Abstract
Background
Although directly administered antiretroviral therapy (DAART) has demonstrated impressive biological benefits compared with self-administered therapy (SAT) among drug users, the persistence of DAART after transition to SAT has not been examined.
Methods
We conducted a community-based, prospective, randomized controlled trial of 6 months of DAART compared with SAT. The primary outcome was the proportion of subjects who achieved virological success at 6 months postintervention, defined as either a 1.0 log10 reduction from baseline or HIV-1 RNA <400 copies per milliliter. Secondary outcomes included the change from baseline in HIV-1 RNA and CD4 lymphocyte count.
Results
Of the 53 subjects in the SAT arm and 88 subjects in the DAART arm, 52 and 82, respectively, provided blood samples at 6 months postintervention. The DAART (n = 88) and SAT (n = 53) arms did not differ on virological success (DAART 58.0% vs. SAT 56.6%, P = 0.64), mean reduction in log10 HIV-1 RNA (-0.79 vs. -0.31 log10 copies/mL, P = 0.53), or mean change in CD4 lymphocyte count (+60.2 vs. -15.4 cells/mL, P = 0.12). In the multivariate analysis, only high levels of social support significantly predicted virological success.
Conclusions
This analysis, from the first randomized controlled trial of DAART among active drug users, failed to show the persistence of the DAART intervention at improving virological outcomes. Additional strategies are needed to ensure the on-treatment benefits persist following the cessation of DAART.
Keywords: adherence, AIDS, directly administered antiretroviral therapy, directly observed therapy, HIV, substance abuse, social support
INTRODUCTION
There is a growing body of evidence suggesting that, across a variety of clinical settings, directly administered antiretroviral therapy (DAART) for poorly adherent active drug users provides virological and immunological benefits during the duration of the intervention.1-8 Available evidence also suggests that these benefits are conferred without producing higher rates of antiretroviral drug resistance.9 DAART is thus emerging as a critical tool for improving outcomes in this highly vulnerable population that is at high risk for lower prescription10,11 and adherence to antiretroviral therapy,12,13 faster HIV progression,14,15 and increased transmission of HIV, including drug-resistant strains.16
Existing data suggest that, for active drug users during the period of observed therapy, virological suppression is more likely, viral loads are lower, and CD4 lymphocyte counts are higher. These findings have been demonstrated in clinical case series using community outreach3 and within a methadone program,1 in a case-control study within a methadone maintenance program,17 and in a 3-month randomized controlled trial (RCT) of DAART using community outreach.8
Our RCT of a 6-month DAART intervention using mobile community outreach compared with self-administered therapy (SAT) demonstrated that 71% of DAART subjects vs. 55% achieved the primary outcome of virological success, defined as a viral load ≤400 copies per milliliter or >1.0 log10 reduction in viral load.18 Adjusted for censoring, the viral load decrease from baseline was also significantly decreased in the DAART group (-1.16 vs. -0.29 log10 copies/mL) (F. L. Altice, D. Smith-Rohrberg, R. D. Bruce, S. A. Springer, G. H. Friedland, unpublished data, 2006). Additional analyses have demonstrated that provision of medical and case management services “enhanced DAART” were associated with better outcomes19 and that resistance did not increase in the DAART arm20 despite increased levels of adherence.21
Unlike short-course directly observed therapy for tuberculosis, antiretroviral therapy is required lifelong. Limited resources and the intensiveness of the intervention, however, make it unlikely that DAART will become a lifelong intervention. Because adherence to medication, irrespective of disease type, duration of treatment, and type of intervention tends to wane,22 it is likely that the on-treatment benefits of DAART will decrease over time. Indeed, public health and clinical practitioners may be less likely to provide DAART, or any adherence intervention, if long-term outcomes are less promising. Additionally, long-term outcomes are important in determining the duration of DAART necessary to optimize effectiveness and minimize costs. Finally, the determinants of postintervention success are important in assessing which particular subject-specific and program-specific factors need to be enhanced or modified.
In nearly all trials of adherence interventions, there is limited published data on this postintervention period. Several other antiretroviral medication adherence interventions that have shown excellent on-treatment effectiveness have suffered from nonpersistent long-term outcomes, including cell phone reminders,23 cognitive behavioral counseling,24 and contingency management.25 As such, the persistence of biological benefits is crucial in evaluating and designing DAART and other adherence programs and in deciding upon the optimal duration of the intervention.
Although much of the data described above strongly support the on-treatment benefits of DAART, to date, only one small study exists on the persistence of the effects of DAART.26 That study analyzed 9 patients in a 15-patient cohort on follow-up of biological outcomes several months after treatment. The results suggested the persistence of biological effects, although minimal conclusions can be made owing to the study's small sample size. To address whether DAART results in sustained clinical benefit to HIV-infected drug users, we examined biological outcomes for 6 months after the end of DAART among subjects enrolled in a RCT.
METHODS
Study Population and Design
We conducted a RCT of a 6-month intervention of DAART compared with SAT among HIV+ drug users in New Haven, CT, from 2001 to 2006. The nature of the DAART program and the design of the study have been previously published as part of an interim report27 and in the primary18 and secondary19,20 analyses. Inclusion criteria included the following: (1) being HIV seropositive; (2) being eligible for and/or being prescribed highly active antiretroviral therapy; (3) living within the city of New Haven; (4) actively using heroin and/or cocaine in the previous 6 months; and (5) receiving no more than a twice-daily regimen.
After informed consent, eligible subjects were randomized 2:1 to DAART or SAT stratified on the following criteria: (1) antiretroviral experience, (2) problematic alcohol use, (3) baseline HIV-1 RNA level, and (4) baseline CD4 lymphocyte count.
Testing for HIV-1 RNA (Amplicor 1.5; Roche, Basel, Switzerland) and CD4 lymphocyte count (Fluorescence-Activated Cell Sorter; Quest, Madison, NJ) was conducted at baseline and at 1, 3, 6, 9, and 12 months thereafter. Laboratory results were available for 108 (77%) and 122 (87%) subjects at 9 and 12 months, respectively. The primary outcome of this analysis was the same as in the original trial: virological success, defined as an HIV RNA level reduction of 1.0 log10 or an HIV-1RNA <400 copies per milliliter at the end of the 6-month intervention.28 This outcome was achieved in the original trial (F. L. Altice, D. Smith-Rohrberg, R. D. Bruce, S. A. Springer, G. H. Friedland, unpublished data, 2006). The 9-month and 12-month postbaseline analyses were part of the planned secondary outcomes of the trial. Standardized scales were used for measuring depression using the Center for Epidemiological Studies Depression score ≥16, social support according to Huba et al,29 and addiction severity using the Drug Abuse Screening Test ≥6 for high severity. These measures were all analyzed from the baseline interview. All multivariate analyses used these baseline values as covariates.
The study was approved by the Yale University Institutional Review Board and had a Certificate of Confidentiality. The study is registered at ClinicalTrials.gov with identifier: NCT00367172.
Statistical Analysis
Statistical analysis proceeded similarly to that for the initial report.7 The probability of virological success was assessed using a logistic regression model. Change from baseline in log10 HIV-1 RNA level data were fitted using the SAS procedure LIFEREG with the dist = normal option. This robustly accounts for the large number of censored values owing to viral loads at the lower limits of detection at baseline and at follow-up.30-33 Normal probability plots confirmed that data fit the parametric assumptions of the regression. Mean change in CD4 count from baseline to 6 months post-intervention was assessed using a general linear model including baseline CD4 count as a covariate.
For multivariate modeling to assess the impact of various demographic and services covariates on long-term outcomes, a logistic regression model was constructed to predict the 6-month postintervention proportion achieving virological success. The demographic and social covariates have been previously described19 and age, sex, education, addiction severity, depression, social support, homelessness status, and income level. These were categorized through assessing the distribution of responses and to achieve consistency with the published literature and clinical practice. For both models, all the covariates were initially fit to a model consisting only of the covariate in question, adjusted only for baseline viral load. In these unadjusted analyses, several functional forms of each covariate, including linear and various polytomous and dichotomous forms, were explored; Akaike's information criterion was used to choose the optimal form, and this form was then used in the multivariate analysis. Subsequently, a multivariate model was fit to the data, using both backward and forward stepwise regression approaches, using P values of ≤0.20 to enter and leave the model. Akaike's information criterion was again used to assess model fit, with attention additionally to the impact of each covariate on the effect of the main health services exposure covariates; the optimal model was chosen at the convergence of the forward and backward models, with attention to parsimony so as to avoid overfitting the model.
RESULTS
The disposition and demographic characteristics of the study population have been previously reported (F. L. Altice, D. Smith-Rohrberg, R. D. Bruce, S. A. Springer, G. H. Friedland, unpublished data, 2006). Fourteen subjects randomized to DAART (16%) refused DAART immediately postrandomization, and these subjects had lower viral loads and higher CD4 counts at baseline. This resulted in the significant differences in biological covariates at baseline between the two groups. It was thus necessary to control for these covariates in subsequent analyses. The demographic and social characteristics of the study participants are presented in Table 1, along with the associated odds ratios, adjusted for baseline virological level.
TABLE 1.
Predictors of Virological Success at 6 Months Postintervention
| Variables | n | n (%) Success | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Randomization arm | ||||
| SAT | 53 | 30 (57) | Referent | 0.64 |
| DAART | 88 | 51 (58) | 1.18 (0.58 to 2.41) | |
| Age | ||||
| <45 yrs | 79 | 45 (57) | Referent | 0.97 |
| ≥45 yrs | 62 | 36 (58) | 1.01 (0.51 to 2.01) | |
| Gender | ||||
| Female | 44 | 25 (57) | Referent | 0.98 |
| Male | 97 | 56 (58) | 1.01 (0.49 to 2.1) | |
| Ethnicity | ||||
| Black, not Hispanic | 82 | 49 (60) | Referent | 0.48 |
| Hispanic or white | 59 | 32 (54) | 0.78 (0.39 to 1.55) | |
| Education | ||||
| Not high school graduate | 52 | 25 (48) | Referent | 0.13 |
| High school or beyond | 89 | 56 (63) | 1.72 (0.85 to 3.47) | |
| Primary language | ||||
| English | 117 | 70 (60) | Referent | 0.17 |
| Spanish | 24 | 11 (46) | 0.53 (0.21 to 1.31) | |
| Homelessness | ||||
| No | 87 | 52 (60) | Referent | 0.35 |
| Yes | 54 | 29 (54) | 0.71 (0.35 to 1.44) | |
| Monthly income | ||||
| <$500 | 64 | 30 (47) | Referent | 0.03 |
| ≥$500 | 77 | 51 (66) | 0.46 (0.23 to 0.92) | |
| Addiction severity | ||||
| Low | 84 | 48 (57) | Referent | 0.80 |
| High | 57 | 33 (58) | 1.09 (0.55 to 2.19) | |
| Depression | ||||
| No | 53 | 33 (62) | Referent | 0.48 |
| Yes | 86 | 46 (53) | 1.29 (0.63 to 2.64) | |
| Social support | ||||
| Low | 46 | 19 (41) | Referent | 0.01 |
| High | 95 | 62 (65) | 2.64 (1.27 to 5.5) | |
| Trust in physician | ||||
| Low | 67 | 36 (54) | Referent | 0.48 |
| High | 73 | 44 (60) | 1.28 (0.65 to 2.53) |
Two subjects were missing the Center for Epidemiological Studies-Depression (CES-D) score, and 1 subject was missing the trust in physician score.
OR, odds ratio; CI, confidence interval; SAT, self-administered therapy.
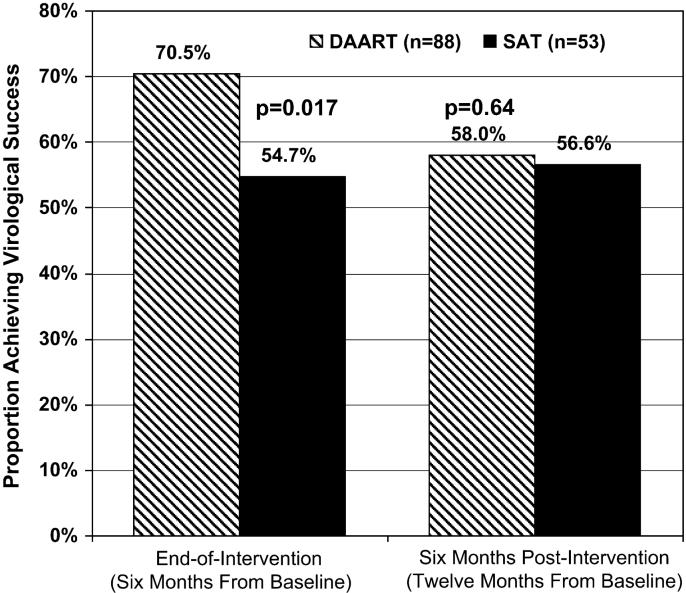
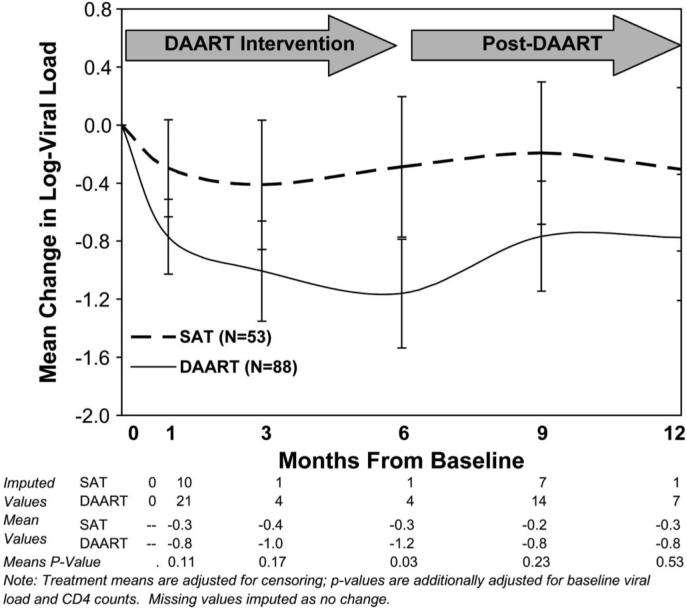
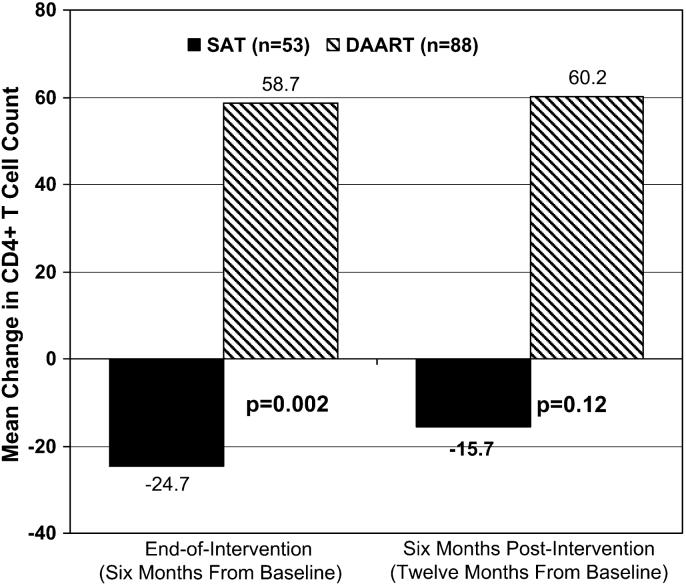
At 6 months postintervention (12 months postbaseline) in the intention-to-treat analysis, the DAART and SAT arms did not differ with respect to virological success (DAART 58.0% vs. SAT 56.6%, P = 0.64; Fig. 1), the proportion <400 copies per milliliter (48.9% vs. 52.8%, P = 0.64), mean reduction in log10 HIV-1 RNA copies (-0.79 vs. -0.31, P = 0.53; Fig. 2), or mean change in CD4 count (+60.2 vs. -15.7 cells/mL, P = 0.12; Fig. 3). The results on viral load and CD4 count changes from baseline did not differ in available case, last observation carried forward (LOCF), or missing = no change analyses.
FIGURE 1.
Virological outcomes at the end of the intervention and at 6 months postintervention. Proportion of subjects achieving virological success (HIV-1 RNA ≤400 copies/mL or a1.0 log10 reduction in HIV-1 RNA). Note: reported P values are adjusted for baseline viral load and CD4 counts.
FIGURE 2.
Mean change in log HIV-1 RNA during the intervention and follow-up period. Raw values are shown, adjusted for censoring at the lower limits of detection. Note: P values are adjusted for baseline HIV-1 viral level and CD4+ T-cell count.
FIGURE 3.
Immunological outcomes at the end of the intervention and at 6 months postintervention. Mean change in CD4 count. Note: reported P values are adjusted for baseline viral load and CD4 counts and represent that from the log-transformed model, which better fits the normality assumption.
The per-protocol analysis, among the 58 (78%) patients who completed all 6 months of the DAART intervention, also failed to show persistence of the effects in the 12-month period (data not shown). These results also did not change when analyzing the subset of participants with amplifiable samples at baseline.
Among the 75 patients who achieved virological suppression of less than or equal to 400 copies at 6 months, 15 of 20 SAT subjects (75%) and 34 of 55 DAART subjects (62%) exhibited virological suppression at 12 months (P = 0.41).
The results of the multivariate analysis are presented in Table 1. Only low social support was significantly associated with virological failure. Low income was associated with high addiction severity (Pearson correlation coefficient ρ = 0.20, P = 0.01) and with homelessness (ρ = 0.34, P < 0.001).
DISCUSSION
Similar to other adherence interventions, the benefits of DAART wane after discontinuation of the intervention and highlight the need for optimizing outcomes in the post-DAART period. Certainly DAART represents a highly intensive structured intervention, and the change to the less structured self-administration in the post-DAART may be particularly difficult for some drug users, particularly for those with low social support. This phenomenon has been seen among HIV-infected prisoners, where the virological benefits seen while on structured therapy during incarceration do not persist after release.34 A better characterization of the postintervention process and the specific barriers to adherence are needed, as are additional strategies that might improve the durability of virological and immunological benefit.
Several possibilities to improve upon long-term outcomes of DAART are worth consideration, including longer durations of the intervention, provision of `booster' DAART when problematic adherence recurs, and more effective transitions from DAART. Hybrid models, whereby patients are initiated on DAART during an undefined stabilization period and then are transitioned to a somewhat less intensive program for some time is worthy of consideration. Such programs might include tapering DAART from daily to thrice weekly and then weekly DAART, with adherence case management, or weekly group adherence counseling sessions. Mitty et al3 described a transition process whereby those subjects terminating DAART continued to receive adherence support with phone calls and assistance with pill organization. Determining the optimal period of transition would require the prospective identification of factors that predict retention in DAART programs. DAART itself may be extended for longer periods of time; in methadone-based program of Lucas et al, subjects remained for up to 1 year on DAART, with persistently good outcomes. Continuation of DAART for protracted periods of time may be particularly necessary for individuals who are more cognitively impaired or with low social support. Those with severe substance use disorders and/or severe mental illness are more likely to be socially destabilized and would be most likely to benefit from longer periods of supervision.4
Another important strategy is the provision of colocated medical and case management services to DAART. In a separate analysis, we demonstrated the impact of these “enhanced services” on short-term clinical outcomes.19 Getting active drug users into effective drug treatment, such as on methadone or buprenorphine, may be part of the process to bring greater stability into these patient's lives. The greater availability of buprenorphine and the ability of HIV practitioners to comanage drug dependence may further improve outcomes for this population, with or without DAART.35 Assessing the impact of these services on longer term outcomes will require further prospective cohort studies andclinicaltrials. Theresultthathighsocialsupport was associated with better outcomes indicates that integrated interventions that address complex social factors may be necessary to achieve better long-term results. Although active substance use was not associated with negative biological outcomes, this may reflect the impact of the DAART intervention with its associated social support at effectively interacting with these individuals.
There are several important limitations of the study. Methodologically, the RCTwas designed with sufficient power to detect differences in the primary outcome; this difference at 6 months was 71% vs. 55%. Because the calculations are the same at the 6-month postintervention sample, so too is the power. It is possible that a study with greater power would be able to detect a more subtle benefit of DAART, particularly with CD4 counts. It is unlikely, however, that such a small benefit would be clinically significant. The conclusions from the multivariate analyses must be tempered by the fact that the measures were taken only at baseline and were not assessed longitudinally. They should be viewed as hypothesis generating rather than as definitively providing a causal link.
In summary, this analysis of the 6-month postintervention outcomes of an RCT comparing DAARTwith SAT among active HIV-infected drug users suggests that DAART programs must seriously consider adherence strategies in the post-DAART period if the benefits of DAART are to persist beyond the intervention itself. Although virological benefit did not persist beyond the intervention, DAART remains an effective strategy to improve adherence among drug users with problematic adherence as previously reported. The next step is in determining how DAART might be improved upon to make its benefits more persistent in the postintervention period.
ACKNOWLEDGMENTS
Author contributions—Conception and design: D.S.R.M., R.D. B., M.W., S.A.S., and F.L.A.; analysis and interpretation of the data: F.L.A. and D.S.R.M.; drafting of the article: D.S.R.M.; critical revision of the article for important intellectual content: D.S.R.M., R.D.B., M.W., S.A.S., and F.L.A.; final approval of the article: D.S.R.M., R.D.B., M.W., S.A.S., and F.L.A.; provision of study materials or patients: R.D.B., M.W., and F.L.A.; statistical expertise: D.S.R.M.; obtaining of funding: F.L.A.; collection and assembly of data: F.L.A., D.S.R.M., and R.D.B. F.L.A. and D.S.R.M. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by National Institutes on Drug Abuse (R01 DA13805) by funding this study and providing career development awards for F.L.A. (K24 DA 0170720), S.A.S. (K23 DA 019381), and R.D.B. (K23 DA 022143). D.S. R.M. receives funding from the National Institutes of Health Medical Science Training Program (GM07205).
The funding sources played no role in design of the study, data collection, analysis or interpretation of results, or in the writing of the report.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to report.
REFERENCES
- 1.Conway B, Prasad J, Reynolds R, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38:S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- 2.Lucas GM, Weidle PJ, Hader S, et al. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38:S409–S413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- 3.Mitty JA, Macalino GE, Bazerman LB, et al. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. J Acquir Immune Defic Syndr. 2005;39:545–550. [PubMed] [Google Scholar]
- 4.Behforouz HL, Kalmus A, Scherz CS, et al. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. J Acquir Immune Defic Syndr. 2004;36:642–645. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- 5.Tinoco I, Giron-Gonzalez JA, Gonzalez-Gonzalez MT, et al. Efficacy of directly observed treatment of HIV infection: experience in AIDS welfare homes. Eur J Clin Microbiol Infect Dis. 2004;23:331–335. doi: 10.1007/s10096-003-1099-8. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg B, Berkman A, Thomas R, et al. Evaluating supervised HAART in late-stage HIV among drug users: a preliminary report. J Urban Health. 1999;76:468–480. doi: 10.1007/BF02351504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altice FL, Maru DS, Bruce RD, et al. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 9.Maru DSR, Kozal MJ, Bruce RD, et al. Directly administered antiretroviral therapy for HIV-infected drug users does not have an impact on antiretroviral resistance: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:555–563. doi: 10.1097/qai.0b013e318158c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strathdee SA, Palepu A, Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 11.Celentano DD, Galai N, Sethi AK, et al. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 12.Lucas GM, Gebo KA, Chaisson RE, et al. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 13.Lucas GM, Griswold M, Gebo KA, et al. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 14.Lucas GM, Cheever LW, Chaisson RE, et al. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 17.Lucas GM, Mullen BA, Weidle PJ, et al. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42:1628–1635. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- 18.Altice FL, Smith-Rohrberg D, Bruce RD, et al. Superiority of directly administered antiretroviral therapy compared to self-administered therapy among HIV-infected drug users: a randomized, controlled trial. Clin Infect Dis. 2007;45:770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith-Rohrberg D, Mezger J, Walton M, et al. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. J Acquir Immune Defic Syndr. 2006;43:S48–S53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- 20.Smith-Rohrberg Maru D, Kozal MJ, Springer SA, et al. Directly administered antiretroviral therapy for HIV-infected drug users does not impact antiretroviral resistance: results from a randomized, controlled trial. J Acquir Immune Defic Syndr. 2007;46:555–563. doi: 10.1097/qai.0b013e318158c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maru DS, Bruce RD, Walton M, et al. Initiation, adherence, and retention in a randomized controlled trial of directly administered antiretroviral therapy. AIDS Behav. 2007;12:284–293. doi: 10.1007/s10461-007-9336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 23.Puccio JA, Belzer M, Olson J, et al. The use of cell phone reminder calls for assisting HIV-infected adolescents and young adults to adhere to highly active antiretroviral therapy: a pilot study. AIDS Patient Care STDS. 2006;20:438–444. doi: 10.1089/apc.2006.20.438. [DOI] [PubMed] [Google Scholar]
- 24.Morin SF, Chesney MA, Ehrhardt AA, et al. Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007;44:213–221. doi: 10.1097/QAI.0b013e31802c0cae. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88:54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitty JA, Huang D, Loewenthal HG, et al. Modified directly observed therapy: sustained self-reported adherence and HIV health status. AIDS Patient Care STDS. 2007;21:897–899. doi: 10.1089/apc.2007.0066. [DOI] [PubMed] [Google Scholar]
- 27.Altice FL, Mezger JA, Hodges J, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38:S376–S387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- 28.Smith-Rohrberg D, Altice FL. Randomized controlled trials of DAART for HIV+ patients: questions about study population and analytical approach. Clin Infect Dis. 2006;43:1221–1222. doi: 10.1086/508357. [DOI] [PubMed] [Google Scholar]
- 29.Huba GJ, Melchior LA, Staff of The Measurement Group, and HRSA/HAB's SPNS Cooperative Agreement Steering Committee . Module 64: Self-Efficacy Form. The Measurement Group; Culver City, CA: 1996. Available at: http://www.themeasurementgroup.com/modules/mods/cov_mod64.htm. Accessed on April 25, 2008. [Google Scholar]
- 30.Marschner IC, Betensky RA, DeGruttola V, et al. Clinical trials using HIV-1 RNA-based primary endpoints: statistical analysis and potential biases. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:220–227. doi: 10.1097/00042560-199903010-00002. [DOI] [PubMed] [Google Scholar]
- 31.Journot V, Chene G, Joly P, et al. Viral load as a primary outcome in human immunodeficiency virus trials: a review of statistical analysis methods. Control Clin Trials. 2001;22:639–658. doi: 10.1016/s0197-2456(01)00158-1. [DOI] [PubMed] [Google Scholar]
- 32.Hammer SM, Vaida F, Bennett KK, et al. Dual vs single protease inhibitor therapy following antiretroviral treatment failure: a randomized trial. JAMA. 2002;288:169–180. doi: 10.1001/jama.288.2.169. [DOI] [PubMed] [Google Scholar]
- 33.Smith-Rohrberg D, Altice FL. Randomized, controlled trials of directly administered antiretroviral therapy for HIV-infected patients: questions about study population and analytical approach. Clin Infect Dis. 2006;43:1221–1222. doi: 10.1086/508357. [DOI] [PubMed] [Google Scholar]
- 34.Springer S, Pesani E, Hodges J, et al. Effectiveness of antiretroviral therapy among HIV infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38:1754–1760. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 35.Altice FL, Sullivan LE, Smith-Rohrberg D, et al. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clin Infect Dis. 2006;43:S178–S183. doi: 10.1086/508181. [DOI] [PubMed] [Google Scholar]