD subjects met criteria for BD-I and 38 for BD-II. The mean age of onset of first depressive episode was 15.8 ± 6.2 years. The mean duration of illness was 19.1 ± 10.4 years. BD subjects were recruited if they were aged between 18 and 60 years at the time of testing. The majority of BD subjects had suffered from at least three depressive episodes. All apart from three BD subjects had experienced three or more manic or hypomanic episodes. One had suffered from psychotic symptoms during a manic episode. Sixteen patients had been hospitalized for mood symptoms [range 1–7 hospitalizations]. Control subjects had no personal or family history (in first-degree relatives) of a major psychiatric disorder. Exclusion criteria for all subjects included: a major medical illness or neurological disorder; a history of head injury with loss of consciousness; substance abuse within the past six months; substance dependence within the past five years; exposure to psychotropic drugs within the three weeks prior to testing (eight weeks for fluoxetine; medications were not discontinued for the purpose of this study); electrolyte disturbance; anemia; or positive urine drug screen on laboratory testing. Additional exclusion criteria for the BD subjects were having an age at onset for the first mood episode of ≥ 40 years or psychotic manifestations to an extent that would impair the capacity to provide informed consent. After the procedures had been fully explained, all subjects gave written informed consent to participate, as approved by the NIMH Institutional Review Board.
Table 1.
Demographics and clinical characterization of study sample
| Healthy controls | Bipolar disordera | |
|---|---|---|
| Number of subjects (% female) | 55 (65%) | 49 (77%) |
| Age (years) | 34.9 (8.1) | 33.6 (8.9) |
| FSIQ | 115.7 (10.5) | 114.3 (13.4) |
| MADRS | 0.18 (0.61) | 24.8 (10.2) |
| IDS | 0.47 (1.6) | 31.6 (12.2) |
| HAM-A | 0.43 (1.2) | 16.9 (8.5) |
| Age of onset of first depressive episode | – | 15.8 (6.2) |
| Years of illness | – | 19.1 (10.4) |
Values are expressed as mean (SD) except where indicated otherwise.
FSIQ = Full-Scale Intelligence Quotient from Wechsler Abbreviated Scale of Intelligence; IDS = Inventory of Depressive Symptomatology; MADRS = Montgomery-Åsberg Depression Rating Scale; HAM-A = Hamilton Anxiety Scale.
n = 11 bipolar I disorder and 38 bipolar II disorder.
Clinical rating scales
Depression severity was assessed using the Montgomery-Åsberg Depression Rating Scale (MADRS) (42) and the Inventory of Depressive Symptomatology (43). Anxiety severity was assessed using the Hamilton Anxiety Rating Scale (44).
Neuropsychological assessment
IQ was estimated using the Wechsler Abbreviated Scale of Intelligence (WASI) (45). Neuropsychological testing was performed using the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition Ltd., Cambridge, UK). These computerized tasks were presented on an Advantech personal computer (Model PP-120T-RT) with a 10½-inch touch-screen monitor. The battery comprised the following tests: Spatial Span; Spatial Working Memory; Intra-dimensional/Extra-dimensional Set-Shifting; Spatial Recognition Memory; Pattern Recognition Memory; Delayed Match to Sample; Rapid Visual Information Processing; Cambridge Gamble task; Affective Go/No-go test; and Probabilistic Reversal Learning. For test descriptions and outcome measures, see Appendix 1. We excluded from the analyses data on the Spatial Working Memory, Pattern Recognition Memory, Affective Go/No-go and Rapid Visual Information Processing tests from four subjects whose data were included in a separate report on these measures (10), in order to ensure independence of samples between this previous study and the current study. However, for the other tests in this study, data from these four subjects were included in the analyses.
Appendix 1.
| Test | Description | Outcome measures |
|---|---|---|
| Working memory | ||
| Spatial Span | Short-term spatial memory storage test, similar to the digit-span subtest of the Wechsler Adult Intelligence Scale. Subjects are required to replicate a randomly arranged spatial sequence of boxes, beginning with two boxes and increasing to a maximum of nine boxes. | Number of boxes successfully replicated; total number of errors. |
| Spatial Working Memory | Working memory test in which subjects search for hidden ‘tokens’ within a spatial array. There are three levels of difficulty, with four, six, and eight boxes to search. An error is recorded when the subject returns to a box in which a token previously has been found within a given trial (between-search error) or when the subject returns to a box within the same search (within-search error). An estimate of the use of strategy (strategy score) is calculated by summing the number of times that the subject begins a new search from the same box for the six- and eight-box trials. | Between-search errors; within-search errors; strategy score; latency. |
| Executive function | ||
| Intra-dimensional/Extra-dimensional set-shifting (ID/ED) | Visual discrimination task indexing set learning, reversal learning, and an extra-dimensional set-shift, in which a shift of attention is required from one class of visual stimuli to another, previously irrelevant class of stimuli. | Number of errors; trials to reach criterion; mean response latency. |
| Episodic memory | ||
| Spatial Recognition Memory | Recognition memory test for spatial locations. | Percent correct; correct response latency. |
| Pattern Recognition Memory | Visuospatial recognition test in which 12 abstract colored visual patterns are presented sequentially. The subject must then recognize those presented (in reverse order) from a pair of patterns in which the incorrect pattern has not previously been presented. | Percent correct; correct response latency. |
| Delayed Match to Sample | Visuospatial recognition test in which a complex abstract pattern is presented to the subjects for 4.5 seconds and then, either immediately or following a 4- or 12-second delay, subjects are required to identify the pattern they initially viewed from a set of four patterns (i.e., the target pattern appears among three distractor patterns). Simultaneous match to sample trials are included to control for motor speed. | Percent correct; correct response latency. |
| Attention | ||
| Rapid Visual Information Processing | Continuous performance test lasting four minutes involving recognizing a specific or target sequence of three digits from a sequential presentation of single digits presented at a rate of 100 digits per minute in a pseudo-random order. | Correct response latency; number of targets correctly detected; number of commission errors; target sensitivity (A′); response bias (B″). |
| Reward and emotional processing | ||
| Cambridge Gamble task | Decision-making test in which subjects are presented with an array of 10 colored boxes, either red or blue, and are required to decide whether a yellow token is hidden inside a red or blue box. The number of blue boxes varies from one to nine. Subjects then place a bet on their decision. The amount of the bet is then added to (if correct) or subtracted from (if incorrect) the subject’s total point score. Bets are either offered in ascending order, beginning with a small bet until the subject chooses a bet, or in descending order. | Latency to make a decision; the number of times the subject chooses the most likely outcome; percentage of the available points put at risk. |
| Affective Go/No-go test | Emotional processing test in which subjects respond to either happy or sad words while ignoring words of the other valence. Every two blocks, the targets and the distractors change (‘shift’) blocks; on ‘non-shift’ blocks, the targets and distractors are of the same emotion as in the previous block. | Correct response latency; number of commission errors; number of omission errors. |
| Probabilistic Reversal Learning | Probabilistic reinforcement acquisition and reversal test in which subjects are presented with two stimuli differing only in color (one green, the other red). One stimulus is correct and the other incorrect, indicated by positive and negative feedback. After 40 trials (Stage 1), the contingencies reverse for the subsequent 40 trials (Stage 2); i.e., the stimulus that was previously correct becomes incorrect, and vice versa. | Total errors; latency; perseverative errors; maintenance error score; probability matching score (see 36, 47). |
Statistical analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences version 15.0 (SPSS, Inc., Chicago, IL, USA). Prior to analysis, data were transformed appropriately to reduce skew (46). Data conforming to the assumptions of parametric analysis were analyzed using t-tests, except where tasks had more than one stage or level of difficulty, where repeated-measures analysis of variance (ANOVA) was employed. Where the assumption of sphericity was violated in repeated-measures ANOVA, within-subjects degrees of freedom were adjusted using the Huynh-Feldt correction (46). Data not conforming to the assumptions of parametric analyses were analyzed using either Mann-Whitney U-tests or Kruskall-Wallis tests. All tests were two-tailed and p < 0.05 was considered significant. Correlations with clinical variables were carried out using Pearson’s r, or Spearman’s ρ for nonparametric variables. Given the large number of correlations performed, we adopted a more stringent threshold of p < 0.005 for correlations between neuropsychological outcome measures and clinical variables.
Results
The groups were well matched for gender balance, age and IQ. The BD subjects were significantly more depressed and anxious than healthy controls (see Table 1). Data from neuropsychological measures are presented in Table 2. Due to occasional instances of computer failure, data were not recorded from all subjects on all tests.
Table 2.
Performance on tests of cognitive function in unmedicated depressed subjects with bipolar disorder and controls
| Test | Measure/stage of test | Healthy controls | Bipolar disordera |
|---|---|---|---|
| Spatial Span | |||
| Span | 6.7 (1.4) | 6.1 (1.7)b | |
| Spatial Working Memory | |||
| Strategy | 31.6 (5.7) | 32.6 (6.7) | |
| Between-search errors | 4-box | 1.3 (2.7) | 0.73 (1.9) |
| 6-box | 5.0 (6.8) | 6.8 (8.1) | |
| 8-box | 15.4 (12.0) | 16.0 (14.0) | |
| Within-search errors | 4-box | 0.23 (0.64) | 0.11 (0.75) |
| 6-box | 0.42 (1.1) | 0.73 (2.4) | |
| 8-box | 1.6 (3.2) | 1.8 (3.0) | |
| Intra-dimensional/Extra-dimensional (ED) shift | |||
| Percent completing task | 82% | 78% | |
| Total number of errors | 16.2 (11.7) | 18.8 (11.2) | |
| Total reversal errors | 5.8 (5.1) | 5.5 (4.9) | |
| Errors at ED shift | 8.6 (9.5) | 11.1 (10.3) | |
| Spatial Recognition Memory | |||
| Percent correct | 81.3 (12.7) | 81.5 (11.6) | |
| Latency | 2144 (710) | 1972 (670) | |
| Pattern Recognition Memory | |||
| Percent correct | 91.5 (8.0) | 89.6 (10.3) | |
| Latency | 1957 (622) | 1853 (434) | |
| Delayed Match to Sample | |||
| Simultaneous | Percent correct | 95.4 (15.3) | 94.0 (14.6) |
| Delayed | 0 sec percent correct | 88.6 (14.1) | 86.9 (13.6) |
| 4 sec percent correct | 89.4 (12.6) | 85.4 (17.4) | |
| 12 sec percent correct | 82.9 (14.3) | 77.3 (21.9) | |
| Latency | 4046 (1020) | 3747 (1233) | |
| Probability of error following error | 0.05 (0.12) | 0.10 (0.14)b | |
| Rapid Visual Information Processing | |||
| A′ | 0.93 (0.04) | 0.92 (0.05) | |
| B″ | 0.97 (0.06) | 0.96 (0.05) | |
| Latency | 493 (179) | 462 (89) | |
| Gamble | |||
| Choice of most likely outcome | 6:4 ratio | 0.95 (0.073) | 0.94 (0.096) |
| 7:3 ratio | 0.97 (0.060) | 0.95 (0.083) | |
| 8:2 ratio | 0.99 (0.040) | 0.98 (0.043) | |
| 9:1 ratio | 0.99 (0.019) | 0.99 (0.035) | |
| Percentage bet | 6:4 ratio | 38.4 (16.3) | 40.5 (16.0) |
| 7:3 ratio | 50.1 (14.5) | 50.2 (14.1) | |
| 8:2 ratio | 64.8 (13.9) | 62.4 (14.2) | |
| 9:1 ratio | 73.1 (14.4) | 66.9 (18.1) | |
| Risk-adjustment | 34.7 (17.9) | 26.4 (21.2)b | |
| Latency | 6:4 ratio | 2324 (1135) | 2542 (944) |
| 7:3 ratio | 2103 (1121) | 2242 (836) | |
| 8:2 ratio | 2034 (951) | 2305 (968) | |
| 9:1 ratio | 2248 (1340) | 2608 (2072) | |
| Probabilistic reversal learning | |||
| Discrimination | Total errors | 1.7 (3.3) | 1.5 (2.8) |
| Probability matching score | 0.06 (0.10) | 0.05 (0.12) | |
| Maintenance error score | 0.04 (0.09) | 0.03 (0.07) | |
| Latency | 816 (328) | 724 (157) | |
| Reversal | Total errors | 8.3 (7.8) | 7.7 (5.5) |
| Perseveration errors | 4.6 (4.3) | 3.9 (3.3) | |
| Probability matching score | 0.18 (0.24) | 0.19 (0.16) | |
| Maintenance error score | 0.03 (0.08) | 0.08 (0.15)c | |
| Latency | 750 (246) | 672 (123) | |
| Affective Go/No-go | |||
| Happy shift | Latency | 505 (54) | 481 (50) |
| Commission errors per block | 2.5 (2.3) | 2.4 (1.6) | |
| Omission errors per block | 0.92 (1.4) | 0.88 (1.5) | |
| Happy non-shift | Latency | 500 (63) | 484 (56) |
| Commission errors per block | 1.8 (2.1) | 1.9 (1.6) | |
| Omission errors per block | 0.41 (0.72) | 0.94 (1.5) | |
| Sad shift | Latency | 501 (51) | 485 (73) |
| Commission errors per block | 2.9 (2.8) | 2.8 (2.1) | |
| Omission errors per block | 0.84 (1.6) | 0.74 (1.0) | |
| Sad non-shift | Latency | 510 (56) | 487 (56) |
| Commission errors per block | 2.1 (2.6) | 0.94 (1.5) | |
| Omission errors per block | 0.73 (1.2) | 2.2 (2.1) | |
Values are expressed as mean (SD) except where indicated otherwise.
n = 11 bipolar I disorder and 38 bipolar II disorder.
p < 0.05.
p < 0.1.
Working memory/executive function
BD subjects scored significantly lower on spatial span than controls [F(1,102) = 6.2, p = 0.015]. As expected, subjects made more errors at the more difficult levels of the spatial working memory test for both between-search errors [F(1.9,184.2) = 154.89, p < 0.001, ε = 0.97] and within-search errors [F(1.8,166.3) = 21.4, p < 0.001, ε = 0.88]. However, the main effect of group and the group-by-difficulty interaction were nonsignificant for both measures (p > 0.1). The groups also did not differ in terms of strategy score (F < 1).
On the Intra-dimensional/Extra-dimensional shift test, there was no significant group difference in the overall number of subjects completing the task (χ2<1) or in the number of total errors committed (F < 1). The groups also did not differ in terms of the total number of reversal errors (U = 1053, p = 0.6) or errors at the extra-dimensional shift stage (t < 1).
Episodic memory
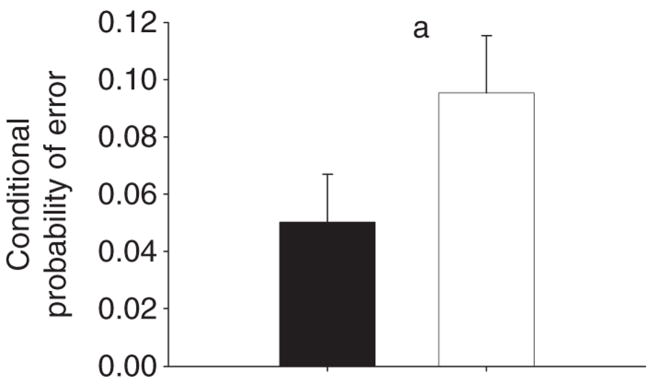
The groups did not differ in terms of accuracy or latency on the Spatial Recognition Memory or Pattern Recognition Memory tests: Spatial Recognition Memory percent correct: [t(98) = 1.3, p = 0.2]; latency t < 1; Pattern Recognition Memory t < 1 for both percent correct and latency. On the Delayed Match to Sample, BD subjects and controls did not differ in terms of error rate on simultaneous trials (U = 1076, p = 0.17) or delayed trials [F(2,97) = 1.9, p = 0.18] compared to healthy controls. As expected, subjects made more errors at longer delays [F(2,194) = 11.7, p < 0.001], though the group-by-delay interaction was nonsignificant (p > 0.1). The groups did not differ on response latency [t(97) = 1.3, p = 0.19]. Despite similar accuracy on the delayed trials, BD subjects were significantly more likely to make an error if they had made an error on the preceding trial (i.e., the conditional probability of making an error, given a preceding error) relative to controls (U = 961.5, p = 0.024) (Fig. 1).
Fig. 1.
Increased conditional probability of error in subjects with bipolar disorder. The conditional probability of making an error having made an error on the previous trial on the Delayed Match to Sample test was higher in depressed bipolar disorder subjects than controls
ap < 0.05 comparing patients to controls. Error bars represent one standard error of the mean.
Sustained attention
The groups did not differ in terms of target sensitivity [t(90) = 1.4, p = 0.16], response bias (U = 1023, p = 0.8), or response latency [t(90) = 1.1, p = 0.3) on the Rapid Visual Information Processing test.
Cambridge Gamble task
Analysis of proportionate choice of the most likely outcome data revealed that BD subjects did not make more disadvantageous choices than controls in terms of choosing the option most likely to result in a win (F < 1).
However, analysis of the amount of points subjects were prepared to stake on their decisions revealed that BD subjects demonstrated impaired risk-adjustment, increasing their bets at increasingly favorable chances of winning at a slower rate than controls [group × ratio interaction: F(1.5, 144.8) = 3.7, p = 0.040, ε = 0.5] (Fig. 2). As expected, subjects also increased their bets with increasing probability of winning [main effect of ratio: F(1.5,144.8) = 216.1, p < 0.001, ε = 0.5] and bet more in the ascending than the descending condition [main effect of condition: F(1,97) = 71.3, p < 0.001]. The main effect of group, group × condition interaction, and group × condition × ratio interaction were all nonsignificant (p > 0.1 for all).
Fig. 2.

Impaired risk-adjustment in depressed subjects with bipolar disorder. Depressed subjects with bipolar disorder exhibited impaired risk-adjustment on the Cambridge Gamble task relative to controls. Error bars represent one standard error of the mean.
Analysis of deliberation time data revealed that subjects made their decisions more quickly at more favorable gambles [F(2.7,263.1) = 6.2, p = 0.001, ε = 0.9]. BD subjects showed a strong trend toward responding more slowly than controls [F(1,97) = 3.9, p = 0.051], but all interactions with group were nonsignificant (p > 0.1).
Affective shifting
Analysis of latency data on the Affective Go/No-go test revealed no significant effects (p > 0.1), including emotional bias score. Analysis of commission error data revealed that subjects made more commission errors on shift than nonshift blocks [F(1,69) = 12.4, p = 0.001). However, the main effect of group and all interactions with group were nonsignificant. Analysis of omission error data revealed no significant effects (p > 0.1).
Probabilistic Reversal Learning
To ensure the demands of the Probabilistic Reversal Learning task had been learned, only subjects who passed Stage 1 (discrimination) were included in subsequent analyses (47). Pass rates for the discrimination stage were similar for the BD patient (87%) and control (93%) groups ( ). Pass rates for the whole task were also similar for the BD patient (87%) and control (83%) groups. Subjects made more errors [F(1,63) = 176.9, p < 0.001] and responded more quickly [F(1,63) = 5.4, p = 0.023] at the reversal stage of the Probabilistic Reversal Learning task than at the discrimination stage, but the main effect of group and group × stage interaction were both nonsignificant for both measures (p > 0.1). The groups also did not differ in terms of the number of consecutive perseverative responses immediately following reversal (t < 1).
The maintenance error score, which measures the tendency to switch to the inappropriate response once the correct stimulus set has been acquired, increased from the discrimination stage to the reversal stage [F(1,57) = 5.2, p = 0.026]. However, this effect was qualified by a significant group × stage interaction [F(1,57) = 7.9, p = 0.007]. Post hoc analysis of the simple main effects revealed that the BD subjects exhibited increased maintenance error score at the reversal stage [F(1,23) = 7.3, p = 0.013], while the controls did not (F < 1) (Fig. 3). Subjects exhibited increased probability matching score at the reversal stage relative to the discrimination stage [F(1,63) = 31.3, p < 0.001], but the main effect of group and group × stage interaction were both nonsignificant (p > 0.1).
Fig. 3.

Increased sensitivity to negative feedback in depressed subjects with bipolar disorder. Depressed subjects with bipolar disorder exhibited increased maintenance error score at the reversal stage of the Probabilistic Reversal Learning test relative to controls
ap < 0.05 at Stage 2 relative to Stage 1. Error bars represent one standard error of the mean.
Correlations with clinical variables
No correlations with clinical variables achieved statistical significance.
Discussion
This is the first study to assess neuropsychological performance on measures of decision-making, short-term spatial memory storage capacity, and sensitivity to negative feedback in a large group of unmedicated depressed BD subjects. This study also included the largest sample of unmedicated depressed BD subjects assessed to date with respect to neuropsychological performance. In accordance with previous studies conducted in medicated BD samples, we identified deficits on measures of spatial memory and decision-making. In addition, we found that the exaggerated sensitivity to negative feedback identified previously in unipolar depressed subjects also extends to unmedicated bipolar depressed subjects. However, we did not identify deficits on tests of attention, visual episodic memory, or ‘cold’ executive function.
Performance on tests of ‘hot’ cognition
Exaggerated response to negative feedback was apparent on two tests in this study; BD subjects made a disproportionate number of errors immediately following an incorrect response on the Delayed Match to Sample test, and exhibited elevated maintenance error scores on the Probabilistic Reversal Learning task, though only at the reversal stage. These findings extend previous work suggesting that both depressed unipolar subjects and manic BD-I subjects show an exaggerated response to negative feedback (36, 38, 39, 48). These data therefore provide further support for the hypothesis proposed by Sahakian and colleagues (49) that cognitive deficits in mood disorders might be causally related to a ‘catastrophic response to perceived failure’. This hypothesis is supported by a recent study that demonstrated enhanced error-related negativity, thought to be related to activity in the anterior cingulate, in depressed subjects (50).
The performance of this depressed BD group on the Cambridge Gamble task was also strikingly similar to that reported previously in manic BD-I subjects and depressed subjects with unipolar depression (35), though another recent study did not identify this pattern of performance (9). The depressed BD subjects exhibited impaired risk-adjustment, reflecting a failure to adapt behavior in response to increasingly favorable scenarios. The only other study assessing gambling performance in depressed BD subjects reported impaired quality of decision-making and increased deliberation times without an effect on risk-adjustment (29). However, the subjects tested by Rubinsztein and colleagues (29) were medicated at the time of testing, older than our subjects, and were all diagnosed with BD-I, which might explain these discrepant findings. Consistent with the recent study of Holmes and colleagues (10), we did not find any evidence of emotional bias on the Affective Go/No-go test in unmedicated depressed BD subjects.
The finding of abnormal performance on measures related to ‘hot’ cognition is consistent with structural and functional neural changes observed in BD subjects using magnetic resonance imaging and positron emission tomography. For example, it has been reported that depressed subjects with BD showed reduced gray matter volume in the subgenual anterior cingulate cortex (51) and increased metabolism in the amygdala and several other subcortical structures in both medicated and unmedicated samples (52–54). Furthermore, consistent with the finding of Chiu and Deldin discussed above (50), using a probabilistic reversal learning task very similar to that employed in the present study, Taylor Tavares and colleagues (41) recently reported that unmedicated depressed subjects with BD exhibited enhanced response to spurious negative feedback in the anterior cingulate, though this result only approached significance in their sample. Therefore, convergent evidence supports the hypothesis that both emotion-dependent cognitive processes and the neural circuits that support them are dysfunctional in depressed subjects with BD, and that this is a feature of the illness itself as opposed to a confounding effect of mood-stabilizing medication.
Lack of attentional deficit in BD subjects
Deficits on continuous performance tests have been observed in the manic and euthymic states in medicated BD subjects (1, 18–20). Therefore, the lack of difference between these depressed BD subjects and controls on the Rapid Visual Information Processing test was surprising. Our negative result may partially be explained by the fact that the version of the Rapid Visual Information Processing test used in the present study was only four minutes long. Previous studies, which used a seven-minute version, noted that the deficit in performance increases over the course of the Rapid Visual Information Processing test, and is only significant after the first minute of the test (1). Therefore, ceiling effects may have been observed in this task. However, other studies reported that the deficit in BD subjects does not change over the course of the continuous performance test (20), and also that manic subjects were more impaired than euthymic subjects (6). Furthermore, the recent study by Holmes and colleagues (10) that used the same version of the Rapid Visual Information Processing test identified a deficit in medicated but not unmedicated depressed patients, predominantly of the BD-II type, relative to controls (10). Therefore, it is also possible that attentional deficits previously identified in BD subjects using continuous performance tasks may be, at least in part, related to the effects of medication (55).
Memory and executive function performance
The finding of impaired performance on the Spatial Span test in BD subjects replicates previous findings (4). However, the lack of differences between the BD and control groups on the Pattern Recognition Memory, Spatial Working Memory, Delayed Match to Sample and Intra-dimensional/Extra-dimensional shift tests was surprising. Deficits in both BD-I and BD-II subjects in manic, euthymic, and depressed states previously have been reported on these and other similar tests (1, 3, 4, 7, 8, 28). However, these studies tested medicated samples; previously, Frangou and colleagues (56) reported that executive dysfunction was related to current neuroleptic treatment, and a more recent large study reported that deficits in recognition memory and cognitive flexibility in BD subjects were related to lithium treatment (16).
Study weaknesses and potential improvements
Clinical and demographic characteristics of the BD cohort
Nevertheless, differences other than medication effects may have existed between our patient sample and those of previous studies, which may have contributed to the discrepant results across studies. For example, the medicated versus unmedicated status of BD depressed subjects may be associated with complex effects on illness severity. Because subjects were untreated, their mood and anxiety symptoms may have been more severe, and may thus have interfered to a greater extent with cognitive function (57). On the other hand, the requirement for volunteers to be unmedicated at study entry may have introduced a selection bias, as cases requiring imminent or involuntary treatment due to disruptive or threatening manic behavior, agitation, psychosis, or suicidal ideation were excluded from participation.
Moreover, while all subjects met criteria for a current major depressive episode and were subsequently entered into treatment programs, they were recruited from outpatient clinical or community settings. Compared to BD subjects recruited from inpatient facilities, outpatients with BD generally show less social and occupational impairment, and are less likely to manifest agitation, psychosis, danger to self or others, and comorbid conditions; such clinical features may influence neuropsychological performance. Furthermore, the majority of our subjects met criteria for BD-II, which by definition implied that they had never been hospitalized, incarcerated, psychotic, or severely impaired during manic episodes. Unfortunately, we were only able to include 11 BD-I subjects in the present study, which reflects the difficulty faced in recruiting unmedicated BD-I subjects. This low sample size makes any direct comparison between the BD-I and BD-II groups difficult to interpret. Therefore, it is possible that the subjects we tested were less severely ill than in previous studies, which might affect cognitive performance (58). However, the MADRS scores of the BD subjects we studied indicated depression severity in the moderate-to-severe range, and many of our subjects were occupationally disabled due to severe depression.
We note that the BD patients included in this study were of above-average IQ. Therefore, our sample is not representative of BD patients generally; high premorbid IQ or other unknown factors may have exerted protective effects, resulting in relatively unimpaired cognitive performance on ‘cold’ processing tests. The clinical and demographic heterogeneity within BD samples and the non-availability of biological measures of disease pathophysiology render comparisons of illness severity across BD samples difficult, and elucidating the extent to which psychotropic drugs influence neuropsychological function in BD will ultimately depend upon longitudinal studies.
Statistical concerns
Another potential limitation of our study was that, to reduce the risk of Type II error, we did not employ any correction for multiple statistical comparisons. Nevertheless, many of our findings replicated those of previous studies, making the probability of Type I error lower. Moreover, the novel findings from the current study that on both the Delayed Match to Sample and Probabilistic Reversal Learning tasks the BD subjects showed exaggerated sensitivity to negative feedback (i.e., higher conditional probability of making an error, given a preceding error) constitute an extension of previous findings obtained in unipolar depressed subjects, which may have been limited to unipolar depressed subjects previously because of the low statistical power of previous studies of BD. For example, in a previous study involving a subset of the depressed BD-II subjects studied herein (n = 17), we reported that depressed BD-II subjects did not differ from controls on a number of neuropsychological measures (9). However, these seemingly inconsistent results may be attributable to the increased statistical power of our current analyses, since in our earlier study (9) scores of the depressed BD subjects were intermediate between those of controls and unipolar depressives. In the present study we were able to include data from more than twice as many BD-II subjects and controls, and had 80% power to detect an effect size of 0.55 at a two-tailed alpha of 0.05.
Cognitive measures included in this study
We did not include a measure of verbal memory in this study, since a verbal memory test was not available in the CANTAB battery when we began the study. This was unfortunate, since verbal memory appears to be a reliably impaired cognitive process in BD and the strongest candidate for a cognitive endophenotype (59). We also included few measures of executive function. Future studies including unmedicated subjects with BD should include measures of verbal memory and executive function to assess whether these measures are impaired in patients with BD regardless of medication status.
Summary
In summary, we identified deficits on measures of ‘hot’ cognitive processing, including decision-making and responses to negative feedback, in depressed BD subjects who were unmedicated at the time of testing. However, with the exception of impaired spatial span, there was relative sparing of performance on tests of ‘cold’ cognition, including sustained attention, memory, and ‘cold’ executive function. These data suggest that deficiencies on tests that involve reward and punishment processing in BD subjects are unrelated to medication. While the lack of deficit on other tests raises the possibility that some neuropsychological impairments reported in the literature in BD may be related to the confounding effects of psychotropic medication, longitudinal studies in more representative samples of unmedicated BD patients are required to test this hypothesis.
Acknowledgments
This research was supported by the Intramural Research Program of the NIMH. JPR and JTT were supported by the NIH-Cambridge Health Science Scholars Program.
Footnotes
JPR, LC and BJS have performed consultancy work for Cambridge Cognition, owners of the CANTAB. DMC, SKG, JTT, KE, SW, JMK, CAZ and WCD have no competing interests to declare.
References
- 1.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- 2.Clark L, Kempton MJ, Scarna A, Grasby PM, Goodwin GM. Sustained attention-deficit confirmed in euthymic bipolar disorder but not in first-degree relatives of bipolar patients or euthymic unipolar depression. Biol Psychiatry. 2005;57:183–187. doi: 10.1016/j.biopsych.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 5.Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med. 2000;30:1025–1036. doi: 10.1017/s0033291799002664. [DOI] [PubMed] [Google Scholar]
- 6.Clark L, Sahakian BJ. Neuropsychological and biological approaches to understanding bipolar disorder. In: Jones SH, Bentall RP, editors. The Psychology of Bipolar Disorder New Developments and Research Strategies. Oxford: Oxford University Press; 2006. pp. 139–178. [Google Scholar]
- 7.Simonsen C, Sundet K, Vaskinn A, et al. Neurocognitive profiles in bipolar I and bipolar II disorder: differences in pattern and magnitude of dysfunction. Bipolar Disord. 2008;10:245–255. doi: 10.1111/j.1399-5618.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- 8.Torrent C, Martínez-Arán A, Daban C, et al. Cognitive impairment in bipolar II disorder. Br J Psychiatry. 2006;189:254–259. doi: 10.1192/bjp.bp.105.017269. [DOI] [PubMed] [Google Scholar]
- 9.Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Holmes MK, Erickson K, Luckenbaugh DA, et al. A comparison of cognitive functioning in medicated and unmedicated patients with bipolar depression. Bipolar Disord. 2008;10:806–815. doi: 10.1111/j.1399-5618.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fountoulakis KN, Vieta E, Bouras C, et al. A systematic review of existing data on long-term lithium therapy: neuroprotective or neurotoxic? Int J Neuropsychopharmacol. 2008;11:269–287. doi: 10.1017/S1461145707007821. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. New York: Oxford University Press; 2007. [Google Scholar]
- 13.Pachet AK, Wisniewski AM. The effects of lithium on cognition: an updated review. Psychopharmacology. 2003;170:225–234. doi: 10.1007/s00213-003-1592-x. [DOI] [PubMed] [Google Scholar]
- 14.Quiroz JA, Gould TD, Manji HK. Molecular effects of lithium. Mol Interv. 2004;4:259–272. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson S, Goldstein LH, Landau S, Raymont V, Frangou S. The Maudsley Bipolar Disorder Project: the effect of medication, family history, and duration of illness on IQ and memory in bipolar I disorder. J Clin Psychiatry. 2003;64:86–93. [PubMed] [Google Scholar]
- 16.Savitz JB, van der Merwe L, Stein DJ, Solms M, Ramesar RS. Neuropsychological task performance in bipolar spectrum illness: genetics, alcohol abuse, medication and childhood trauma. Bipolar Disord. 2008;10:479–494. doi: 10.1111/j.1399-5618.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 17.Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry. 1989;154:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- 18.Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry. 2001;158:1605–1611. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- 19.Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE, Jr, Hawkins JM. Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry. 1999;156:139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JM, Gallagher P, Hughes JH, et al. Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- 21.Harmer CJ, Clark L, Grayson L, Goodwin GM. Sustained attention deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologia. 2002;40:1586–1590. doi: 10.1016/s0028-3932(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 22.Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. J Affect Disord. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 23.DelBello MP, Adler CM, Amicone J, et al. Parametric neurocognitive task design: a pilot study of sustained attention in adolescents with bipolar disorder. J Affect Disord. 2004;82(Suppl 1):S79–S88. doi: 10.1016/j.jad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Pavuluri MN, Schenkel LS, Aryal S, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- 25.Sahakian BJ, Morris RG, Evenden JL, et al. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111:695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- 26.Murphy FC, Sahakian BJ, Rubinsztein JS, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 27.Badcock JC, Michiel PT, Rock D. Spatial working memory and planning ability: contrasts between schizophrenia and bipolar I disorder. Cortex. 2005;41:753–763. doi: 10.1016/s0010-9452(08)70294-6. [DOI] [PubMed] [Google Scholar]
- 28.Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007;9:114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 29.Rubinsztein JS, Michael A, Underwood BR, Tempest M, Sahakian BJ. Impaired cognition and decision-making in bipolar depression but no ‘affective bias’ evident. Psychol Med. 2006;36:629–639. doi: 10.1017/S0033291705006689. [DOI] [PubMed] [Google Scholar]
- 30.Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neuro-cognitive approach. Psychol Med. 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- 31.Gourovitch ML, Torrey EF, Gold JM, Randolph C, Weinberger DR, Goldberg TE. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol Psychiatry. 1999;45:639–646. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 32.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 33.Zalla T, Joyce C, Szoke A, et al. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Res. 2004;121:207–217. doi: 10.1016/s0165-1781(03)00252-x. [DOI] [PubMed] [Google Scholar]
- 34.Erickson K, Drevets WC, Clark L, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- 35.Murphy FC, Rubinsztein JS, Michael A, et al. Decision-making cognition in mania and depression. Psychol Med. 2001;31:679–693. doi: 10.1017/s0033291701003804. [DOI] [PubMed] [Google Scholar]
- 36.Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. 2003;33:455–467. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- 37.Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med. 1998;28:559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- 38.Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel ES. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J Neurol Neurosurg Psychiatry. 1997;63:74–82. doi: 10.1136/jnnp.63.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minassian A, Paulus MP, Perry W. Increased sensitivity to error during decision-making in bipolar disorder patients with acute mania. J Affect Disord. 2004;82:203–208. doi: 10.1016/j.jad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Robinson LJ, Thompson JM, Gallagher P, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Taylor Tavares JV, Clark L, Williams GB, Furey ML, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–1126. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 43.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 45.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 46.Howell DC. Statistical Methods for Psychology. London: Duxbury Press; 2002. [Google Scholar]
- 47.Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 48.Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med. 1996;26:975–989. doi: 10.1017/s0033291700035303. [DOI] [PubMed] [Google Scholar]
- 49.Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26:591–603. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- 50.Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. Am J Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- 51.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 52.Mah L, Zarate CA, Jr, Singh J, et al. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 54.Bauer M, London ED, Rasgon N, et al. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Mol Psychiatry. 2005;10:456–469. doi: 10.1038/sj.mp.4001647. [DOI] [PubMed] [Google Scholar]
- 55.Bora E, Vahip S, Akdeniz F. Sustained attention deficits in manic and euthymic patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1097–1102. doi: 10.1016/j.pnpbp.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 56.Frangou S, Donaldson S, Hadjulis M, Landau S, Goldstein LH. The Maudsley Bipolar Disorder Project: executive dysfunction in bipolar disorder I and its clinical correlates. Biol Psychiatry. 2005;58:859–864. doi: 10.1016/j.biopsych.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 57.Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interactions between emotion and cognition. Cogn Emot. 1998;12:353–385. [Google Scholar]
- 58.Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: evidence of modest impairment. Biol Psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]