Abstract
Purpose
Oncolytic herpes simplex virus 1 (oHSV) vectors treat tumors in preclinical models and have been used safely in phase I clinical trials for patients with cancer. Infection of tumors with oHSV also induces specific antitumor immunity. We investigated whether this immunotherapeutic effect is enhanced by combining oHSV infection with intratumoral administration of immature myeloid dendritic cells (iDC).
Experimental Design
Subcutaneous neuroblastoma tumors were established in syngeneic immunocompetent mice and sequentially treated with oHSV(G47Δ) and intratumoral iDCs. Tumor volumes and survival were monitored. Antitumor immune responses were evaluated by immunohistochemistry, IFN-γ ELISPOT, and CTL assay. Treatment was also evaluated in immunodeficient NOD-SCID mice.
Results
We observed significant reductions in tumor volumes in mice receiving G47Δ + iDCs compared with those treated with G47Δ or iDC monotherapy. Survival was prolonged, with ~90% of tumors eradicated in the combination group. Combination therapy led to enhancement of antitumor immune responses, confirmed by increases in IFN-γ expression by splenocytes harvested from G47Δ + iDC-treated mice. Splenocytes harvested from G47Δ + iDC-treated mice were effective against neuroblastoma tumor cells in a CTL assay. Immunohistochemistry of combination-treated tumors revealed robust lymphocytic infiltrates. Adding iDCs to G47Δ infection in tumors in NOD-SCID mice did not reduce the rate of growth. Substitution of lipopolysaccharide-matured dendritic cells abrogated the enhanced tumor volume reduction seen with combination therapy with iDCs.
Conclusions
Combination treatment of murine tumors with oHSV and iDCs reduces the volume of established tumors and prolongs survival via enhancement of antitumor immunity.
Treatment of malignant tumors with oncolytic herpes simplex virus 1 (oHSV) is promising because of the opportunity to selectively target cancerous cells while sparing neighboring normal tissues. Clinical trials for patients with solid tumors both inside and outside of the central nervous system have proceeded without evidence of treatment-associated toxicity and with some objective clinical responses (1, 2). G207 is a second-generation oHSV with an inactivating Escherichia coli lacZ insert in the gene for the large subunit of ribonucelotide reductase, rendering it replication “conditional,” and deletions in both copies of the γ34.5 gene, attenuating its neurovirulence (3). Although the initial effect of tumor inoculation with G207 is lysis of the infected tumor cells with spread of viral progeny to adjacent cells, the resultant cell death and associated inflammatory response appears to set off a cascade through which effectors of innate immunity are activated and an adaptive, specific antitumor immune response follows (4). Modifying the virus to express proinflammatory cytokines such as interleukin (IL)-12 or soluble costimulatory molecules further enhances antitumor immunity and improves tumor control (5). As antiviral immunity may limit the spread and the ultimate replication capacity of oHSV, the antitumor immune effect may be the most effective and durable aspect of the treatment in immunocompetent hosts. With this in mind, a third-generation oHSV, G47Δ, was created from the G207 backbone by deleting the viral α 47 gene, which interferes with peptide assembly and antigen presentation by MHC I (6).
Dendritic cells are professional antigen-presenting cells that traffic to sites of inflammation. Plasmacytoid dendritic cells are associated with the type I IFN response to viral infection and are functionally and phenotypically distinct from myeloid dendritic cells, which are better suited for antigen processing, migration, and stimulation of naive T lymphocytes in lymph nodes (7). Immature myeloid dendritic cells (iDC) are efficient at phagocytosis and receptor-mediated uptake of antigenic material but require a maturation stimulus before they have migratory and full antigen-presenting capacity. Antigen presentation by iDCs, associated with a lack of costimulation, induces tolerance (7).
Translational Relevance
Effective therapies are direly needed for patients with cancer. Both treatment of tumors with oHSV and dendritic cell vaccination have been effective in preclinical cancer models and have been translated into clinical trials. Here, we show that combination treatment of tumors in immunocompetent mice by intratumoral injection of G47Δ, an oHSV-1 that has a deletion in the gene that interferes with peptide assembly and subsequent presentation on MHC I, followed by injection of iDCs results in potent antitumor immunity in a relatively nonimmunogenic murine neuroblastoma model. This approach involves elements that, individually, are ready for clinical use and/or study, and our intention is to pursue clinical application of the combination.
We show here that combining G47Δ infection of tumors with intratumoral injection of iDCs is an effective treatment for established subcutaneous neuroblastoma tumors in syngeneic immunocompetent mice.
Materials and Methods
Virus and cells
Purified G47Δ, containing a lacZ insertion in ICP6 and deletions in the ICP34.5 and ICP47 genes (6), was provided by MediGene. The N18 cell line is a subclone of C1300 murine neuroblastoma cells derived from an A/J mouse and obtained from K. Ikeda (Tokyo Institute of Psychiatry). N18 cells were cultured in DMEM supplemented with 10% FCS at 37°C and 5% CO2.
Dendritic cells
Murine dendritic cells were generated by harvesting the bone marrow from the long bones of 6-week-old A/J mice (Jackson Laboratory) as described previously (8). Precursor cells were cultured in RPMI 1640 with 10% inactivated FCS, 50 μmol/L 2-ME, 2 mmol/L glutamine, 20 mmol/L HEPES, and penicillin-streptomycin supplemented with recombinant granulocyte-macrophage colony-stimulating factor (Sigma-Aldrich; 10 ng/mL). Monocytes were cultured for 6 days and received fresh medium supplemented with serum and cytokines on day 3. On day 6 of culture, iDCs were isolated using magnetic beads coated with anti-CD11c monoclonal antibodies (Miltenyi Biotech). When appropriate, dendritic cell maturation was induced by adding lipopolysaccharide (1 μg/mL; Sigma-Aldrich) for the last 24 h of culture. iDC and mature dendritic cell phenotypes were confirmed by flow cytometry antibody detection of CD80, CD86, CD40, and MHC II (eBioscience).
Subcutaneous tumor models
N18 cells (1 × 106 in 50 μL DMEM) were subcutaneously injected in the flanks of immunocompetent A/J mice. When tumors reached ~5 mm in diameter, mice were treated with 1 × 106 plaque-forming units G47Δ by direct intratumoral inoculation. Two days following viral treatment, 1 × 106 iDCs or mature dendritic cells were injected into the tumors in 50 μL PBS followed by a second G47Δ (1 × 106 plaque-forming units) intratumoral inoculation on day 5. Tumors were measured using calipers, and volumes were derived by the formula: v = [(length) × (width2)]/2. Mock-treated mice received equivalent intratumoral injections of PBS or medium, whereas other experimental groups received either intratumoral G47Δ alone or dendritic cells alone (plus PBS or medium as appropriate). Mice were sacrificed when their maximal tumor dimension reached 2.1 cm according to the protocol approved by the Institutional Animal Care and Use Committee. An identical treatment protocol was employed for N18 tumors established subcutaneously in NOD-SCID mice (Jackson Laboratory).
Immunohistochemistry
Subcutaneous N18 tumors were excised on day 15 and snap-frozen in embedding medium (OCT; Miles). Tumors were sectioned (5 μm) and fixed with cold acetone for 20 min and peroxidase blocked for 5 min. Sections were incubated with primary mouse monoclonal antibodies against CD4 (L3T4) and CD8a (Ly-2; eBioscience) for 2 h at room temperature followed by incubation with a biotinylated secondary antibody for 45 min. Immunostaining was visualized with BCIP-NBT substrate-chromagen (Dako). Tissue sections were counterstained with H&E or hematoxylin alone and examined using an optical microscope (Nikon).
IFN-γ ELISPOT assay
Splenocytes were harvested from tumor-bearing mouse spleens 15 days after the first G47Δ treatment. Splenocytes (5 × 106) were restimulated for 48 h in vitro with 1 × 105 irradiated (40 Gy) N18 cells in RPMI 1640 supplemented with 10% inactivated FCS, 50 μmol/L 2-ME, 2 mmol/L glutamine, 20 mmol/L HEPES, penicillin-streptomycin, and 10 ng/mL IL-2 (eBioscience) in 24-well tissue culture plates (Costar). After restimulation, 1 × 105 splenocytes from the various treatment group mice were loaded in triplicate onto Millipore MultiScreen-HA 96-well filter plates coated overnight at 4°C with anti-IFN-γ monoclonal antibody (eBioscience). Plates were incubated at 37°C and 5% CO2 for 24 h, washed three times with wash buffer, and incubated with biotinylated anti-IFN-γ monoclonal antibodies for 2 h at 37°C followed by incubation with streptavidin-horseradish peroxidase conjugate for 45 min. Cytokine-producing cells were developed with BCIP/NBT substrate (Sigma-Aldrich) for 10 min and counted on an AID version 3.1.1 ELISPOT reader.
CTL assay
Single-cell suspensions of splenocytes were cultured in upright flasks at a concentration of 2.5 × 107 cells/mL in RPMI 1640 with 10% IFCS and restimulated by adding 1 × 106 irradiated N18 cells to the medium, and splenocytes were harvested after 5 days of in vitro culture (9). N18 (1 × 105) target cells labeled with 0.3 μmol/L DDAO-SE (Molecular Probes) for 15 min at 37°C were mixed with restimulated splenocyte effector cells at E:T ratios of 3:1, 1:1, and 0.3:1 in conical polypropylene tubes, centrifuged at 200 rpm for 1 min, incubated at 37°C, 5% CO2 for 3 h, fixed with 2% paraformaldehyde, and stored at 4°C overnight. Cells were permeabilized using Fix/Perm buffer (BD Biosciences) for 20 min, washed twice, stained for 30 min at room temperature with phycoerythrin-labeled anti-cleaved caspase-3 monoclonal antibody (BD Biosciences), washed, and resuspended in Dulbecco’s PBS/1% bovine serum albumin for analysis on a FACSCalibur flow cytometer (Becton Dickinson) with FlowJo software. The percentage of T-expressing cleaved caspase-3 was determined by gating on the DDAO-SE-labeled N18 target cells in the FL4 channel and measuring phycoerythrin labeling in the FL2 channel.
Statistical analysis
Significance of data comparisons between treatment groups was done via paired Student’s t test or by ANOVA. Kaplan-Meier survival curves were compared via Mantel-Cox analyses. P values < 0.05 were considered statistically significant.
Results
Sequential intratumoral injection of G47Δ and iDCs causes regression of established subcutaneous neuroblastomas and prolongs survival of tumor-bearing mice
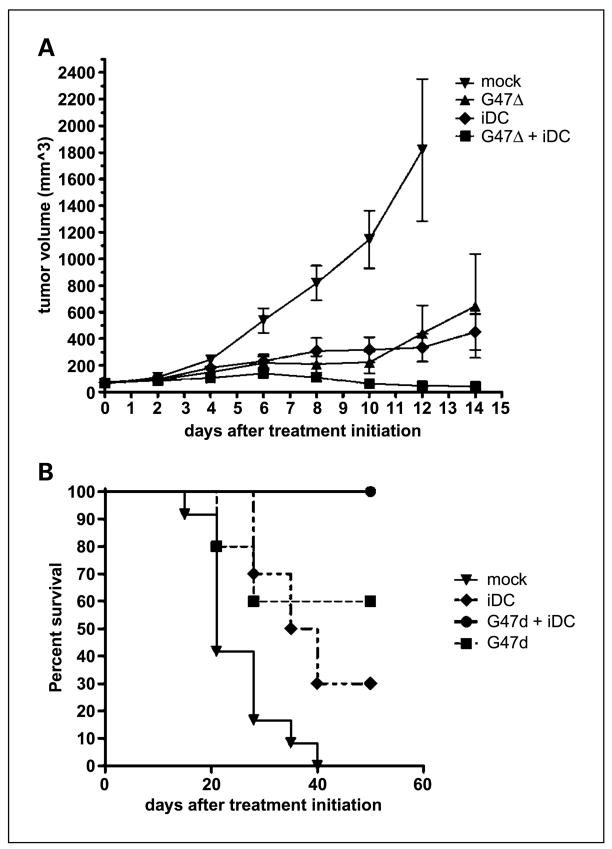
We sought to determine whether combining intratumoral G47Δ injection with local delivery of large numbers of iDCs could effectively treat subcutaneous tumors in immunocompetent mice. Small tumors underwent an injection of virus once they became established (day 0). To deliver iDCs into the tumor milieu when viral oncolysis and the initial inflammatory response were prominent, they were injected 2 days later. A second injection of G47Δ was administered on day 5 to slow the growth of these aggressive tumors, giving time for the additional immune effect of the iDCs to take effect. Whereas treatment with oHSV and iDCs alone were each superior to mock, sequential intratumoral delivery of G47Δ and iDCs led to significant improvements in tumor control (Fig. 1A). Nearly all of the tumors treated with this combination (n = 13) regressed and were no longer measurable. Accordingly, all of the animals treated in this fashion were long-term survivors (Fig. 1B). Comparisons of survival curves are displayed in Table 1. Two combination-treated mice succumbed to slow tumor growth at 15 weeks. The remaining mice had no residual evidence of tumor and were sacrificed at 20 weeks.
Fig. 1.
Inhibition of tumor growth and increased survival. Subcutaneous tumor volumes. A, subcutaneous N18 tumors were significantly smaller in mice in the combination G47Δ + iDC treatment group. Mice in the mock-treated group were sacrificed at day 12 because tumor size exceeded 2,100 mm3, in accordance with the Institutional Animal Care and Use Committee protocol. Statistical significance was achieved at this point (P < 0.0001, ANOVA). Bars, SD. In the experiment depicted, only G47Δ+ iDC combination therapy was significantly better than mock by Tukey’s post hoc multiple comparison tests (95% confidence interval, −1,053 to −134.1). In multiple experiments, combination therapy with G47Δ and iDCs was significantly more effective at controlling tumor growth than monotherapy with virus or iDCs (P < 0.05, Student’s t test). B, Kaplan-Meier curves showing enhanced survival in mice treated with combination G47Δ and iDCs. By log-rank (Mantel-Cox) analyses, combination treatment was superior to each monotherapy.
Table 1.
Comparisons of survival curves
| (A) Both combination therapy and monotherapy with either G47Δ or iDCs enhanced survival as measured by log-rank (Mantel-Cox) analyses | |
|---|---|
| Treatment group vs mock | P* |
| G47Δ + iDC | <0.0001 |
| G47Δ | 0.002 |
| iDC | 0.002 |
|
(B) Combination therapy with G47Δ and iDCs significantly enhanced survival relative to monotherapy with either agent | |
| Treatment group comparisons | Hazard ratio (95% confidence interval) |
|
| |
| G47Δ vs G47Δ + iDC | 9.483 (1.254–71.2) |
| iDC vs G47Δ + iDC | 13.56 (2.792–65.88) |
P < 0.017, Bonferroni adjustment for multiple comparisons.
Subcutaneous tumors treated with sequential intratumoral injection of G47Δ and iDCs are heavily infiltrated with CD4+ and CD8+ T lymphocytes
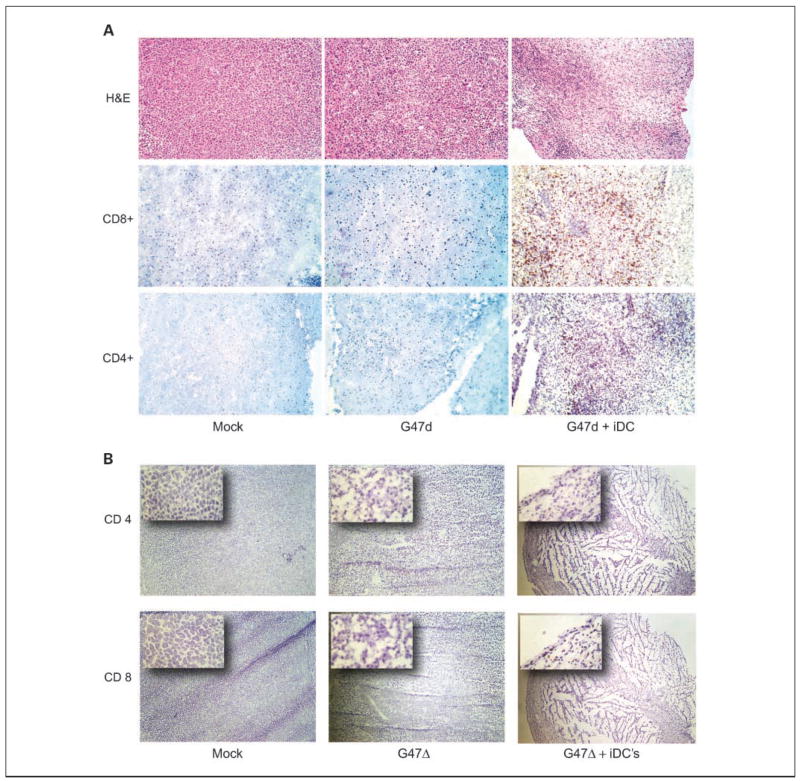
Representative tumors from all treatment groups were harvested at day 15, frozen, and subjected to immunohistochemistry. On H&E staining, combination-treated tumors showed reduced cellularity and scattered areas of necrosis. This correlated with increased CD4+ and particularly CD8+ intratumoral lymphocyte infiltration (Fig. 2). Neither control-treated tumors nor those treated with virus alone exhibited significant inflammatory cell infiltration.
Fig. 2.
Lymphocytic infiltration of tumors. A, immunohistochemistry of representative N18 tumors from the indicated treatment groups. Combination-treated tumors show reduced cellularity and areas of necrosis on H&E (top) and increased CD4+ (bottom) and CD8+ (middle) T lymphocyte infiltration. B, low-magnification images (×4) show sheets of polygonal neuroblastoma cells in the mock-treated tumors, whereas cellularity is decreased somewhat in G47Δ-treated tumors. Combination-treated tumors were smaller at day15 (the entire lesion can be seen at this magnification) and centrally necrotic with a rim of viable tumor tissue infiltrated by CD4+ and CD8+ T lymphocytes as seen in the insets (×20).
Efficacy of sequential treatment of subcutaneous tumors with G47Δ and dendritic cells is dependent on the presence of lymphocytes and the maturation status of the dendritic cells
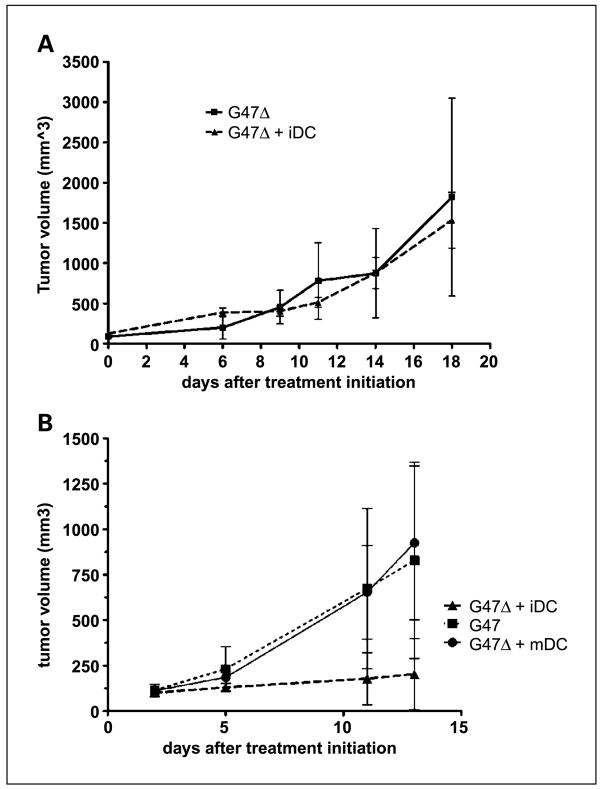
To verify the contribution of antitumor immunity to the enhanced effect of combining G47Δ and iDCs, we examined the treatment in NOD-SCID mice, which lack functional T and B lymphocytes as well as NK cells. As seen in Fig. 3A, injecting iDCs into tumors in the NOD-SCID model did not enhance the antitumor activity of oHSV-1.
Fig. 3.
Requirement for lymphocytes and iDCs. A, combining G47Δ infection of subcutaneous N18 tumors with intratumoral iDC injection does not slow progression relative to treatment with G47Δ alone in immunodeficient NOD-SCID mice. B, combining G47Δ injection of subcutaneous N18 tumors with intratumoral injection of mature dendritic cells does not slow progression relative to treatment with G47Δ alone in syngeneic A/J mice (G47Δ/mature dendritic cells versus G47Δ, P = 0.8; G47Δ versus G47Δ/iDC, P = 0.02; G47Δ/iDC versus G47Δ/mature dendritic cells, P = 0.15, Student’s t test). Enhanced antitumor effect is dependent on use of iDCs.
We hypothesize that the effect of combining G47Δ infection of tumors with intratumoral dendritic cells occurs due to release of tumor-associated antigens and antigen chaperones such as heat shock proteins and that, therefore, iDCs would be more effective than mature cells, which are less capable of processing exogenous antigen. Injection of mature dendritic cells, however, could augment antitumor effects, even without presenting antigen, by expressing large amounts of IL-12, which promotes Th1 lymphocyte responses and is antiangiogenic (10). However, intratumoral injection of mature dendritic cells following G47Δ treatment of subcutaneous N18 neuroblastomas did not slow the growth of these tumors (Fig. 3B). The addition of iDCs to oHSV-1 infection of tumors had a statistically significant effect on tumor volume, whereas the addition of mature dendritic cells had no effect.
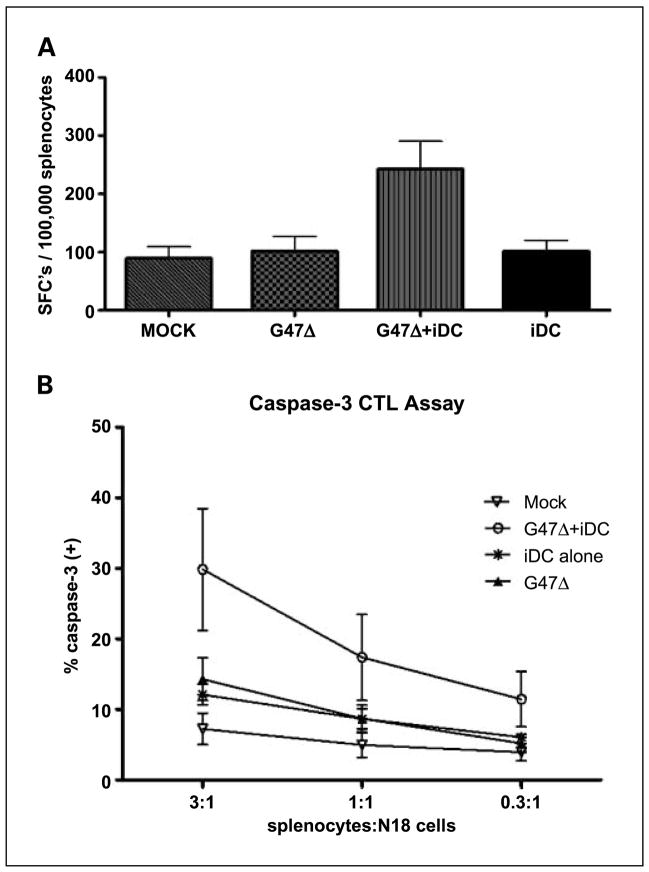
Splenocytes harvested from N18 tumor-bearing mice treated with G47Δ and iDCs have functional activity against in vitro N18 neuroblastoma
To show that combination treatment results in more vigorous antitumor lymphocyte activity, we harvested spleens from experimental animals on post-treatment day 15 and examined both cytokine production in response to stimulation by N18 tumor cells and splenocyte capacity, once restimulated, to kill N18 cells in culture. Splenocytes harvested from combination-treated animals were more than twice as likely to express IFN-γ as those harvested from animals treated with either G47Δ or iDCs alone (Fig. 4A). Monotherapy with either virus or iDCs did not significantly increase the fraction of IFN-γ-expressing splenocytes (Fig. 4A). Similarly, harvested splenocytes from combination-treated animals, after 5 days of in vitro stimulation by irradiated N18 cells, led to greater cleaved caspase-3 expression in N18 target cells, reflective of lymphocyte-induced cytolysis (Fig. 4B). Again, splenocytes harvested from tumor-bearing animals treated with G47Δ or iDC monotherapy did not enhance CTL activity in a statistically significant fashion (Fig. 4B).
Fig. 4.
Splenocytes from combination-treated mice are active in vitro. A, ELISPOT analysis for IFN-γ. Splenocytes harvested from mice treated with both G47Δ and iDCs were more likely to produce IFN-γ on stimulation with irradiated N18 cells. Average of three mice from each treatment group. SFC, spot-forming cells. * P < 0.05. B, cleaved caspase-3 CTL assay. A significantly higher percentage of DDAO-labeled N18 target cells expressed cleaved caspase-3 after coincubation with splenocyte effectors harvested from mice whose tumors had been treated with G47Δ and iDCs than when coincubated with cells from animals in other treatment groups. Average of four mice from each treatment group. E, effector cells; T, target cells. Bars, SD.
Discussion
oHSV-1 was originally conceived as a local therapy, with cell killing at the site of injection by selective lytic infection of tumor cells and subsequent propagation of viral progeny. As more studies are done in immunocompetent animal models, understanding of the complex relationship between viral oncolysis and immunity is evolving. Postinfection antiviral immunity limits intratumoral viral spread and associated tumor lysis (11). Accordingly, strategies that oppose elements of innate immunity, such as complement, are associated with enhanced intratumoral viral replication and antitumor effect (11). Cyclophosphamide has been used to this effect, and in cyclophosphamide-pretreated animals, oHSV replication is enhanced and tumor growth is inhibited (12). Low-dose cyclophosphamide also reduces the proportion of circulating regulatory T lymphocytes, and it may be that this relative boost in the number of effector T lymphocytes contributes to antitumor activity, raising the possibility that the drug works by enhancing antitumor immunity (13). Previous data confirm the “vaccination” effect of oHSV infection of tumors, as a systemic antitumor immune response, which is cell-specific and has memory, follows (4). This antitumor immune response is an important benefit of the therapy, transforming it from a mechanism for local control of cancer into a systemic treatment for multifocal disease, the effect of which may be durable.
Numerous efforts have been undertaken to enhance this antitumor immune effect. Although not definitively proven, dendritic cell subsets likely play central roles in transforming oHSV infection of tumors into systemic antitumor immunity. Viral infection and oncolysis may lead to recruitment and activation of plasmacytoid dendritic cells through toll-like receptors, generating a robust type I IFN response. As immunity responds, iDCs may infiltrate the inflammatory milieu associated with dying tumor, engulf and process cellular debris, and then, having been matured by the “danger signals” associated with the oncolytic environment, migrate to draining lymph nodes where antigen presentation to naive lymphocytes occurs.
Although presentation of tumor-associated antigens by dendritic cells is necessary for antitumor immunity, these cells and other antigen-presenting cells such as macrophages are components of tumor-derived immunosuppressive networks (14). Many tumors are sources of vascular endothelial growth factor, cyclooxygenase-2, and prostaglandin E2, all of which suppress dendritic cell differentiation and maturation. Furthermore, tumors, tumor-associated macrophages, and regulatory T lymphocytes express IL-10 and transforming growth factor-β, which suppress dendritic cell maturation and function (14). Some tumors, including human and murine neuroblastomas, express gangliosides, which are suppressive of dendritic cell maturation and migration (15, 16). Cytokines associated with dendritic cell differentiation, such as granulocyte-macrophage colony-stimulating factor, IL-4, IL-12, and IFN-γ, are rare in the tumor environment. This cytokine imbalance in the tumor milieu leads to overrepresentation of immature, partially differentiated dendritic cells, which, rather than leading to tumor-specific immunity, is associated with T-cell anergy or with induction of suppressive T lymphocytes (14).
However, in the context of cytodestructive and/or inflammatory stimuli, the tumor milieu may be altered to allow iDCs to overcome tumor-associated suppression of function. HSV-1 infection is associated with up-regulated expression of cytokines classically associated with dendritic cell maturation, such as IL-1 and tumor necrosis factor-α (17).
Simultaneously, antigen-presenting cells may be exposed to a wider array of tumor-associated antigens and immune adjuvants such as heat shock proteins. Our demonstration that injection of mature dendritic cells into the post-oHSV infection environment does not add to tumor control (Fig. 3B) suggests that antigen uptake and processing by iDC is a necessary first step before in situ maturation and subsequent migration to regional lymph nodes.
Combination therapy for tumors with G47Δ and iDCs is an attractive and promising clinical strategy. Safety and feasibility have been shown for oHSV-1 injection of tumors and antigen-pulsed dendritic cell vaccination is a commonly studied strategy in patients (18). We have shown that sequential treatment of established subcutaneous tumors with intratumoral injection of G47Δ and iDCs is curative and should be developed as a feasible clinical strategy for cancer patients.
Acknowledgments
Grant support: Brain Tumor Society (S.D. Rabkin and C.J. Farrell), NIH grant RO1 NS032677 (R.L. Martuza), American Brain Tumor Association (C.J. Farrell and W.T. Curry, Jr.), Rappaport Fellowship in the Neurosciences (W.T. Curry, Jr.), and Amos Medical Faculty Development Award of the Robert Wood Johnson Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
R.L. Martuza and S.D. Rabkin serve on the Scientific Advisory Board of MediGene, USA.
References
- 1.Shen Y, Nemunaitis J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006;13:975–92. doi: 10.1038/sj.cgt.7700946. [DOI] [PubMed] [Google Scholar]
- 2.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 3.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–43. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 4.Todo T, Rabkin SD, Sundaresan P, et al. Systemic anti-tumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther. 1999;10:2741–55. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 5.Ino Y, Saeki Y, Fukuhara H, Todo T. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy. Clin Cancer Res. 2006;12:643–52. doi: 10.1158/1078-0432.CCR-05-1494. [DOI] [PubMed] [Google Scholar]
- 6.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cools N, Ponsaerts P, Van Tendeloo VFI, Berneman ZN. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol. 2007;82:1365–74. doi: 10.1189/jlb.0307166. [DOI] [PubMed] [Google Scholar]
- 8.Wells JW, Darling D, Farzaneh F, Galea-Lauri J. Influence of interleukin-4 on the phenotype and function of bone marrow-derived murine dendritic cells generated under serum-free conditions. Scand J Immunol. 2005;61:251–9. doi: 10.1111/j.1365-3083.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 9.He L, Hakimi J, Salha D, Miron I, Dunn P, Radvanyi L. A sensitive flow cytometry-based cytotoxic T-lymphocyte assay through detection of cleaved caspase 3 in target cells. J Immunol Methods. 2005;304:43–59. doi: 10.1016/j.jim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–85. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 11.Wakimoto H, Johnson PR, Knipe DM, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10:983–90. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- 12.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12873–8. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J-Y, Wu Y, Zhang X-S, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM., Jr Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–9. [PubMed] [Google Scholar]
- 16.Walker SR, Ogagan PD, DeAlmeida D, Aboka AM, Barksdale JEM. Neuroblastoma impairs chemokine-mediated dendritic cell migration in vitro. J Pediatr Surg. 2006;41:260–5. doi: 10.1016/j.jpedsurg.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 17.Sergerie Y, Rivest S, Boivin G. Tumor necrosis factor-α and interleukin-1β play a critical role in the resistance against lethal herpes simplex virus encephalitis. J Infect Dis. 2007;196:853–60. doi: 10.1086/520094. [DOI] [PubMed] [Google Scholar]
- 18.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–50. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]