Abstract
Objective
To compare measures of body fat and lean mass and the prevalence of abnormal body composition phenotypes (sarcopenia, overfat, and sarcopenic obesity) in men and women with rheumatoid arthritis (RA) versus matched controls, and to explore the disease-related predictors of abnormal body composition in patients with RA.
Methods
A total of 189 men and women with RA and 189 age-, sex-, and race-matched non-RA controls underwent dual-energy x-ray absorptiometry for measurement of total and regional body fat and lean mass. Continuous and categorical measures of body composition were compared between RA and control subjects by sex and according to categories of body mass index (BMI). Within the group of RA patients, demographic, lifestyle, and RA disease and treatment characteristics were compared for RA patients with healthy body composition versus those with abnormal body composition phenotypes.
Results
Compared with non-RA controls, RA status was significantly associated with greater odds of sarcopenia, overfat, and sarcopenic obesity in women, but not in men. Relative differences in body composition phenotypes between RA and control subjects were greatest for patients in the normal weight BMI category (<25 kg/m2). Among RA characteristics, increasing joint deformity, self-reported disability scores, C-reactive protein levels, rheumatoid factor seropositivity, and a lack of current treatment with disease-modifying antirheumatic drugs were significantly associated with abnormal body composition.
Conclusion
Abnormal body composition phenotypes are overrepresented in patients with RA, particularly in those in the normal weight BMI range. RA-associated disease and treatment characteristics contribute to this increase in abnormal body composition.
INTRODUCTION
Although the first description of abnormal body composition (BC) in rheumatoid arthritis (RA) patients was published in 1873 (1), subsequent investigations of BC in patients with RA have been sparse. Over a decade ago, Roubenoff and colleagues described an association of reduced body cell mass with inflammatory cytokine levels in patients with RA (2), coining the term “rheumatoid cachexia.” Additionally, increased fat mass in patients with RA has been reported (3), but not uniformly confirmed, in clinical studies.
Emerging evidence suggests that the proportions and bodily distribution of fat and lean mass have important implications for health. Reduced lean mass, at its extreme termed “sarcopenia,” and excess body fatness are predictors of poor health outcomes in the general population. Loss of lean mass may lead to weakness, disability, and metabolic abnormalities (4,5). Gain of fat mass may predispose to diabetes, hypertension, and risk for cardiovascular disease (6). Adipose itself is a potent source of inflammatory cytokines that may contribute to systemic inflammation (7). The combination of muscle loss and fat mass gain, at its extreme referred to as “sarcopenic obesity,” is theorized to compound these individual risks (8). Interestingly, many of these same adverse health outcomes are features of RA (9,10), yet the potential for abnormal BC to mediate their effects in RA is unknown. Understanding these relationships is important to identifying factors that are potentially modifiable.
Most studies have used body mass index (BMI), a method of adjusting body weight for height, as a proxy measure of BC. However, the validity of this approach has been questioned, because individuals with the same BMI may have dramatically different BCs (11). The few prior studies of BC in patients with RA (2,3,12,13) provide little information on the prevalence of sarcopenia, excess body fatness (hereafter referred to as “overfat”), or sarcopenic obesity in RA, nor do they identify the disease-related predictors of these abnormal phenotypes. In addition, these reports largely predate the introduction of biologic disease-modifying antirheumatic drugs (DMARDs) and the emerging emphasis on tight control of disease activity. The same time period has witnessed population increases in obesity and sedentary lifestyles in both the US and Europe. Given these temporal trends, an updated appraisal of BC in RA patients is warranted.
In the present investigation, we examined dual x-ray absorptiometry (DXA)–derived BC characteristics and phenotypes in RA subjects compared with matched non-RA controls. Disease-associated predictors of BC phenotype were examined in the RA group, adjusting for pertinent confounding demographic and lifestyle characteristics.
SUBJECTS AND METHODS
Study subjects
RA subjects were participants in the Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis (ESCAPE RA), a cohort study investigating the prevalence, progression, and risk factors for subclinical cardiovascular disease in RA patients. ESCAPE RA participants were ages 45–84 years, met American College of Rheumatology (formerly the American Rheumatism Association) 1987 classification criteria for RA (14), and were excluded if they reported a prior physician-diagnosed cardiovascular event or procedure. Subjects weighing >300 pounds were also excluded due to limitations of imaging equipment used.
RA participants were recruited from patients followed at the Johns Hopkins Arthritis Center and by referral from local rheumatologists. The study was approved by the Institutional Review Board of the Johns Hopkins Hospital, with all subjects providing written informed consent. Enrollment occurred between October 2004 and May 2006.
Each RA subject was matched by sex, race, and age (within 1 year) to 1 subject without RA from the Baltimore Longitudinal Study of Aging (BLSA), but otherwise fulfilled entry criteria for ESCAPE RA. The BLSA is an ongoing longitudinal study of normative aging conducted at the Clinical Research Branch of the National Institute on Aging, for which a large cohort of volunteers undergoes comprehensive examinations every 1–4 years. DXA and other BC data were obtained during 1 of these examinations between 2003 and 2006.
Measures
BC assessments
All subjects underwent total body DXA scanning on a Lunar Prodigy DXA scanner (GE/Lunar Radiation, Madison, WI), RA subjects at the Johns Hopkins Bayview General Clinical Research Center and BLSA subjects at the Clinical Research Branch facility at Harbor Hospital, Baltimore, Maryland. Fat, lean, and bone mass for the total body (minus the head) and per region (arms, legs, and trunk) were measured and analyzed using Prodigy software, version 05.60.003 (GE/Lunar Radiation). This method uses an x-ray tube to direct 2 energy levels of photons through the body that, when attenuated as they pass through body tissues, are measured by a detector. Fat, lean, and bone masses are quantified using complex calculations based on the attenuation properties of the photons measured by the detector. Automated quantification of total and regional body tissue masses using the manufacturer’s validated software is in real time, with the entire acquisition and analysis process accomplished by the scanner and technician at the time of subject scanning (15). Daily quality control and calibration procedures were performed on each DXA scanner using the manufacturer’s standard.
To ensure comparability of DXA-assessed data, cross-calibration of the 2 scanners was performed using the same phantom standard. The phantom was scanned 30 times per scanner, with a mean difference of 0.9%. This negligible difference required no correction factor.
All subjects underwent height (in cm) and weight (in kg) measurements per standardized protocols, with the subject standing and wearing light clothing without shoes.
BC definitions
BMI was calculated as weight (in kg) divided by height (in m2). Body fat percentage was calculated as the proportion of total fat mass to total mass. Skeletal muscle mass was estimated using the method proposed by Kim et al (16), and when adjusted for height, yields the relative skeletal muscle index. This method has been validated and is highly correlated (r = 0.97) with total skeletal muscle mass as measured by total body magnetic resonance imaging (16). Appendicular fat and lean tissue masses were computed as the sum of the tissue compartment (fat or lean) of both arms and legs combined.
BC phenotypes
Sex-stratified cut points for defining sarcopenia were based on the criteria proposed by Janssen et al (17), defining sarcopenia as a relative skeletal muscle index ≤5.75 kg/m2 in women and ≤8.50 kg/m2 in men. These cut points were highly predictive of physical disability in older participants in the Third National Health and Nutrition Examination Survey (5).
The term obesity is commonly used to refer to a BMI ≥30 kg/m2 (18). To differentiate terms, overfat was adopted to denote excess body fatness from DXA. Overfat was defined with the criteria proposed by Cesari et al (19), using age-, sex-, and race-stratified cut points of body fat percentage from a large cohort of healthy adults (20). Sarcopenic obesity was defined as fulfilling the criteria for both sarcopenia and overfat. For the within-RA comparisons, subjects were further divided into subgroups: healthy BC (neither sarcopenic nor overfat), overfat but not sarcopenic, sarcopenic but not overfat, and sarcopenic obesity.
Patient assessments
Demographic, lifestyle, and RA characteristics were assessed on the same day as DXA by trained examiners performing a structured interview with formalized questionnaires.
RA disease characteristics
Forty-four joints (10 meta-carpophalangeal, 10 proximal interphalangeal, 2 wrists, 2 elbows, 2 shoulders, 2 hips, 2 knees, 2 tibiotalar, 2 subtalar, and 10 metatarsophalangeal) were examined for swelling, tenderness, deformity, and surgical replacement or fusion by a single trained assessor (MT). The RA Disease Activity Score in 28 joints (DAS28) was calculated using C-reactive protein (CRP) level (21). RA disease duration was assessed by patient self-report from the date of diagnosis. RA disease severity was estimated using the sum of joints exhibiting deformity or surgical replacement/fusion (22). Functional limitation was assessed with the Health Assessment Questionnaire (HAQ) (23). Current and past use of glucocorticoids and biologic and nonbiologic DMARDs was ascertained by detailed examiner-administered questionnaires. Cumulative glucocorticoid exposure was calculated based on subject self-report, with confirmation from medical records when available.
Lifestyle characteristics
Medical comorbidity was assessed using the Charlson Index of Comorbidity (24), depressive symptoms by the Center for Epidemiologic Studies Depression Scale (25), and physical activity by the 7-Day Physical Activity Recall questionnaire (26). Regular exercise was defined according to current recommendations for leisure-time physical activity (27), i.e., daily average of the weekly total of self-reported physical activity for intentional exercise activities (moderate or brisk walking for exercise, and moderate or vigorous sports and conditioning activities) of ≥30 minutes on ≥5 days per week.
Laboratory assessments
Fasting serum and plasma samples were collected on the day of BC analysis and stored at −70°C. Serum CRP level was measured by nephelometry (Dade Behring, Deerfield, IL). Rheumatoid factor (RF) was assessed by enzyme-linked immunosorbent assay, with seropositivity defined as ≥40 units.
Statistical analysis
Differences in means for continuous BC characteristics were compared for RA subjects against their matched non-RA counterparts using paired t-tests, and differences in proportions for categorical variables (phenotypes) were compared using the chi-square test in groups stratified by sex and World Health Organization categories of BMI. Conditional logistic regression models were constructed and odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to investigate the association of RA status on the prevalence of sarcopenia, overfat, and sarcopenic obesity according to sex and BMI category, adjusting for pertinent characteristics shared between RA patients and non-RA matched controls (e.g., current smokers and menopausal women).
To investigate the predictors of BC phenotype within the RA group, differences in mean characteristics were compared among the 4 BC subgroups using analysis of variance (for normally distributed continuous variables) or the Kruskal-Wallis test (for nonparametric continuous variables). Bonferroni correction was used to adjust for the effects of multiple comparisons. Multivariable ordinary logistic regression models were constructed and ORs and 95% CIs were calculated to investigate the independent associations of individual RA-related characteristics for the group comprising the combined abnormal BC subgroups compared with the healthy BC subgroup, adjusting for factors with potential confounding effects on BC, including demographics (age, sex, race, education), comorbidities (total comorbidity, depression), and lifestyle characteristics (current smoker, physical activity). Logarithmic transformation was used for non-normal variables (e.g., CRP level).
Statistical calculations were performed using Intercooled Stata 9 software (StataCorp, College Station, TX) and SAS 9 software (SAS Institute, Cary, NC). In all tests, a 2-tailed alpha level of 0.05 was used.
RESULTS
Comparison of RA subjects with matched non-RA controls
A total of 189 RA subjects (117 women and 72 men) were compared with 189 matched non-RA controls. Baseline characteristics of the RA subjects are shown in Table 1. Compared with the non-RA control group, the RA group had similar proportions of postmenopausal women (78.6% versus 77.8%; P = 0.8742), but a larger proportion of current smokers (11.1% versus 2.1%; P < 0.001). The majority (94%) of RA subjects were currently treated with biologic or nonbiologic DMARDs. Overall, the proportions of participants with high (DAS28 score >5.1), moderate (DAS28 score 3.2–5.1), and low (DAS28 score <3.2) disease activity were 10.2%, 54.1%, and 35.7%, respectively.
Table 1.
Characteristics of study subjects*
| RA subjects (n = 189) |
Non-RA controls (n = 189) |
P | |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD years | 59.9 ± 8.4 | 60.7 ± 8.8 | 0.367 |
| Women | 117 (61.9) | 117 (61.9) | 1.000 |
| White | 165 (87.3) | 165 (87.3) | 1.000 |
| Current smoker | 21 (11.1) | 4 (2.1) | < 0.001 |
| Postmenopausal women | 92 (78.6) | 91 (77.8) | 0.874 |
| RA disease characteristics | |||
| RA duration, median (IQR) years | 9 (5–17) | - | - |
| Rheumatoid factor seropositivity | 124 (65.5) | - | - |
| Swollen joint count (range 0–42), median (IQR) | 7 (3–11) | - | - |
| Tender joint count (range 0–44), median (IQR) | 6 (3–13) | - | - |
| DAS28 score, mean ± SD | 3.67 ± 1.07 | - | - |
| HAQ score (range 0–3), mean ± SD | 0.85 ± 0.75 | - | - |
| Any joint deformity/replacement/fusion | 132 (69.8) | - | - |
| Deformed and replaced joint count, median (IQR) | 2 (0–7) | - | - |
| C-reactive protein level, median (IQR) mg/liter | 2.78 (1.13–7.69) | - | - |
| Current RA therapeutics | |||
| Any nonbiologic DMARD use | 158 (84.0) | - | - |
| Any biologic DMARD use | 85 (45.0) | - | - |
| Combination biologic and nonbiologic DMARD use | 66 (34.9) | - | - |
| Glucocorticoid use | 83 (43.9) | - | - |
| No DMARD use | 12 (6.3) | - | - |
Values are the number (percentage) unless otherwise indicated. RA = rheumatoid arthritis; IQR = interquartile range; DAS28 = Disease Activity Score in 28 joints; HAQ = Health Assessment Questionnaire; DMARD = disease-modifying antirheumatic drug.
Comparisons of BC characteristics
BC characteristics for RA subjects and non-RA controls are shown in Table 2. Mean weight and BMI were significantly higher and the proportion in the normal weight category was significantly lower in women with RA compared with women without RA. Although the proportions of women in the overweight and obese BMI categories were not different between the 2 groups, women with RA nonetheless had significantly higher mean values for all measures of body fat mass. Absolute measures of lean mass did not differ significantly between women with RA and controls, but the muscle mass to fat mass ratio was significantly lower in women with RA, suggesting a selective reduction of lean mass. Men with RA did not differ significantly from controls in any measure of BC except for appendicular lean mass, which was lower for men with RA.
Table 2.
Anthropometric and dual x-ray absorptiometry–derived body composition characteristics and phenotypes for women and men with RA versus matched non-RA controls*
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| RA (n = 117) |
Non-RA (n = 117) |
P | RA (n = 72) |
Non-RA (n = 72) |
P | |
| Weight, kg | 74.4 ± 16.0 | 70.1 ± 13.9 | 0.031 | 88.2 ± 17.0 | 87.7 ± 13.7 | 0.837 |
| Body mass index, kg/m2 | 28.3 ± 5.6 | 26.2 ± 4.8 | 0.002 | 28.7 ± 5.0 | 28.1 ± 4.2 | 0.416 |
| Underweight, no. (%)† | 1 (0.9) | 1 (0.9) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| Normal weight, no. (%)‡ | 36 (30.8) | 58 (49.6) | 0.002 | 16 (22.2) | 20 (27.8) | 0.441 |
| Overweight, no. (%)§ | 42 (35.9) | 29 (24.8) | 0.065 | 30 (41.7) | 31 (43.1) | 0.866 |
| Obese, no. (%)¶ | 38 (32.5) | 29 (24.8) | 0.193 | 26 (36.1) | 21 (29.2) | 0.374 |
| Total fat mass, kg | 31.5 ± 10.7 | 28.0 ± 10.3 | 0.012 | 27.3 ± 10.3 | 25.9 ± 9.9 | 0.414 |
| Fat mass index, kg/m2 | 12.0 ± 4.0 | 10.4 ± 3.7 | 0.002 | 8.9 ± 3.3 | 8.3 ± 3.2 | 0.280 |
| Truncal fat mass, kg | 16.0 ± 14.1 | 14.1 ± 5.9 | 0.015 | 16.5 ± 6.4 | 15.4 ± 6.2 | 0.312 |
| Body fat percentage | 42.1 ± 7.2 | 38.7 ± 7.6 | < 0.001 | 30.4 ± 7.5 | 28.9 ± 8.0 | 0.294 |
| Total lean mass, kg | 39.3 ± 6.9 | 40.0 ± 4.9 | 0.419 | 56.9 ± 8.4 | 58.8 ± 8.3 | 0.196 |
| Appendicular lean mass, kg | 16.1 ± 3.3 | 16.4 ± 2.4 | 0.531 | 24.7 ± 3.9 | 26.1 ± 3.9 | 0.039 |
| Relative skeletal muscle index, kg/m2 | 6.8 ± 1.2 | 6.8 ± 0.9 | 0.811 | 9.2 ± 1.2 | 9.5 ± 1.4 | 0.130 |
| Skeletal muscle mass to fat mass ratio | 0.63 ± 0.23 | 0.72 ± 0.26 | 0.006 | 1.24 ± 0.74 | 1.26 ± 0.50 | 0.850 |
| Sarcopenia, no. (%) | 25 (21.4) | 9 (7.7) | 0.004 | 24 (33.3) | 16 (22.2) | 0.157 |
| Overfat, no. (%) | 67 (57.3) | 41 (35.0) | 0.001 | 41 (56.9) | 36 (50.0) | 0.423 |
| Sarcopenic obesity, no. (%) | 13 (11.1) | 3 (2.6) | 0.008 | 11 (15.3) | 8 (11.1) | 0.467 |
Values are the mean ± SD unless otherwise indicated. RA = rheumatoid arthritis.
BMI 18.50 kg/m2.
BMI 18.50–24.99 kg/m2.
BMI 25.00–29.99 kg/m2.
BMI ≥30 kg/m2.
Comparisons of BC phenotypes
The distributions of BC phenotypes in RA subjects versus non-RA controls are shown in Table 2. ORs for RA subjects compared with non-RA controls for each phenotype, with adjustment for smokers and menopause status, are shown according to sex in Figure 1, and according to sex and BMI category in Figure 2.
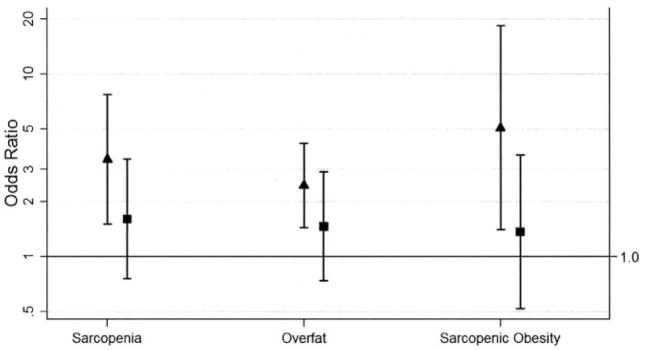
Figure 1.
Adjusted odds of sarcopenia, overfat, and sarcopenic obesity for rheumatoid arthritis (RA) subjects compared with non-RA controls, by sex. Adjusted for current smokers and menopausal women. Values are shown as the odds ratio (95% confidence interval). Solid triangles = female; solid squares = male.
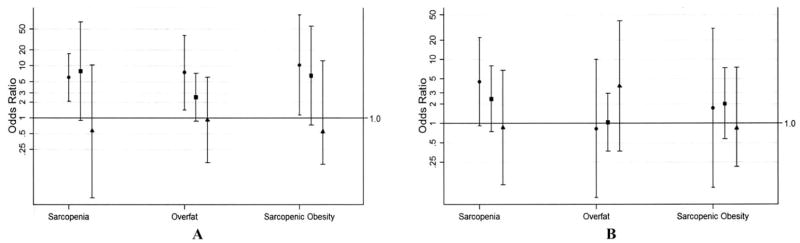
Figure 2.
Adjusted odds of sarcopenia, overfat, and sarcopenic obesity for rheumatoid arthritis (RA) subjects compared with non-RA controls according to body mass index (BMI) category, by sex. Adjusted for current smokers and menopausal women. A, Female. B, Male. Values are shown as the odds ratio (95% confidence interval). Solid circles = BMI <25 kg/m2; solid squares = BMI 25–29.9 kg/m2; solid triangles = BMI ≥30 kg/m2.
Women
The prevalence of sarcopenia was significantly higher in women with RA than in controls (Table 2). The adjusted odds of sarcopenia was >3 times greater in women with RA compared with controls (OR 3.41, 95% CI 1.51–7.69) (Figure 1). The greatest difference in the adjusted OR was observed in the normal weight BMI category, in which women with RA had 6 times greater odds of sarcopenia compared with controls (OR 6.00, 95% CI 2.11–17.11) (Figure 2). For women in the overweight and obese BMI categories, the adjusted odds of sarcopenia did not differ significantly between the groups.
The prevalence of overfat was significantly higher in women with RA than in controls (Table 2). The adjusted odds of overfat was >2 times greater in women with RA compared with controls (OR 2.45, 95% CI 1.44–4.16) (Figure 1). The greatest difference in the adjusted OR for overfat was observed in the normal weight BMI category (Figure 2), in which women with RA had 7 times greater odds of overfat compared with controls (OR 7.42, 95% CI 1.43–38.29). Women with RA in the overweight and obese BMI categories did not differ significantly in the adjusted odds of overfat compared with controls.
The prevalence of sarcopenic obesity was significantly higher in women with RA than in controls (Table 2). The adjusted odds of sarcopenic obesity was 5 times greater in women with RA compared with controls (OR 5.07, 95% CI 1.40–18.33; P = 0.013) (Figure 1). The greatest difference in the adjusted OR for sarcopenic obesity was observed for women in the normal weight BMI category (Figure 2), in which women with RA had >10 times greater odds of sarcopenic obesity compared with controls (OR 10.33, 95% CI 1.13–94.60; P = 0.039). For women in the overweight and obese BMI categories, the adjusted odds of sarcopenic obesity did not significantly differ between women with RA and controls.
Men
Men with and without RA did not differ significantly in the prevalence of sarcopenia, overfat, or sarcopenic obesity (Table 2), even after adjusting for smoking (Figure 1). Furthermore, in analyses stratified by BMI category (Figure 2), no significant differences in the adjusted odds of sarcopenia, overfat, or sarcopenic obesity were observed for men with RA versus controls, although a trend in sarcopenia was observed in the normal weight BMI category of men with RA (OR 4.50, 95% CI 0.92–21.93; P = 0.063).
Predictors of BC phenotypes in RA subjects
Comparisons of characteristics for RA subjects according to BC subgroup are shown in Table 3. Demographic and lifestyle characteristics, for the most part, did not differ significantly between RA patients with abnormal BC versus those with healthy BC, with the exception of more television watching in sarcopenic obese subjects and a lower frequency of regular intentional exercise in sarcopenic but not overfat subjects. Among RA characteristics, joint deformity was significantly higher in the sarcopenic but not overfat subgroup versus those with healthy BC, and HAQ score was significantly associated with sarcopenic obesity. Interestingly, CRP levels were significantly higher in overfat RA subjects (with and without sarcopenia) compared with those with healthy BC, whereas increasing DAS28 score was not significantly associated with an increased odds of abnormal BC. Among RA therapeutics, a significantly higher proportion of sarcopenic and sarcopenic obese subjects were treated with DMARDs. However, specific medications (current and ever prednisone, current biologic and nonbiologic DMARDs) did not differ between those with and without abnormal BC phenotypes.
Table 3.
Characteristics of RA subjects according to body composition phenotype*
| Healthy body composition (n = 56) |
Overfat but not sarcopenic (n = 84) |
Sarcopenic but not overfat (n = 25) |
Sarcopenic and overfat (n = 24) |
P† | |
|---|---|---|---|---|---|
| Demographic and lifestyle characteristics | |||||
| Age, mean ± SD years | 59.8 ± 9.5 | 59.1 ± 7.7 | 62.3 ± 8.0 | 61.0 ± 8.7 | 0.359 |
| Women | 38 (67.9) | 54 (64.3) | 12 (48) | 13 (54.2) | 0.295 |
| White | 50 (89.3) | 74 (88.1) | 21 (84.0) | 20 (83.3) | 0.841 |
| Current smoker | 9 (16.1) | 8 (9.5) | 4 (16.0) | 0 (0) | 0.157 |
| CES-D score, median (IQR) | 4.5 (2–10.5) | 6 (2–12) | 5 (3–6) | 7 (5–14) | 0.161 |
| Regular intentional exercise | 41 (73.2) | 41 (48.8) | 10 (40.0)‡ | 11 (45.8) | 0.009 |
| Daily TV watching, mean ± SD hours | 1.9 ± 1.5 | 2.4 ± 1.5 | 2.7 ± 1.7 | 2.9 ± 2.0‡ | 0.039 |
| RA disease characteristics | |||||
| RA duration, mean ± SD years | 10.0 ± 8.7 | 12.7 ± 10.8 | 15.7 ± 12.4 | 15.8 ± 11.3 | 0.051 |
| RF seropositivity | 30 (53.6) | 62 (73.8) | 17 (68.0) | 15 (62.5) | 0.099 |
| DAS28 score, mean ± SD | 3.44 ± 0.95 | 3.79 ± 1.04 | 3.48 ± 1.31 | 4.00 ± 1.05 | 0.084 |
| Joint deformity, median (IQR) | 1 (0–3) | 3 (0–9) | 5 (1–12)‡ | 3 (2–10) | < 0.001 |
| HAQ score (range 0–3), mean ± SD | 0.59 ± 0.64 | 0.92 ± 0.74 | 0.86 ± 0.79 | 1.22 ± 0.84‡ | 0.004 |
| CRP level, median (IQR) mg/liter | 1.3 (0.8–3.1) | 4.0 (1.9–8.5)§ | 2.0 (0.9–6.7) | 4.3 (1.6–12.0)§ | < 0.001 |
| RA treatment characteristics | |||||
| Current prednisone | 24 (42.9) | 36 (42.9) | 14 (56.0) | 9 (37.5) | 0.583 |
| Ever prednisone | 44 (78.6) | 62 (73.8) | 16 (64.0) | 18 (75.0) | 0.588 |
| Current biologic DMARD use | 22 (39.3) | 41 (48.8) | 11 (44.0) | 11 (45.8) | 0.726 |
| Current nonbiologic DMARD use | 48 (85.7) | 71 (84.5) | 20 (80.0) | 18 (75) | 0.815 |
| No current DMARD use | 0 (0) | 5 (5.6) | 3 (12.0)‡ | 5 (20.8)§ | 0.006 |
Values are the number (percentage) unless otherwise indicated. RA = rheumatoid arthritis; CES-D = Center for Epidemiologic Studies Depression Scale; IQR = interquartile range; RF = rheumatoid factor; DAS28 = Disease Activity Score in 28 joints; HAQ = Health Assessment Questionnaire; CRP = C-reactive protein; DMARD = disease-modifying antirheumatic drug.
From global test of all 4 body composition subtypes (analysis of variance).
P < 0.05 for pairwise comparison with the healthy body composition group (adjusted for multiple comparisons using Bonferroni correction).
P < 0.001 for pairwise comparison with the healthy body composition group (adjusted for multiple comparisons using Bonferroni correction).
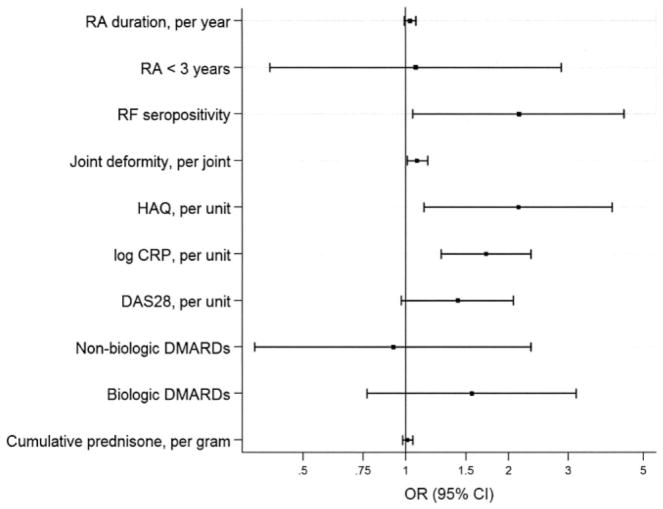
In multivariable analyses comparing subjects with any of the abnormal BC phenotypes as a combined group with those with healthy BC (Figure 3), abnormal BC was significantly associated with RF seropositivity (OR 2.15, 95% CI 1.05–4.38), increasing joint deformity (OR 1.08, 95% CI 1.01–1.16 per joint), HAQ score (OR 2.14, 95% CI 1.13–4.03 per unit), and CRP level (OR 1.72, 95% CI 1.27–2.33 per log unit), but not DAS28 score, even after adjusting for pertinent demographic and lifestyle characteristics. Increasing RA duration was not associated with abnormal BC and, interestingly, there was no significant difference in the adjusted odds of abnormal BC in subjects with early RA (disease duration <3 years) versus those with longer disease duration. Because all subjects with healthy BC were treated with DMARDs, it was not possible to explore the relative odds of abnormal BC versus healthy BC in DMARD-untreated subjects. Although DMARD treatment was associated with healthy BC, treatment with neither biologic nor nonbiologic DMARDs was independently associated with lower odds of abnormal BC. Interestingly, increasing cumulative glucocorticoid exposure was not significantly associated with greater odds of abnormal BC.
Figure 3.
Adjusted associations of rheumatoid arthritis (RA) disease and treatment characteristics with abnormal versus healthy body composition. Odds ratios (ORs) >1.0 are associated with abnormal body composition. ORs <1.0 are associated with healthy body composition. Analyses are adjusted for demographic and lifestyle factors (age, sex, race, highest education level attained, comorbidity, depression, current smoker, and physical activity). RF =rheumatoid factor; HAQ = Health Assessment Questionnaire; CRP = C-reactive protein; DAS28 = Disease Activity Score in 28 joints; DMARDs = disease-modifying antirheumatic drugs; 95% CI = 95% confidence interval.
To investigate the differential associations of DMARD therapy on abnormal BC, we compared clinical characteristics of subjects treated and untreated with DMARDs. Subjects not receiving any current DMARDs had significantly higher joint deformity, HAQ scores, and CRP levels compared with those receiving treatment with biologic and nonbiologic DMARDs (data not shown). Mean age, RA duration, and DAS28 scores did not significantly differ between groups treated and untreated with DMARDs.
DISCUSSION
To our knowledge, this study represents the largest investigation of BC in patients with RA and is the first to investigate the association of RA characteristics with abnormal BC phenotypes. We observed, in general, greater fatness and lower relative lean mass in women with RA, most pronounced in women of normal weight. These BC changes were significantly associated with RF seropositivity, greater joint deformity and HAQ scores, and higher CRP concentrations, independent of the confounding effects of less exercise and greater sedentariness in the RA cohort. Furthermore, subjects treated with DMARDs were less likely to exhibit abnormal BC. However, glucocorticoids were not associated with abnormal BC.
The finding that RA subjects, particularly women, with BMI in the normal weight range were at greater risk for abnormal BC phenotypes than those in the overweight and obese BMI categories is novel and should caution against the use of BMI as a surrogate for BC in subjects with RA, as has been recently suggested (28). This should alert the practitioner to suspect unhealthy BC in patients who may appear normal based on weight and BMI. Additionally, an image of the typical RA patient as cachectic appears outdated, because <1% of subjects demonstrated a BMI <18.5 kg/m2, the same as in the non-RA group. Previously, a J-shaped relationship has been described in relating BMI to adverse health outcomes (29), with the extremes of BMI demonstrating greatest risk. Our findings suggest that the effects of abnormal BC on health outcomes may be shifted in RA into the normal weight BMI range and may actually diminish with increasing BMI. This may explain, in part, recently reported paradoxical protective effects of increasing BMI on cardiovascular (30) and all-cause mortality (31) in patients with RA. In contrast to prior studies (2,12), we did not observe an association between reduced lean mass and current levels of RA disease activity. The fact that accumulated disease activity and inflammation over time negatively affect lean mass is suggested, however, by the association of sarcopenia with joint deformity, disability, and resulting sedentary lifestyle. This is supported by the observation that subjects lacking current DMARD therapy had the highest prevalence of these characteristics. Longitudinal analyses in progress will aid in discerning causality. Although our findings suggest that the cumulative effects of RA are associated with abnormal BC, these effects appear to develop relatively early in the disease, because early RA was not found to be protective against abnormal BC.
Normally, an increase in lean mass is expected to accompany an increase in fat mass by a ratio of approximately 1:4 (32). Due to this dependency of lean mass on fat mass, the adequacy of an individual’s lean mass cannot be gauged in isolation. In our study, although continuous measures of lean mass did not differ between the RA and non-RA groups, the greater prevalence of sarcopenia, despite significantly higher fat mass and the lower ratio of muscle mass to fat mass in women with RA compared with controls, suggests that lean mass was selectively reduced in women with RA. Why this effect was more pronounced in women than in men with RA is likely related to a higher prevalence of key predictors of sarcopenia (e.g., joint deformity, physical inactivity) in the women versus men (data not shown). However, the influence of hormonal factors is possible. In contrast, we identified a strong relationship between CRP level and fat mass. Body fat is a known producer of tumor necrosis factor α, interleukin-6, and other cytokines capable of inducing hepatic CRP production (7). These data suggest that fat mass should be considered as a contributor to CRP levels in RA.
The relationship between BC and glucocorticoid use in subjects with RA has not been extensively studied. In this study, we did not observe a relationship between abnormal BC phenotypes and either current or cumulative glucocorticoid use. Due to the cross-sectional design, it is not possible to decipher whether this lack of association is due to a true lack of effect or, alternatively, whether any weight-promoting effect of glucocorticoids was counterbalanced by an ability to suppress the catabolic effects of inflammation.
Due to their potent antiinflammatory effects, biologic DMARDs could be expected to reduce the catabolic effect of hypercytokinemia on muscle. In this cross-sectional analysis, neither biologic nor nonbiologic DMARD treatment alone was associated with a decreased risk of abnormal BC. However, the finding that the lack of any DMARD treatment was associated with abnormal BC suggests that beneficial effects may be mediated through RA treatment in general. Although the number of RA subjects not treated with DMARDs was low, the association with abnormal BC was striking. A recent trial (33) suggested that treatment with etanercept was associated with a gain in lean mass in a subset of patients with early RA. This interesting finding deserves confirmation in larger cohorts and raises the question as to whether anti–tumor necrosis factor therapy has a direct anabolic effect on muscle or exerts its effects indirectly by reducing RA disease activity and pain, thus enabling increased physical functioning and activity.
Several limitations to our investigation should be acknowledged. Subjects were derived from a study of subclinical cardiovascular disease, in which those with prevalent cardiovascular disease and those weighing >300 pounds were excluded. Due to links between cardiovascular disease and abnormal BC, these exclusions might select out subjects with the most aberrant BC. However, it is likely that the inclusion of these excluded subjects would strengthen, not diminish, the effects observed. Because different DXA scanners were used to assess BC, a systematic measurement bias is possible. However, our extensive quality control and calibration procedures render this unlikely. Finally, several of the measures used in the study (i.e., cumulative glucocorticoid exposure and physical activity) were assessed by patient self-report, and may be more variable than direct measurement.
In summary, we observed greater than expected abnormal body fat and lean composition in RA subjects age >45 years compared with matched controls, the effect being most pronounced in women and particularly in those in the normal weight BMI category. RA and non-RA factors were associated with abnormal BC. Because aberrant BC is increasingly implicated as a key determinant of health, further investigation is needed to determine the causal factors for these BC changes and to devise and test interventional strategies to reduce their effects on health outcomes in patients with RA.
Acknowledgments
We would like to thank the Johns Hopkins Bayview Medical Center General Clinical Research Center and staff for providing support for the DXA scans for RA subjects in this study. We are indebted to the dedication and hard work of the ESCAPE RA staff: Marilyn Towns, Michelle Jones, Patricia Jones, Marissa Hildebrandt, and Shawn Franckowiak. We thank Drs. Uzma Haque, Clifton Bingham, III, Carol Ziminski, Jill Ratain, Ira Fine, Joyce Kopicky-Burd, David McGinnis, Achini Perera, Peter Holt, Megan Clowse, Gordon Lam, and others for generously recommending their patients for this study.
Supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grants AR-050026-01 and 1-K23-AR-054112-01), a Clinical Investigator Fellowship Award from the Research and Education Foundation of the American College of Rheumatology, the Johns Hopkins Bayview Medical Center General Clinical Research Center, and the NIH Intramural Research Program, National Institute on Aging.
Footnotes
Dr. Bartlett has received consultant fees (less than $10,000) from Centocor.
AUTHOR CONTRIBUTIONS
Dr. Giles had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Giles, Ferrucci, Bathon.
Acquisition of data. Giles, Ferrucci, Towns, Bathon.
Analysis and interpretation of data. Giles, Ling, Ferrucci, Bartlett, Andersen, Towns, Fontaine, Bathon.
Manuscript preparation. Giles, Ling, Ferrucci, Bartlett, Andersen, Fontaine, Bathon.
Statistical analysis. Giles, Muller, Bathon.
References
- 1.Paget J. Clinical lectures on the nervous mimicry of diseases: mimicry of diseases of joints. Lancet. 1873;102:727–9. [Google Scholar]
- 2.Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379–86. doi: 10.1172/JCI117244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westhovens R, Nijs J, Taelman V, Dequeker J. Body composition in rheumatoid arthritis. Br J Rheumatol. 1997;36:444–8. doi: 10.1093/rheumatology/36.4.444. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 6.Abbasi F, Brown J, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–43. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–8. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 9.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel SE, Crowson CS, O’Fallon WM. Comorbidity in arthritis. J Rheumatol. 1999;26:2475–9. [PubMed] [Google Scholar]
- 11.Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–75. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 12.Walsmith J, Abad L, Kehayias J, Roubenoff R. Tumor necrosis factor-α production is associated with less body cell mass in women with rheumatoid arthritis. J Rheumatol. 2004;31:23–9. [PubMed] [Google Scholar]
- 13.Munro R, Capell H. Prevalence of low body mass in rheumatoid arthritis: association with the acute phase response. Ann Rheum Dis. 1997;56:326–9. doi: 10.1136/ard.56.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Genton L, Hans D, Kyle UG, Pichard C. Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–83. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 17.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–21. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute, National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. 1998 URL: http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdls.pdf. [PubMed]
- 19.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation: results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–34. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 21.Paulus HE, Ramos B, Wong WK, Ahmed A, Bulpitt K, Park G, et al. the Western Consortium of Practicing Rheumatologists. Equivalence of the acute phase reactants C-reactive protein, plasma viscosity, and Westergren erythrocyte sedimentation rate when used to calculate American College of Rheumatology 20% improvement criteria or the Disease Activity Score in patients with early rheumatoid arthritis. J Rheumatol. 1999;26:2324–31. [PubMed] [Google Scholar]
- 22.Navarro-Cano G, del Rincon I, Pogosian S, Roldan JF, Escalante A. Association of mortality with disease severity in rheumatoid arthritis, independent of comorbidity. Arthritis Rheum. 2003;48:2425–33. doi: 10.1002/art.11127. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe F, Kleinheksel SM, Cathey MA, Hawley DJ, Spitz PW, Fries JF. The clinical value of the Stanford Health Assessment Questionnaire functional disability index in patients with rheumatoid arthritis. J Rheumatol. 1988;15:1480–8. [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64:507–21. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 26.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. Physical activity and health: a report of the Surgeon General. Atlanta: National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 28.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Nevill AM, Douglas KM, Jamurtas A, et al. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis. 2007;66:1316–21. doi: 10.1136/ard.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 30.Maradit Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450–7. doi: 10.1002/art.20612. [DOI] [PubMed] [Google Scholar]
- 31.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 32.Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527–58. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- 33.Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr. 2006;84:1463–72. doi: 10.1093/ajcn/84.6.1463. [DOI] [PubMed] [Google Scholar]