Abstract
It was recently asserted that the voltage-dependent anion channel (VDAC) serves as a global regulator, or governor, of mitochondrial function (Lemasters and Holmuhamedov, Biochim Biophys Acta 1762:181–190, 2006). Indeed, VDAC, positioned on the interface between mitochondria and the cytosol (Colombini, Mol Cell Biochem 256:107–115, 2004), is at the control point of mitochondria life and death. This large channel plays the role of a “switch” that defines in which direction mitochondria will go: to normal respiration or to suppression of mitochondria metabolism that leads to apoptosis and cell death. As the most abundant protein in the mitochondrial outer membrane (MOM), VDAC is known to be responsible for ATP/ADP exchange and for the fluxes of other metabolites across MOM. It controls them by switching between the open and “closed” states that are virtually impermeable to ATP and ADP. This control has dual importance: in maintaining normal mitochondria respiration and in triggering apoptosis when cytochrome c and other apoptogenic factors are released from the intermembrane space into the cytosol. Emerging evidence indicates that VDAC closure promotes apoptotic signals without direct involvement of VDAC in the permeability transition pore or hypothetical Bax-containing cytochrome c permeable pores. VDAC gating has been studied extensively for the last 30 years on reconstituted VDAC channels. In this review we focus exclusively on physiologically relevant regulators of VDAC gating such as endogenous cytosolic proteins and mitochondrial lipids. Closure of VDAC induced by such dissimilar cytosolic proteins as pro-apoptotic tBid and dimeric tubulin is compared to show that the involved mechanisms are rather distinct. While tBid mostly modulates VDAC voltage gating, tubulin blocks the channel with the efficiency of blockage controlled by voltage. We also discuss how characteristic mitochondrial lipids, phospatidylethanolamine and cardiolipin, could regulate VDAC gating. Overall, we demonstrate that VDAC gating is not just an observation made under artificial conditions of channel reconstitution but is a major mechanism of MOM permeability control.
Keywords: Apoptosis, Mitochondria, Mitochondria outer membrane, Voltage dependent anion channel, VDAC, Channel gating, Tubulin, tBid, Cardiolipin, Lipid packing stress
Introduction
A conserved property of VDAC channels in vitro is the ability to adopt a unique fully open state and multiple “closed” states of significantly smaller conductance. Application of voltage to VDAC channels reconstituted into planar lipid membranes promotes these “closed” states—the phenomenon known as voltage-induced gating (Colombini et al. 1996). The states differ in their ability to pass non-electrolytes and to conduct ions (Hodge and Colombini 1997). “Closed” states are characterized by weak cationic selectivity, as compared with weak anionic selectivity in the open state, and are virtually impermeable to negatively charged metabolites such as ATP (Rostovtseva and Colombini 1996, 1997). The gating mechanism is still under discussion, but most of the experimental and theoretical evidence supports a model proposed by Colombini and coauthors (Peng et al. 1992; Thomas et al. 1993; Song et al. 1998a, b) wherein the existence of a positively charged mobile domain in the wall of the channel, called voltage sensor, is postulated. According to the model, VDAC responds to the electric field applied to the membrane by moving this domain to the surface of the membrane, which results in a pore of smaller diameter and inverted selectivity (Fig. 1).
Fig. 1.
Schematics of VDAC gating. Upon VDAC closure a positively charged voltage sensor domain moves out of the channel to the membrane surface. This process is accompanied by decreasing of the pore volume and reversing of the channel selectivity. Closed states are impermeable for ATP/ADP fluxes but still conduct small ions and Ca2+. There are multiple stimuli which regulate VDAC gating or induce transient block of the open state
By using the luciferin/luciferase method the fluxes of ATP through open VDAC channels were directly measured confirming that VDAC is sufficient to provide all ATP efflux from mitochondria (Rostovtseva and Colombini 1996, 1997). When channels were closed by applying high voltage, the ATP flux was shut down. This was a straightforward demonstration that VDAC closure could greatly diminish metabolite flux across the mitochondrial outer membrane. However, VDAC closed states are still rather conductive and able to transport small ions, such as K+, Na+, Cl−, and Ca2+ (Tan and Colombini 2007). When reconstituted into planar lipid membranes, a VDAC monomer forms an aqueous pore of 2.5–3 nm in diameter (Mannella et al. 1989), permeable for uncharged polymers with molecular weight of 3,400–6,000 kDa (Colombini 1980; Krasilnikov et al. 1992). However, the effective size of the channel appears to be rather different for neutral and charged solute molecules and even sensitive to their structure. By using current noise analysis, it was found that VDAC channels could distinguish between such comparable molecules as ATP and UTP and that the selectivity among molecules of similar size and charge is based on their 3-dimensional structure (Rostovtseva et al. 2002a). The evidence strongly favors the existence of a binding site (or a region of binding sites) in the channel that discriminates between purines and pyrimidines. Furthermore, it was found that VDAC can differentiate between natural metabolites and synthetic molecules (Rostovtseva et al. 2002b). These findings suggest that the electrostatic environment within the channel has been evolutionary selected to favor the passage of adenine nucleotides. Certainly, the positively charged cytochrome c molecules of 12 kDa molecular weight and ~3.4 nm diameter cannot permeate through the VDAC pore under normal conditions.
Implication of VDAC gating in apoptosis
As the most abundant and large channel in MOM, VDAC has been an attractive candidate for a pathway for cytochrome c release. It was suggested that pro-apoptotic Bax directly interacts with VDAC and forms a novel large cytochrome c permeable pore (Shimizu et al. 1999, 2000a, b). However, the subsequent studies did not confirm either a formation of the VDAC/Bax large pore in electrophysiological experiments (Rostovtseva et al. 2004) or a direct interaction of these two proteins by co-immunoiprecipitation and cross-linking (Mikhailov et al. 2001). In the generally accepted model of the permeability transition pore (PTP), VDAC in MOM together with ANT in the inner membrane are considered to be the major components of the PTP supramolecular complex that spans both mitochondrial membranes. By definition, PTP opening allows the solutes up to ~1.5 kDa to permeate through mitochondrial membranes. This results in dissipation of the mitochondrial inner membrane potential and matrix swelling and leads to cytochrome c release which is followed by necrosis or apoptosis (see reviews Crompton 1999; Zoratti et al. 2005; Bernardi et al. 2006). In this scheme, opening and closing of PTP (for instance, by Ca2+) is associated with VDAC opening and closing. However, a number of experimental results contradict this model (see discussion Rostovtseva et al. 2005; Bernardi et al. 2006). First of all, whether VDAC is a part of the PTP complex or not, solutes permeating through PTP could cross MOM through open VDAC channels. Second, it was demonstrated recently that Ca2+, the main stimulus of PTP opening, does not change VDAC gating properties (Tan and Colombini 2007). Ca2+ is not able to open or close VDAC channels. Importantly, Ca2+ flux is actually higher through VDAC closed states than through its open state due to the reversed cation selectivity of the closed states (Tan and Colombini 2007). Finally, recent experiments with genetical knock-out or knock-down of three VDAC isoforms provided strong evidence that VDAC is not an essential part of PTP (Baines et al. 2007). It was demonstrated that VDAC-deficient mitochondria and cells (one isoform, different combinations of two isoforms, or all three isoforms) exhibit both PTP and Bcl-2 family member-driven cell death. Similar conclusions were reached in previous studies with a yeast strain lacking VDAC1 (Priault et al. 1999; Pavlov et al. 2001; Polcic and Forte 2003) and with mitochondria from VDAC−/− mice (Krauskopf et al. 2006). These data undermine the initial belief that PTP or Bax-mediated apoptosis would depend on physical interaction of Bax with VDAC (Galluzzi and Kroemer 2007).
Does it mean that VDAC is “dispensable” for the mitochondria-dependent cell death as was declared recently by Baines and coworkers (Baines et al. 2007)? We believe that there is a third mechanism, in which VDAC closure leads to the initiation or promotion of apoptosis and cell death (Vander Heiden et al. 2000, 2001; Lemasters and Holmuhamedov 2006; Tan et al. 2007a). It was shown that the removal of growth-factor leads to a deficiency in the exchange of adenine nucleotides due to the VDAC closure (Vander Heiden et al. 2000, 2001). This loss of MOM permeability for metabolites, such as phosphocreatine and adenine nucleotides, could be restored by the anti-apoptotic Bcl-xL protein. In turn, the pro-apoptotic BH3 domain only protein, tBid, induces irreversible VDAC closure (Rostovtseva et al. 2004). In support of the VDAC closure mechanism, ethanol treatment of permeabilized hepatocytes inhibits mitochondrial respiration and decreases permeability of MOM to ATP and respiratory substrates (Lemasters and Holmuhamedov 2006). Another evidence that VDAC closure favors cytochrome c release was reported recently (Lai et al. 2006; Tan et al. 2007a). It was found that phosphorothioate oligonucleotide, G3139, induces cytochrome c release and apoptosis in melanoma and other cancer cells in a Bcl-2-independent pathway and that G3139 directly binds to MOM and reduces its permeability to ADP by closing VDAC. The addition of G3139 to a reconstituted VDAC channel resulted in channel closure (Lai et al. 2006; Tan et al. 2007b).
Cytosolic and membrane regulators of VDAC
The facts surveyed above strongly suggest that ATP/ADP exchange between mitochondria and the cytosol through the open VDAC channels is a requirement for the efficient mitochondrial energy transfer in healthy cells. On the contrary, excessive VDAC closure promotes apoptotic signals without direct involvement of VDAC in PTP or Bax-containing cytochrome c permeable pores. That is why we believe that gating of VDAC, which has been extensively studied on reconstituted channels, is not just an observation made under artificial conditions but a major mechanism of MOM permeability regulation. However, what are the exact ways of VDAC regulation in the cell? To address this question one first needs to know which cytosolic and mitochondrial agents participate in VDAC regulation in normal conditions and in apoptosis, and what mechanisms of their interactions are involved.
VDAC regulation by Bcl-2 family proteins
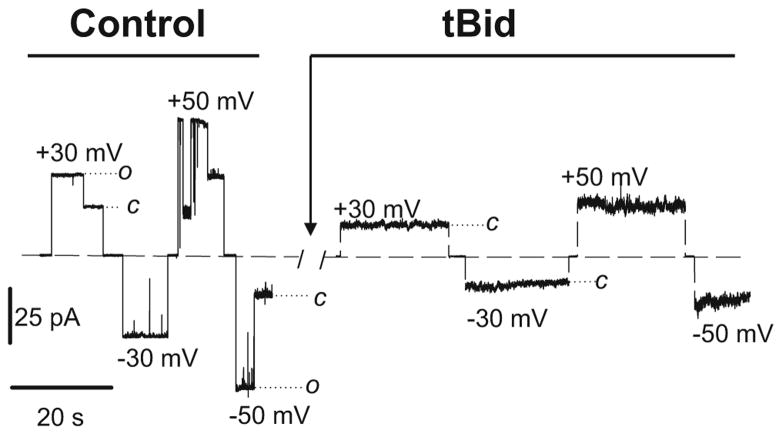
It has been shown that anti-apoptotic Bcl-xL favors the open state of VDAC reconstituted into a planar membrane and also restores the ATP/ADP exchange between mitochondria and the cytosol after it was disrupted by growth factor withdrawal (Vander Heiden et al. 2000, 2001). In accordance with this scheme, pro-apoptotic protein, tBid, affects the voltage-gating properties of VDAC by inducing channel closure (Rostovtseva et al. 2004). It was shown that tBid leads to irreversible closure of VDAC channels in a dose-dependent manner, both on single (Fig. 2) and multichannel membranes. The mechanism by which Bcl-xL and tBid alter the gating properties of VDAC remains to be understood. Noteworthy, any direct interaction of VDAC with both of these Bcl-2 family proteins (as well as with Bax) has never been demonstrated by either co-immunoprecipitation or cross-linking. It was suggested that tBid and Bcl-xL could alter VDAC gating indirectly through the lipid environment surrounding the proteins (Vander Heiden et al. 2001; Rostovtseva et al. 2004). Both proteins interact with the target membrane: they form unspecific ion-conducting channels in the planar lipid membrane (Minn et al. 1997; Rostovtseva et al. 2004), tBid promotes negative membrane curvature and, as a result, destabilizes bilayer membranes (Epand et al. 2002). It was proposed (Basanez et al. 2002) that tBid could act in concert with Bax to form lipidic pore-type non-bilayer structures in the membrane. It was also shown that cardiolipin, a lipid characteristic of mitochondrial membranes, increases binding of tBid to pure lipid vesicles as well as to MOM (Lutter et al. 2000) and promotes formation of large pores by tBid and monomeric Bax (Kuwana et al. 2002).
Fig. 2.
tBid-induced irreversible closure of VDAC. The current traces through the same single VDAC channel reconstituted into planar lipid membrane are shown before and after addition of 20 nM tBid in the cis-side of the membrane chamber. Membrane lipid composition: asolectin/DPhPC/CL/cholesterol; membrane bathing solution: 250 mM KCl, 1 mM CaCl2, 5 mM HEPES buffered at pH 7.2 (from Rostovtseva et al. 2004) (with permission ASBMB Journals)
Considering tBid ability to modify lipid bilayer elastic properties, we proposed a model where tBid inserts into the membrane, alters the local lipid environment in close proximity to VDAC, and thus induces VDAC closure indirectly (Rostovtseva et al. 2004). Indirect protein–protein interactions involving membrane lipids have been proposed for a model system of gramicidin channels (Goforth et al. 2003) and stretch-activated cation channels (Suchyna et al. 2004). Whether tBid alters VDAC gating properties by direct or indirect interactions, these most likely occur within the lipid membrane, so that its lipid composition itself may modify them. Following this logic one could hypothesize that membrane lipid composition would affect VDAC gating.
VDAC regulation by mitochondrial lipids
In order to test this hypothesis, the effect of three key mitochondrial lipids: phosphatidylcholine (PC), lamellar lipid with small spontaneous curvature, and two non-lamellar lipids with high negative spontaneous curvature, phosphatidylethanolamine (PE) and cardiolipin (CL) was investigated (Rostovtseva et al. 2006). The rationale for this study was that when a non-lamellar lipid, such as PE, which spontaneously forms inverted hexagonal phase, is forced into the planar bilayer structure, this results in a significant stress of lipid packing in the region of the acyl chains (Gruner 1985; Keller et al. 1993; Bezrukov et al. 1998; Cantor 1999; Bezrukov 2000; Brink-van der Laan et al. 2004). Estimates show that the difference in the corresponding lateral pressure between PE and PC membranes can reach hundred atmospheres (Bezrukov 2000; Gullingsrud and Schulten 2004). We found that two non-lamellar lipids with the higher lipid packing stress, PE and CL, facilitate VDAC closure at the cis-negative applied potentials (Rostovtseva et al. 2006). This was seen in multi-channel membranes as an extra (in comparison with lamellar PC) reduction in conductance at negative potentials (Fig. 3a). Thus, the presence of non-lamellar lipids changes VDAC conformational equilibrium to promote smaller conductance closed states, suggesting a coupling between the mechanical pressure in the hydrocarbon lipid region and VDAC channel gating. However, it turned out that this coupling takes place not through a change in the gating charge but mostly through a shift in the equilibrium between open and closed states.
Fig. 3.
VDAC gating asymmetry enhanced by non-lamellar mitochondrial lipids, PE and CL. a In the multichannel membranes formed from PE and PC + CL the closed state conductance is two times lower at negative potentials than at positive ones as follows from the conductance versus voltage plots normalized to the maximum conductance, Gmax (from Rostovtseva et al. 2006). Analysis of the plot also shows that for negative potentials the midpoint voltage, that is, the voltage at which half channels are open and half closed, is 10 mV less negative for PE as compared with PC. b A cartoon explaining gating asymmetry under lipid packing stress in PE bilayer. The model of VDAC gating was adopted from (Song et al. 1998a). There are one open and two sets of closed states of VDAC channel depending on the sign of the applied voltage. We hypothesize that the protein’s outer surface has a concave shape when channel adopts the closed state conformations at negative potentials. c Because of the concave shape, the lateral pressure in the hydrocarbon tail area of a planar bilayer shifts conformational equilibrium of VDAC channel in favor of the closed states of this shape. The probability to find these states in non-lamellar PE is much higher than in lamellar PC. Indeed, transition to the concave shape relieves the elastic stress of lipid packing, making the closed conformation energetically more favorable in membranes formed from non-lamellar lipid. A simple estimate illustrated here predicts a significant free energy change (see the text)
These experiments showed that the asymmetry of VDAC gating, an intrinsic property of the channel, can be either catalyzed or suppressed by the membrane lipid composition. According to the working model, transitions between the open and closed states of VDAC are associated with relatively large conformational rearrangements of the protein (Colombini et al. 1996; Song et al. 1998a, b). These rearrangements account for a 50% reduction in the pore diameter and decrease the pore volume by 20–40 nm3 (Zimmerberg and Parsegian 1986). Depending on the applied voltage polarity, VDAC gates by two distinctly different processes (Colombini et al. 1996; Song et al. 1998a) wherein a part of protein is displaced toward one or other side of the membrane (Fig. 3b). This conjecture is strongly supported by the fact that the closed states have different conductance for different signs of the applied voltage (Fig. 3a). Therefore, it is natural to expect that the geometry of the channel’s outer surface which is in contact with the hydrophobic phase of the membrane is different for these states too (Fig. 3b).
The sensitivity of a conformational transition of a membrane protein to the changing pressure in the hydrocarbon area of the membrane can be rationalized in a model wherein the transition changes the shape of the protein molecule in a way that either relieves or increases the pressure. In the case of VDAC gating, one of the possibilities is that the protein surface that is in contact with the hydrocarbon area can be hourglass-shaped in a subset of the closed states at negative applied voltages but nearly cylindrical in the open state and the closed ones at the positive voltages. A crude estimate based on assuming a 0.1 nm difference in the outer radius of the channel (Fig. 3c) shows that substitution of PC for PE is expected to introduce a free energy contribution of about 5 kT per VDAC molecule or 3 kcal/mol.1 The actual free energy change found from the shift in the conformational equilibrium in experiments with PE and PC (Rostovtseva et al. 2006) was in the range of 1 to 2 kT per VDAC molecule, so that its explanation within the framework of the present model would require even smaller deviations from the shape of a cylinder.
We also demonstrated that VDAC is much more sensitive to the presence of CL than gramicidin channels (Rostovtseva et al. 2006). Interestingly, CL is found in high concentrations at the points of contact between the inner and outer mitochondrial membranes (Ardail et al. 1990; Simbeni et al. 1991), and VDAC is believed to be localized in these contact sites (Beutner et al. 1996; Crompton 1999). This suggests that CL affinity for VDAC might be physiologically relevant, namely that VDAC is more sensitive to the presence of CL than gramicidin channels because VDAC inserts into the CL-rich domains. Thus, specific lipid composition of the mitochondria outer membrane and/or of contact sites might influence MOM permeability by regulating VDAC gating.
VDAC regulation by tubulin
Mitochondria have long been known to localize within and move along the tubulin–microtubule network. In isolated mitochondria, respiration is characterized by an apparent Km for exogenous ADP that is about 10-fold lower than in permeabilized cells with oxidative metabolism (Appaix et al. 2003; Saks et al. 1995). In other words, in permeabilized cells with oxidative metabolism ANT is less accessible to cytosolic ADP than in isolated mitochondria. Saks and coauthors (Appaix et al. 2003) have found that mild trypsin treatment of permeabilized cardiomyocytes and cardiac fibers resulted in a decrease in apparent Km for ADP, or an increase of MOM permeability up to the level of the high MOM permeability of isolated mitochondria. They also observed that this processes is accompanied by disintegration of the mitochondrial network, which otherwise is very structured in cardiac cells, and by rearrangement of the microtubule network (Appaix et al. 2003). These observations suggested the existence of a protein factor(s) associated with the cytoskeleton, the so called “Factor-X” introduced by Saks and coauthors in mid-1990s, which controls MOM permeability in vivo (Saks et al. 1995).
Recently we identified this cytoskeleton protein as dimeric tubulin (Rostovtseva et al. 2008). By direct measurements we showed that nanomolar concentrations of mammalian tubulin induce highly voltage-sensitive reversible closure of VDAC reconstituted into planar phospholipid membranes. Analysis of single channel fluctuations in the presence of tubulin showed that already at nanomolar concentrations of this cytosolic protein the channel closure occurs at very low potentials (as low as 10 mV) compared with VDAC gating in control. The tubulin-VDAC interaction requires the presence of negatively charged C-terminal tails of tubulin. Tubulin with the proteolytically removed C-terminus does not induce VDAC closure. We suggested a model of tubulin–VDAC interaction, in which the tubulin C-terminus penetrates into the channel lumen, interacting with VDAC with high specificity and blocking channel conductance (Fig. 4). Experiments with isolated mitochondria strongly confirm these findings. The apparent Km for exogenous ADP increases ten times after addition of 1–10 μM of tubulin to isolated mitochondria from heart or brain (Monge et al. 2008). Thus, tubulin prevents ADP entry into mitochondria. Tubulin added to isolated mitochondria dramatically decreases the availability of ADP to ANT, restoring low permeability of MOM (high apparent Km for ADP) found in permeabilized cells. These results suggest a new general mechanism of regulation of MOM permeability under normal and apoptotic conditions.
Fig. 4.
A model of tubulin-induced VDAC permeation block. One of tubulin negatively charged C-terminal tails partially blocks the channel conductance by entering VDAC pore in its open state and binding to the positively charged channel walls. This is seen on the traces of current through a single channel (bottom inset on the right) as tubulin-induced fast flickering of channel conductance between open and one closed state. This process is voltage-dependent and can be described by the first-order reaction. Interpolated to zero applied voltage the reaction is characterized by an equilibrium binding constant of the order of 1 nM −1. Analysis of the reaction voltage dependence gives an effective charge of about five elementary charges
The high-affinity binding of tubulin to isolated mitochondria was known long ago (Bernier-Valentin and Rousset 1982). Recently, a specific association of VDAC with tubulin was shown by co-immunoprecipitation experiments (Carre et al. 2002). Now we demonstrate the mechanism of VDAC regulation by tubulin in vitro by reconstituting the channel in planar lipid membranes in the presence of dimeric tubulin and relate it to the action of this protein in vivo. Thus we think we have identified a natural cytoplasmic VDAC regulator—the potent but evasive “Factor-X”. By this type of control, tubulin may selectively regulate metabolic fluxes between mitochondria and the cytoplasm (Saks et al. 2006, 2007). Our results not only reveal a novel mechanism of mitochondria respiration regulation, but also discover a new functional role for the cytoskeleton protein, dimeric tubulin.
Another long standing question was how could VDAC voltage gating observed in planar lipid bilayers at relatively high potentials (>30 mV) be relevant to the situation in vivo and in isolated mitochondria, where potential difference across MOM should be very low due to the presence of VDAC channels themselves? This potential is most likely the Donan potential stemming from the high concentration of charged polyions (like cytochrome c) in the intermembrane space. The direct measurement of MOM potential involves serious experimental problems. Indirectly, pH difference between the intermembrane space and the cytosol gave estimations of transmembrane potential varying from 15–20 mV (Cortese et al. 1992) to 43 mV (Porcelli et al. 2005). However, the latter seems to be too high. A theoretical analysis of Donan potential across MOM estimated it as ~12 mV (Lemeshko 2006), which seems more realistic. As it follows from our findings, cytosolic concentrations of tubulin could tremendously increase VDAC sensitivity to voltage and induce significant VDAC closure at transmembrane potentials smaller than 10 mV.
Mechanisms of VDAC closure
There is a quite long list of known stimuli and compounds, physiologically relevant and synthetic, which stimulate VDAC transition from the open to closed states (Rostovtseva et al. 2005). In the present review we focus on the physiologically relevant cytosolic compounds exclusively (Table 1), but in order to get an insight into the mechanisms of VDAC closure, we outline the effects of multiple stimuli. The mechanism of VDAC gating under the applied voltage has been extensively studied by Colombini and coauthors (Colombini 1989; Doring and Colombini 1985; Zizi et al. 1995, 1998). Some cytosolic compounds, like NADH, enhance gating, or decrease it by favoring VDAC open state, like Bcl-xL (Table 1). The enhanced gating, or what we call in Table 1 “favoring closed states,” is manifested in narrowing of the bell-shaped G/V plot (Fig. 3a) and in shifting of the midpoint voltage, Vo (the voltage at which half channels are open and half closed), to smaller values. In some cases this process is accompanied by the changes in another parameter of voltage gating, the effective gating charge n, characterizing the steepness of G/V dependence. The mechanism of VDAC closure induced by tubulin or oligonucleotide G3139 (Tan et al. 2007a, b) is rather different from the above; it is a permeation block, when oligonucleotide or the C-terminus tail of tubulin penetrates into the channel lumen and partially obstructs it. Both gating and tubulin-induced VDAC closure are voltage dependent, but the mechanisms are different, with the voltage dependence of tubulin block originating from the interaction of the negatively charged tubulin tail with the applied voltage. The third mechanism of VDAC closure is a decrease of the average closed state conductance, Gmin, at one or both polarities of applied voltage. This mechanism was found in the presence of non-lamellar lipids, PE or CL (Rostovtseva et al. 2006), and actin (Xu et al. 2001) which, similarly to tubulin, is acidic protein but lacking C-terminal tail (Table 1). All three mechanisms describe a reversible VDAC closure. Finally, there is an irreversible VDAC closure, induced by König polyanion (Colombini et al. 1987) or tBid (Table 1). Most likely the latter is related to the first mechanism of voltage-induced gating. Polyanion traps channel in the closed conformation by interacting with the positive charges of the voltage-sensor domain, which are exposed on the membrane surface upon gating (Fig. 1).
Table 1.
Cytosolic and membrane regulators of VDAC
| Agent | Mechanism of action | References |
|---|---|---|
| Bcl-xL | Favors open state | Li et al. (2001), Vander Heiden et al. (2001) |
| NADH | Favors closed states | Lee et al. (1994, 1996) |
| tBid | Induces irreversible closure | Rostovtseva et al. (2004) |
| Non-lamellar lipids, PE and cardiolipin | Favors closed states of smaller conductance | Rostovtseva et al. (2006) |
| Tubulin | Induces permeation block | Rostovtseva et al. (2008) |
| Actin | Favors closed states of smaller conductance | Xu et al. (2001), Rostovtseva, unpublished data |
| Hexokinase-I | Induces closurea | Azoulay-Zohar et al. (2004) |
Data obtained in vitro, in experiments with reconstituted channels
The detailed mechanism is not investigated.
In addition to the regulation issues briefly reviewed above, understanding mechanisms of the newly discovered tubulin interaction with VDAC is important for identification of potential targets for VDAC and, correspondingly, for mitochondria binding. According to our findings tubulin acts on the open channel state and interacts with binding sites inside the channel lumen. In this process both electrostatics and specific binding play roles, so that the strength of interactions and degree of channel closure should depend on the charge as well as on the length of the tubulin tail.
Conclusions
How could VDAC closure promote the apoptotic signals? Certainly, the answer will be found in future research. Meanwhile, there are only tentative models available. One of the models proposes participation of VDAC in growth factors and Akt-mediated antiapoptotic action (Robey and Hay 2006). Akt regulates hexokinase attachment to mitochondria and this association may prevent the cytochrome c release. It is presumed that hexokinase directly binds to mitochondria through VDAC and, according to the model proposed by Majewski and coauthors (Majewski et al. 2004), supports VDAC open state. Importantly, depending on the state of VDAC, open or closed, hexokinase could be either attached to or detached from VDAC, and consequently could modulate anti- or pro-apoptotic signals (see review Robey and Hay 2006). Tubulin, as a newly discovered and potentially active player, adds another level of complexity to the VDAC regulation of mitochondrial signals, suggesting a possible competition between tubulin and hexokinase for VDAC binding. Interestingly, a well-known anti-tumor drug, paclitaxel, which inhibits the dynamics of microtubules and subsequently induces apoptosis, was found to induce cytochrome c release from mitochondria in intact human neuroblastoma cells and isolated mitochondria (Andre et al. 2002). It is likely, however, that paclitaxel and other microtubule-active anti-tumor drugs might modify interactions of microtubules and/or tubulin with VDAC and thus deliver a signal for mitochondria permeabilization and apoptosis induction (Esteve et al. 2007).
Acknowledgments
The authors are grateful to Adrian Parsegian for fruitful discussions and reading parts of the manuscript. This study was supported by the Intramural Research Program of the NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
The estimate is based on the assumption that the outer radius r of the channel is about 1.5 nm and that the transition from cylindrical to the hypothetical concave shape at negative potentials can be represented by Δr≈0.1 nm (Fig. 3b,c). Then the extra volume that becomes available for hydrocarbon chains upon this transition is ΔV=2πrhΔr, where h is the height of the protein surface exposed to the pressure of the hydrophobic region. The free energy change accompanying this transition is ΔF = ΔVΔP, where ΔP is the increase in the lateral pressure in the hydrocarbon area upon transition from PC to PE, which can be estimated as ΔP≈100 atm or 107 Pa (Bezrukov 2000; Gullingsrud and Schulten 2004). Choosing h≈2 nm, we obtain ΔV≈ 2 nm3 and ΔF≈3 kcal/mol.
References
- Andre N, Carre M, Brasseur G, Pourroy B, Kovacic H, Briand C, Braguer D. FEBS Lett. 2002;532:256–260. doi: 10.1016/s0014-5793(02)03691-8. [DOI] [PubMed] [Google Scholar]
- Appaix F, Kuznetsov AV, Usson Y, Kay L, Andrienko T, Olivares J, Kaambre T, Sikk P, Margreiter R, Saks V. Exp Physiol. 2003;88:175–190. doi: 10.1113/eph8802511. [DOI] [PubMed] [Google Scholar]
- Ardail D, Privat JP, Egretcharlier M, Levrat C, Lerme F, Louisot P. J Biol Chem. 1990;265:18797–18802. [PubMed] [Google Scholar]
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. Biochem J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. J Biol Chem. 2002;277:49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blalchy-Dyson E, Di Lisa F, Forte MA. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Bernier-Valentin F, Rousset B. J Biol Chem. 1982;257:7092–7099. [PubMed] [Google Scholar]
- Beutner G, Ruck A, Riede B, Welte W, Brdiczka D. FEBS Lett. 1996;396:189–195. doi: 10.1016/0014-5793(96)01092-7. [DOI] [PubMed] [Google Scholar]
- Bezrukov SM. Curr Opin Colloid Interface Sci. 2000;5:237–243. [Google Scholar]
- Bezrukov SM, Rand RP, Vodyanoy I, Parsegian VA. Faraday Discuss. 1998;111:173–183. doi: 10.1039/a806579i. [DOI] [PubMed] [Google Scholar]
- Brink-van der Laan EV, Killian JA, de Kruijff B. Biochim Biophys Acta. 2004;1666:275–288. doi: 10.1016/j.bbamem.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Cantor RS. Biophys J. 1999;76:2625–2639. doi: 10.1016/S0006-3495(99)77415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D. J Biol Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- Colombini M. J Membr Biol. 1980;53:79–84. [Google Scholar]
- Colombini M. J Membr Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- Colombini M. Mol Cell Biochem. 2004;256:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- Colombini M, Yeung CL, Tung J, König T. Biochim Biophys Acta. 1987;905:279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- Colombini M, Blachly-Dyson E, Forte M. In: Ion channels. Narahashi T, editor. Vol. 4. Plenum; New York: 1996. pp. 169–202. [DOI] [PubMed] [Google Scholar]
- Cortese JD, Voglino AL, Hackenbrock CR. Biochim Biophys Acta. 1992;1100:189–197. doi: 10.1016/0005-2728(92)90081-c. [DOI] [PubMed] [Google Scholar]
- Crompton M. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Doring C, Colombini M. J Membr Biol. 1985;83:81–86. doi: 10.1007/BF01868740. [DOI] [PubMed] [Google Scholar]
- Epand RF, Martinou JC, Fornallaz-Mulhauser M, Hughes DW, Epand RM. J Biol Chem. 2002;277:32632–32639. doi: 10.1074/jbc.M202396200. [DOI] [PubMed] [Google Scholar]
- Esteve MA, Carre M, Braguer D. Curr Cancer Drug Targets. 2007;7:713–729. doi: 10.2174/156800907783220480. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kroemer G. Nat Cell Biol. 2007;9:487–489. doi: 10.1038/ncb0507-487. [DOI] [PubMed] [Google Scholar]
- Goforth RL, Chi AK, Greathouse DV, Providence LL, Koeppe RE, Andersen OS. J Gen Physiol. 2003;121:477–493. doi: 10.1085/jgp.200308797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner SM. Proc Natl Acad Sci USA. 1985;82:3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullingsrud J, Schulten K. Biophys J. 2004;86:3496–3509. doi: 10.1529/biophysj.103.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge T, Colombini M. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- Keller SL, Bezrukov SM, Gruner SM, Tate MW, Vodyanoy I, Parsegian VA. Biophys J. 1993;65:23–27. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasilnikov OV, Sabirov RZ, Ternovsky VI, Merzliak PG, Muratkhodjaev JN. FEMS Microbiol Immunol. 1992;105:93–100. doi: 10.1111/j.1574-6968.1992.tb05891.x. [DOI] [PubMed] [Google Scholar]
- Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Biochim Biophys Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Lai JC, Tan WZ, Benimetskaya L, Miller P, Colombini M, Stein CA. Proc Natl Acad Sci USA. 2006;103:7494–7499. doi: 10.1073/pnas.0602217103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Zizi M, Colombini M. J Biol Chemi. 1994;269:30974–30980. [PubMed] [Google Scholar]
- Lee AC, Xu XF, Colombini M. J Biol Chemi. 1996;271:26724–26731. doi: 10.1074/jbc.271.43.26724. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Holmuhamedov E. Biochim Biophys Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Lemeshko VV. Eur Biophys J. 2006;36:57–66. doi: 10.1007/s00249-006-0101-7. [DOI] [PubMed] [Google Scholar]
- Li XX, Vander Heiden MG, Thompson CB, Colombini M. Biophysi J. 2001;80:239A–239A. [Google Scholar]
- Lutter M, Fang M, Luo X, Nishijima M, Xie XS, Wang XD. Nat Cell Biol. 2000;2:754–756. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Mannella CA, Guo XW, Cognon B. FEBS Lett. 1989;253:231–234. [Google Scholar]
- Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P. J Biol Chem. 2001;276:18361–18374. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Monge C, Beraud N, Rostovtseva T, Sackett D, Vendelin M, Saks V. Biophys J. 2008;94:1562. doi: 10.1007/s11010-008-9865-7. [DOI] [PubMed] [Google Scholar]
- Pavlov EV, Priault M, Pietkiewicz D, Cheng EHY, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA, Kinnally KW. J Cell Biol. 2001;155:725–731. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Blachly-Dyson E, Forte M, Colombini M. Biophys J. 1992;62:123–135. doi: 10.1016/S0006-3495(92)81799-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcic P, Forte M. Biochem J. 2003;374:393–402. doi: 10.1042/BJ20030690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, Rugolo M. Biochem Biophys Res Commun. 2005;326:799–804. doi: 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- Priault M, Chaudhuri B, Clow A, Camougrand N, Manon S. Eur J Biochem. 1999;260:684–691. doi: 10.1046/j.1432-1327.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- Robey RB, Hay N. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T, Colombini M. J Biol Chem. 1996;271:28006–28008. doi: 10.1074/jbc.271.45.28006. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T, Colombini M. Biophys J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtseva TK, Komarov A, Bezrukov SM, Colombini M. Biophys J. 2002a;82:193–205. doi: 10.1016/S0006-3495(02)75386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtseva TK, Komarov A, Bezrukov SM, Colombini M. J Membr Biol. 2002b;187:147–156. doi: 10.1007/s00232-001-0159-1. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Antonsson B, Suzuki M, Youle RJ, Colombini M, Bezrukov SM. J Biol Chem. 2004;279:13575–13583. doi: 10.1074/jbc.M310593200. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Tan WZ, Colombini M. J Bioenerg Biomembranes. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Kazemi N, Weinrich M, Bezrukov SM. J Biol Chem. 2006;281:37496–37506. doi: 10.1074/jbc.M602548200. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Sackett DL, Sheldon K, Monge C, Saks V, Bezrukov SM. Biophys J. 2008;94:1760. [Google Scholar]
- Saks VA, Kuznetsov AV, Khuchua ZA, Vasilyeva EV, Belikova JO, Kesvatera T, Tiivel T. J Mol Cell Cardiol. 1995;27:625–645. doi: 10.1016/s0022-2828(08)80056-9. [DOI] [PubMed] [Google Scholar]
- Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. J Physiol-London. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks V, Vendelin M, Aliev MK, Kekelidze T, Engelbrecht J. In: Brain energetics: integration of molecular and cellular processes. Gibson GF, Dienel G, editors. Springer; Berlin: 2007. pp. 815–860. [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Ide T, Yanagida T, Tsujimoto Y. J Biol Chem. 2000a;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Shinohara Y, Tsujimoto Y. Oncogene. 2000b;19:4309–4318. doi: 10.1038/sj.onc.1203788. [DOI] [PubMed] [Google Scholar]
- Simbeni R, Pon L, Zinser E, Paltauf F, Daum G. J Biol Chem. 1991;266:10047–10049. [PubMed] [Google Scholar]
- Song JM, Midson C, Blachly-Dyson E, Forte M, Colombini M. Biophys J. 1998a;74:2926–2944. doi: 10.1016/S0006-3495(98)78000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Midson C, Blachly-Dyson E, Forte M, Colombini M. J Biol Chem. 1998b;273:24406–24413. doi: 10.1074/jbc.273.38.24406. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- Tan WZ, Colombini M. Biochim Biophys Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WZ, Lai JC, Miller P, Stein CA, Colombini M. Am J Physiol Cell Physiol. 2007a;292:C1388–C1397. doi: 10.1152/ajpcell.00490.2006. [DOI] [PubMed] [Google Scholar]
- Tan WZ, Loke YH, Stein CA, Miller P, Colombini M. Biophys J. 2007b;93:1184–1191. doi: 10.1529/biophysj.107.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L, Blachly-Dyson E, Colombini M, Forte M. Proc Natl Acad Sci USA. 1993;90:5446–5449. doi: 10.1073/pnas.90.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Proc Natl Acad Sci USA. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- Xu X, Forbes JG, Colombini M. J Membr Biol. 2001;180:73–81. doi: 10.1007/s002320010060. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Parsegian VA. Nature. 1986;323:36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]
- Zizi M, Thomas L, Blachly-Dyson E, Forte M, Colombini M. J Membr Biol. 1995;144:121–129. doi: 10.1007/BF00232798. [DOI] [PubMed] [Google Scholar]
- Zizi M, Byrd C, Boxus R, Colombini M. Biophys J. 1998;75:704–713. doi: 10.1016/S0006-3495(98)77560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti M, Szabo D, De Marchi U. Biochim Biophys Acta. 2005;1706:40–52. doi: 10.1016/j.bbabio.2004.10.006. [DOI] [PubMed] [Google Scholar]