Abstract
Study Design
Investigation of the effect of static compression and anisotropy on the apparent diffusivity of glucose in bovine annulus fibrosus.
Objectives
To determine the apparent glucose diffusivity in two directions (axial and radial) of bovine annulus fibrosus under three levels of compressive strain (0%, 10%, 20%).
Summary of Background Data
Knowledge of diffusivity of small molecules is important for understanding nutritional supply in IVD and the mechanisms of disc degeneration. However, little is known regarding the strain-dependent and anisotropic behavior of glucose diffusivity in IVD.
Methods
Apparent glucose diffusivity measurements were performed on 10 axial and 10 radial AF specimens from bovine coccygeal discs. The dependence of diffusivity on compression was determined using three levels of strain (0%, 10% and 20%).
Results
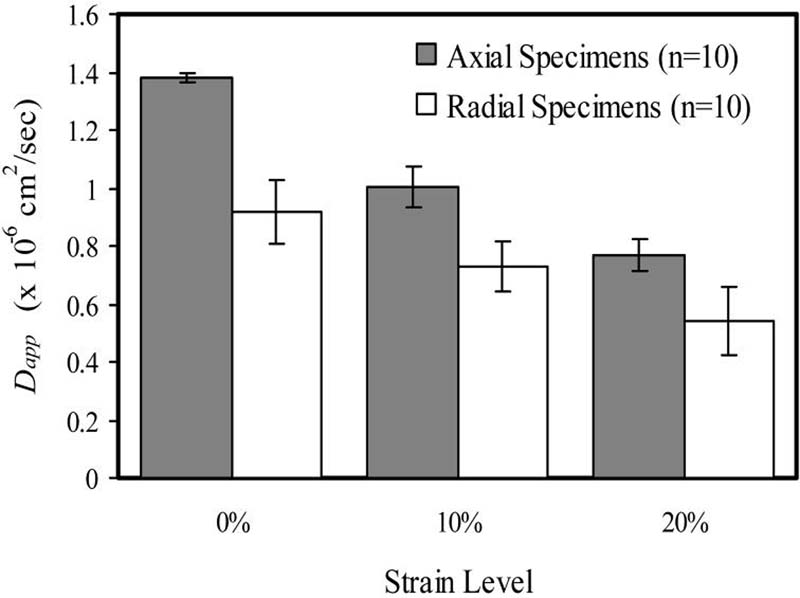
The apparent glucose diffusivity (mean±SD) of the bovine AF in the axial direction was 1.38 ± 0.015 × 10−6 cm2/sec (n=10) at 0%, 1.00 ± 0.070 × 10−6 cm2/sec (n=10) at 10%, and 7.65 ± 0.552 × 10−7 cm2/sec (n=10) at 20% compression. For radial specimens, the apparent glucose diffusivity was determined to be 9.17 ± 1.12 × 10−7 cm2/sec (n=10), 7.29 ± 0.863 × 10−7 cm2/sec (n=10), and 5.43 ± 1.16 × 10−7 cm2/sec (n=10) for 0%, 10% and 20% compression, respectively. A significant decrease in diffusivity with increasing strain was found for both axial and radial specimens (ANOVA, p<0.05). Diffusion in the radial direction was determined to be significantly less than that in the axial direction (ANOVA, p<0.05). A significant interaction was found between level of strain and direction of diffusion (ANOVA, p<0.05).
Conclusions
Diffusion of glucose in bovine AF is dependent on strain and direction of diffusion.
INTRODUCTION
Low back pain is a major socio-economic concern in the United States.1 Each year, the prevalence of low back pain ranges from 15 to 45% of the population, while more than seventy percent of all people experience symptoms at some point in their lifetime. Although the exact cause of low back pain is unclear, degenerative changes in the intervertebral discs (IVD) of the spine have been implicated as a possible primary etiologic factor.2-5 Poor nutritional supply to the disc is believed to be an important mechanism leading to the onset of disc degeneration.6-10
The IVD is the largest avascular structure in the human body. It consists of the nucleus pulposus (NP), the annulus fibrosus (AF) and the cartilaginous end-platen (CEP). The composition and structure of these tissues are distinctly different from each other,11;12 suggesting a unique role for each. In the present study, the transport properties of bovine AF are investigated. The AF surrounds the NP on its periphery. The normal AF consists of a series of concentric lamellae with a highly organized structure of collagen fiber bundles.13;14
Due to the avascular nature of the IVD, transport of fluids and solutes through its matrix plays an integral role in cellular nutrition because blood vessels are not present to carry these solutes. There are two possible pathways through which nutrient transport into the IVD may occur: axially through the cartilage endplate route or radially through the perianular route.6;8;15-23 It is generally believed that diffusion, the passive transport of solutes from a high to low concentration, is the main mechanism of transport for small solutes in avascular cartilaginous tissues.17;24;25 Determining the diffusion coefficient (i.e., diffusivity, which is a measure of the solute mobility) for small solutes (glucose, oxygen, small ions, etc.) is important in the study of transport mechanisms and pathways in IVD. The major factors governing diffusivity in cartilaginous tissues are solute size and pore size of the tissue.15;26
Few studies have been completed to investigate anisotropic transport in IVD tissues. Several have been done using imaging techniques as a method of determining the apparent diffusion coefficient of water in the intervertebral disc. A study by Hsu and Setton (1999) using diffusion tensor imaging techniques found that diffusion in the IVD is anisotropic (i.e., direction-dependent) and preferred directions of diffusion are arranged in discrete layers.27 Likewise, a later study by Chiu et al. (2001) using magnetic resonance imaging measurement of water diffusion found that significant differences in water diffusion were found by region, loading state, diffusion direction, and degenerative (Thompson) grade, again suggesting the anisotropic transport behavior in the IVD.28 A study by Jackson et al. (2006) showed the anisotropic behavior of electrical conductivity in bovine AF.29 In the same study, conductivity values were used to estimate values of ion diffusivity, and the same anisotropic trend was found for Na+ and Cl− ion diffusivities. However, to our knowledge, no study has been reported on the anisotropic behavior of glucose diffusivity in the IVD.15
The major role of the IVD is mechanical, offering load support capabilities. Under compressive stress, the IVD acts to transfer the load from one cartilaginous end plate to the other. Many studies can be found in the literature reporting on the effect of mechanical loading on water content, chemical composition and nutritional levels in IVD.7;9;30 Compressive loading conditions cause a decrease in disc water content and water diffusivity in IVD.31-33 Several studies have reported on the effect of compression on diffusion of water in IVD using MRI techniques. Chiu et al. (2001) found that the diffusion of water in IVD tissues increased with an increase in compressive load applied,28 while Drew et al. (2004) reported a decrease in the apparent diffusion coefficient in IVD with increasing loads.34 The difference in findings in these two studies most likely results from the difference in experimental techniques; Chiu et al. measured diffusion immediately after applying a step load to the IVD, whereas Drew et al. used longer loading times. The effect of compression on the diffusion of several molecules in cartilage has also been investigated, including water and Li+ ions,35 various sized dextran molecules,36;37 and Na+ ions.38 All studies demonstrated a decrease in diffusivity with increasing compression in bovine articular cartilage. However, to our knowledge, no previous study has investigated the effect of static compression on the diffusion of glucose in IVD tissue.
It was hypothesized that the diffusion of glucose in bovine AF is strain-dependent due to changes in water content caused by compression, and is anisotropic for non-degenerated AF due to its layered structure. The objective of this study was to test these hypotheses by measuring the apparent glucose diffusivity of bovine AF in two directions (axial and radial directions) under three levels of static compressive strain (0%, 10% and 20%).
THEORETICAL BACKGROUND
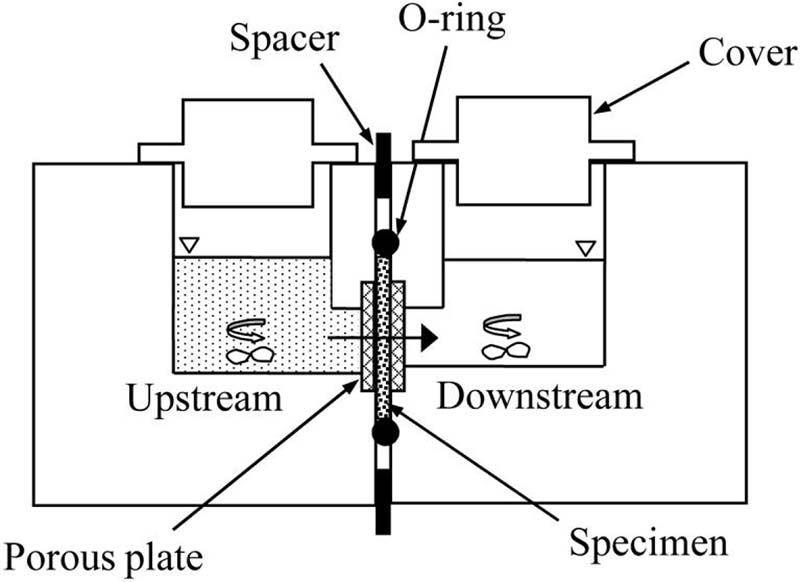
A one-dimensional steady-state diffusion experiment was used to directly yield values of apparent glucose diffusivity in bovine AF. Similar methods have been employed to determine solute diffusivities in cartilage39 and IVD tissues.6;15 A specimen of known thickness is clamped between two compartments of a diffusion chamber, Fig. 1. The solute is introduced into the upstream chamber. The solute then gradually diffuses across the specimen, into the downstream chamber. At some time intervals, the content of the downstream chamber is withdrawn and completely replaced with fresh solution. The solute concentration in the upstream chamber is assumed to be constant due to the high concentration of the solute and large volume of solution. The solute concentration in the downstream chamber is always close to zero due to solution replacement.
Figure 1.
The diffusive flux (J) within the tissue is governed by Fick's law:
| (1) |
where D is the intrinsic diffusion coefficient and C is the concentration of glucose within the tissue. At steady state, the diffusive flux (defined as the mass flow rate of solute, ΔQ, per unit area, A) can be evaluated by the difference in concentration of solute between the upstream and downstream chambers (Cup − Cdown):
| (2) |
where K is the partition coefficient, Dapp (=DK) is the apparent diffusion coefficient, and h is the thickness of tissue sample. The flux is also related to the concentration in the downstream chamber by conservation of mass:
| (3) |
where Vdown is the volume of solution in the downstream chamber. By setting Equation (2) equal to Equation (3), the following differential equation is obtained accordingly:
| (4) |
An expression for the apparent diffusion coefficient can thus be determined by integration over the interval [to, t]:
| (5) |
where Cdown(to) is the concentration of solute in the downstream chamber at time to (initial time) and Cdown(t) is that at time t. The concentration of solute in the upstream chamber is assumed to be constant in arriving at Equation (5). This assumption can be easily achieved experimentally. Equation (5) indicates that the apparent diffusion coefficient can be conveniently calculated from downstream concentrations at two different time points.
MATERIALS AND METHODS
Specimen Preparation
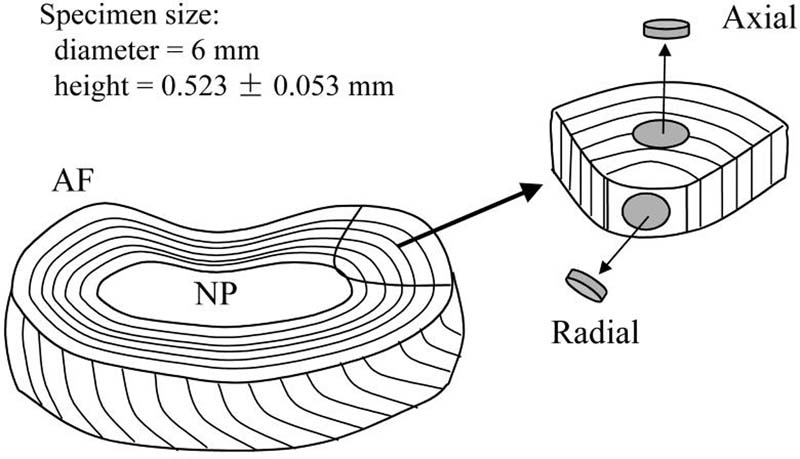
Four coccygeal discs were harvested from two fresh bovine tails (S2-3 and S3-4, ∼6 mo.) obtained from a local supermarket. Cylindrical specimens (6 mm diameter and ∼0.5 mm thickness) were prepared using a stainless steel corneal trephine (Biomedical Research Instruments, Inc., Silver Spring, MD) and sledge microtome (Model SM2400, Leica Instruments, Nussloch, Germany) with freezing stage (Model BFS-30, Physitemp Instruments Inc., Clifton, NJ). AF samples were excised in the axial and radial directions, Fig. 2. A total of two groups of specimens were tested: axial (n=10) and radial (n=10). A total of three tests, corresponding to three levels of compressive strain (0%, 10%, 20%), were performed on each specimen.
Figure 2.
Porosity Measurement
The water volume fraction or porosity of specimens under zero compression was determined using a buoyancy method published in literature.40-42 The weight of the specimens in air, Wwet, and the weight in the bathing solution, Wsol, was measured using the density determination kit of a Sartorius analytical balance (Model LA120S, Goettingen, Germany). In order to reduce the effects of tissue swelling in solution, the weight measurement of specimen in solution was taken in less than 15 seconds. This was done prior to diffusivity measurement (see below). Previous studies indicate swelling has negligible effects in this short duration.40-42 The difference between Wwet and Wsol, which is due to buoyancy force, was related to the specimen volume (V) and the mass density of solution (ρsol) by V = (Wwet − Wsol)/gρsol (g is the gravitational acceleration). After the diffusion measurements, the specimens were lyophilized and the dry weights, Wdry, were recorded. The water volume (Vw) of the specimen is equal to Vw = (Wwet − Wdry)/gρw, where ρw is the mass density of water. The volume fraction of water at 0% strain of the specimens was calculated by:40-42
| (6) |
Water content of a tissue can be related to deformation by:
| (7) |
where J is the ratio of the current tissue volume to the undeformed tissue volume, and is the volume fraction of solid at the undeformed configuration (where J = 1), which is related to the water content of the undeformed tissue by . Tissue dilation, or relative volume change, is related to deformation by e = J − 1. Volume fraction of water in deformed tissue (ϕw) can be estimated from the following relationship:
| (9) |
Diffusivity Measurement
A custom diffusion cell was designed to measure strain-dependent diffusion of glucose in IVD tissues, Fig. 1. The apparatus consists of two acrylic solution chambers divided by a specimen holder in the middle. The specimen is held between two rigid porous plates (hydrophilic polyethylene, 50-90 μm pore size, Small Parts, Inc., Miami Lakes, FL) to inhibit swelling, and sealed with an o-ring (Buna-N metric o-rings, Small Parts, Inc., Miami Lakes, FL). The compressive strain can be controlled by changing of a spacer placed between the two chamber halves.
The AF specimen was initially confined to 0% compressive strain. The upstream chamber was filled with 400 μL of glucose solution (concentration of 20 mg/ml glucose (D(+)-Glucose Monohydrate, Sigma-Aldrich Co., St. Louis, MO) in phosphate buffered saline (PBS) (Sigma-Aldrich Co., St. Louis, MO) solution). The downstream chamber was filled with 200 μL of PBS solution. The contents of both chambers were stirred continuously at low speed using micro-magnetic stirring bars and a magnetic stirring plate. Continuous stirring aided in maintaining constant solute distribution within the solution, and also minimized stagnant layer formation at the tissue boundary.
After glucose was allowed to diffuse through the tissue specimen for a 15-minute time interval, the contents of the downstream chamber were emptied for glucose concentration measurement. Glucose concentrations were determined using a spectrophotometer (Model SmartSpec™ Plus Spectrophotometer, Bio-Rad Laboratories, Inc., Hercules, CA) and glucose assay reagent (G3293, Sigma-Aldrich Co., St. Louis, MO). The glucose assay reagent was capable of measuring glucose concentrations in liquid solutions in the range of 0.05 mg/ml to 5 mg/ml by measuring the spectrophotometric absorbance at 340 nm. A standard curve was constructed prior to each experiment by determining the absorbance of several glucose standard solutions with concentrations encompassing the range to be measured. Experimental glucose concentrations could be calculated directly from absorbance measurements using the relationship derived from the standard curve.
Following each 15-minute time interval, the downstream chamber was rinsed with PBS solution and dried with a cotton swab to ensure no glucose remained in the chamber. It was then filled with 200 μL of fresh PBS solution, while the upstream chamber was filled with 400 μL of fresh glucose solution. The experiment was repeated until the same concentration (within 5%) was obtained for 2-3 consecutive readings, signifying that steady state had been reached. An average of 2 hours (8 intervals of 15 minutes each) was necessary to reach steady state. Once steady state was achieved for specimens at 0% compression, the experiment was repeated for 10% and 20% compressive strains.
Apparent glucose diffusion coefficients were calculated using Equation (5). Due to solution replacement at the start of each 15-minute interval, the value of Cdown(to) is always equal to zero in Equation (5); the value of Cdown(t) is the averaged value of the 2-3 consecutive readings of downstream concentration at steady state. The cross-sectional area, A, of the specimen was calculated based on the area of the tissue through which diffusive flux occurs. This area was calculated as 50% of the area of the porous plates confining the specimen, as the porous material has a 50% open area.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) images of two bovine coccygeal intervertebral discs were obtained in order to relate the collagen fiber structure of AF to the diffusion coefficient data. Axial and radial slices of anterior and posterior regions of AF were fixed with a 2% gluteraldehyde (Electron Microscopy Sciences, Hatfield, PA) in phosphate buffer solution (Sigma-Aldrich Co., St. Louis, MO). They were then dehydrated in a graded series of ethanol (20, 40, 60, 80, 100%) and dried by immersion in hexamethyldisilizane (Electron Microscopy Sciences, Hatfield, Pa, USA).43 Lastly, the samples were sputter-coated with Pd (Sputter Coater 108auto, Cressington, Watford, UK). High-resolution SEM images were obtained using an Environmental Scanning Electron Microscope (XL30 ESEM-FEG, FEI Company, Hillsborg, OR).
Statistical Analysis
A total of 10 specimens were tested for each six groups. There were two independent variables: level of compression and direction of diffusion. Two-way ANOVA analysis of variance (with repeated measures on compression factor) was performed using Excel Spreadsheet software (Microsoft Corp., Seattle, WA) to determine the statistical significance of the differences between the diffusion coefficients found for each of these groups. The significance level was set at p<0.05. All data are given in mean ± standard deviation.
RESULTS
The results for apparent glucose diffusion coefficients can be found in Fig. 3. The apparent glucose diffusivity (mean ± SD) of bovine AF in the axial direction was found to be 1.38 ± 0.015 × 10−6 cm2/sec (n=10) at 0% compression, 1.00 ± 0.070 × 10−6 cm2/sec (n=10) at 10% compression, and 7.65 ± 0.552 × 10−7 cm2/sec (n=10) at 20% compression. For radial specimens, the apparent glucose diffusivity was determined to be 9.17 ± 1.12 × 10−7 cm2/sec (n=10), 7.29 ± 0.863 × 10−7 cm2/sec (n=10), and 5.43 ± 1.16 × 10−7 cm2/sec (n=10) for 0%, 10% and 20% compression, respectively. Tests were carried out at room temperature (23.13 ± 0.79 °C). The mean height and water content of the specimens under zero compression conditions were 0.523 ± 0.053 mm and 0.757 ± 0.014, respectively. There was a significant decrease in apparent glucose diffusivity with increasing compressive strain for both axial and radial specimens (ANOVA, p<0.05, Fig. 3). The value for the apparent glucose diffusion coefficient in the radial direction was significantly lower than the value in the axial direction (ANOVA, p<0.05, Fig. 3). A significant interaction was also found to exist between level of strain and direction of diffusion (ANOVA, p<0.05).
Figure 3.
DISCUSSION
The primary objective of this study was to investigate the effect of both strain and anisotropy on the apparent diffusivity of glucose in bovine AF tissue. The results for the average apparent glucose diffusivity in the axial and radial directions at 0% strain in bovine AF at 23°C (1.15 × 10−6 cm2/sec) were comparable with the averaged value in human AF in the axial and radial directions (1.20 × 10−6 cm2/sec, calculated on the basis of an averaged value of 1.7 × 10−6 cm2/sec obtained at 37°C).15
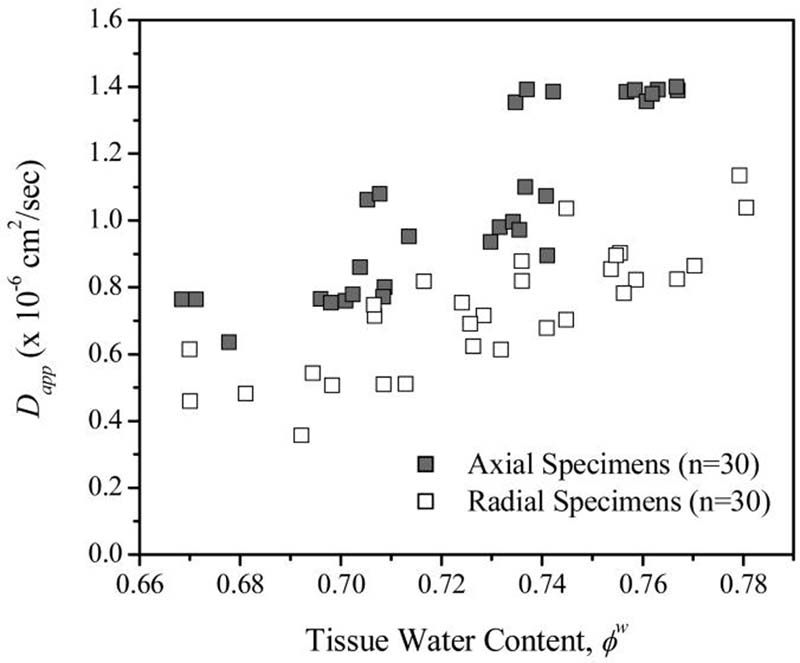
The experimental findings of this investigation show that the apparent glucose diffusivity in bovine annulus fibrosus is significantly (p<0.05) affected by the level of compression applied to the tissue. To our knowledge, this is the first data reporting this effect of compression on diffusion of glucose in IVD tissues. The decrease in diffusivity with increasing strain was anticipated due to the observed changes in water content and water diffusion with compression in previous studies.7;9;30-33 Diffusion of solutes in IVD tissues is highly dependent upon tissue porosity. Therefore, a decrease in tissue water content would likely be accompanied by a decrease in solute diffusivity, as seen in Fig. 4. When the tissue is compressed, water is exuded, resulting in a decrease in water content. According to Equation (8), for 10% compression, the water content is decreased by approximately 4%, and by 8% for 20% compression. This is in agreement with the previous study by Drew et al. (2004) showing a decrease in water diffusivity with increasing strain.34 This is also in agreement with previous studies reporting decreasing diffusivity of solutes with increasing static compressive strain in bovine articular cartilage.35-38
Figure 4.
The findings of this investigation also suggest that the diffusion of glucose in bovine annulus fibrosus is anisotropic, i.e., direction-dependent. It was found that the apparent glucose diffusivity in the radial direction was significantly (p<0.05) less than that in the axial direction at each level of strain. To our knowledge, this is the first data reporting the anisotropic behavior of glucose diffusion in IVD tissues. This anisotropic behavior was anticipated based on the organization of collagen fibers in AF tissue. In order to correlate the structure of the AF with the anisotropic trend for diffusion, scanning electron microscopy (SEM) images were obtained (Fig. 5). Images for radial specimens are shown in Fig. 5(a) and 5(b). These images show the structure of collagen fiber bundles but are absent of microtubules.44 In contrast, the images of axial specimens (Fig. 5(c) and 5(d)) show distinct microtubules that run parallel to the collagen fiber bundles. These microtubules do not appear to be contiguous between adjacent lamellae. A previous study by Iatridis and ap Gwynn (2004) presented SEM images that showed a similar microtubule structure in the AF of rat tail IVD.44 If it is assumed that these microtubules do, in fact, provide a favored pathway for diffusion, their presence would explain the anisotropic transport behavior of glucose in AF; in the axial direction, solutes would preferentially diffuse through the microtubules, whereas this would not be favorable in the radial direction given that the tubules are not contiguous.
Figure 5.
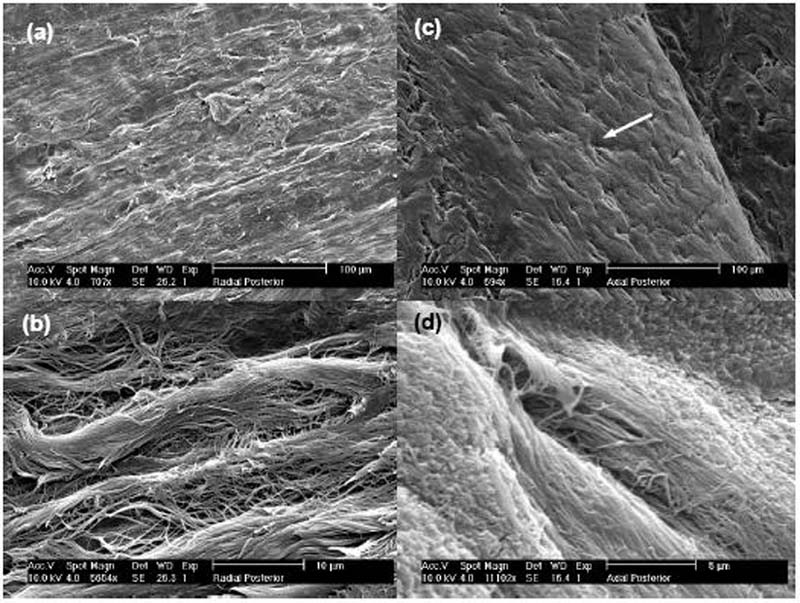
The anisotropic trend found in this study is similar to trends reported in previous studies. For uncompressed tissue, the value of the apparent diffusivity in the radial direction was 66% of that in the axial direction. This is similar to findings for electrical conductivity of bovine annulus fibrosus. The electrical conductivity of radial bovine AF was found to be 61% of that in the axial direction. Na+ and Cl− diffusivities estimated from conductivity data also showed this trend.29 Furthermore, this is similar to findings of our study using fluorescence recovery after photobleaching (FRAP) to determine diffusion of fluorescein in bovine AF, which showed diffusivity in the radial direction was 65% that in the axial direction in the anterior region of the AF and 75% in the posterior AF.45
Results of this investigation suggest that there exists an interaction between compression and direction in bovine annulus fibrosus. That is, the tissue specimen orientation has an influence on how compression affects the diffusion of glucose within the tissue. Two-way ANOVA analysis of variance showed a significant (p<0.05) interaction between direction and compression. While diffusivity decreased with compression in both the axial and radial directions, there was a steeper decrease for diffusion in the axial direction with increasing compression than for that in the radial direction. This interaction may again be related to the microtubule structure viewed in SEM images (Fig. 5). Under the assumption that these tubules provide a preferred route for diffusion in the axial direction, the more marked decrease in diffusivity with increasing compression for axial specimens is likely a result of decreased size of the microtubules resulting from tissue compression. On the other hand, diffusion in the radial direction would not be as significantly effected by compression given that no favored pathway exists for radial diffusion.
The main limitation of this study is the measurement of apparent diffusivity values rather than intrinsic diffusion coefficients. In order to determine the intrinsic diffusion coefficient, it is necessary to know the value of the partition coefficient of glucose in bovine AF, as apparent diffusivity is related to intrinsic diffusivity by Dapp = KD, where K is the partition coefficient. Knowledge of the intrinsic diffusion coefficient is more useful as it can be applied to theoretical modeling of IVD tissues. Studies of partitioning of solutes in cartilage show a dependence of the partition coefficient on level of compression.37;46 Further experimentation is therefore necessary to determine the strain-dependent partition coefficient of glucose in IVD.
The use of porous plates was necessary in order to confine and compress the tissue as desired. However, the presence of these plates may have caused a significant stagnant layer to form between the tissue and the solution in both chambers, resulting in error in diffusion coefficient calculations. Continuous stirring of the solution is known to minimize stagnant layer formation. In order to estimate the effect of the plates with continuous stirring, a study was done using porcine articular cartilage and the experimental setup with and without porous plates. In this study, it was determined that the apparent glucose diffusivity in cartilage with porous plates was 7% less than that without porous plates (data not shown). This error is likely due to stagnant layer formation at the tissue boundary. However, it was necessary to confine the IVD sample due to problems with tissue swelling, and also because of the need to compress the sample in the chamber.
It has been found that the variation in composition, swelling pressure, and synthesis rates across bovine coccygeal IVD are similar to that of human lumbar IVD, making them suitable for use.47 Additionally, since the water content of human IVD tissues are close to that of bovine IVD specimens in the present study, the results are expected to be similar, provided the two have similar structures. Further investigations on human IVD tissues will be performed in the future.
In summary, the effects of static compression and anisotropy on the diffusion of glucose in bovine annulus fibrosus were investigated. A significant variation was found for glucose diffusivity in the axial and radial directions. This anisotropic trend is consistent with anisotropic water diffusion27;28 and electrical conductivity and ion diffusivity29 reported in the literature. A significant decrease in glucose diffusivity with increasing static compressive strain was also found, which is consistent with a previous study on strain-dependent water diffusion in IVD.34 This study provides important insight into solute transport and diffusion in IVD tissue and aids in the understanding of nutrition in avascular cartilaginous tissues.
Acknowledgments
This project was supported by a grant from the NIH (AR50609). The authors also wish to thank Mr. Larry Hazbun and Mr. Andre Castillo for their assistance in apparatus machining.
Footnotes
- Diffusion of glucose in bovine AF is strain-dependent
- Diffusion of glucose in bovine AF is anisotropic
- Results are useful for understanding the transport behavior in IVD tissues
REFERENCES
- 1.NIH Research on low back pain and common spinal disorders. NIH Guide. 1997:26. [Google Scholar]
- 2.Eyre DR, Benya P, Buckwalter J, et al. Intervertebral disk: Basic science perspectives. In: Frymoyer JW, Gordon SL, editors. New Perspectives on Low Back Pain. American Academy of Orthopaedic Surgeons; Park Ridge, IL: 1989. pp. 147–207. [Google Scholar]
- 3.Kelsey JL, Mundt DF, Golden AL. Epidemiology of low back pain. In: Malcolm JIV, editor. The Lumbar Spine and Back Pain. 4th ed. Churchill Livingstone; New York: 1992. pp. 537–49. [Google Scholar]
- 4.White AA. Biomechanics of lumbar spine and sacroiliac articulation: relevance to idiopathic low back pain. In: White AA, Gordon SL, editors. Symposium on Idiopathic Low Back Pain. CV Mosby Co.; St. Louis: 1981. pp. 296–322. [Google Scholar]
- 5.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Nachemson A, Lewin T, Maroudas A, et al. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970;41:589–607. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- 7.Holm S, Nachemson A. Nutritional changes in the canine intervertebral disc after spinal fusion. Clin.Orthop. 1982:243–58. [PubMed] [Google Scholar]
- 8.Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–9. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Bibby SR, Fairbank JC, Urban MR, et al. Cell viability in scoliotic discs in relation to disc deformity and nutrient levels. Spine. 2002;27:2220–8. doi: 10.1097/00007632-200210150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Urban JP. The role of the physicochemical environment in determining disc cell behaviour. Biochem.Soc Trans. 2001;30:858–64. doi: 10.1042/bst0300858. [DOI] [PubMed] [Google Scholar]
- 11.Lundon K, Bolton K. Structure and function of the lumbar intervertebral disk in health, aging, and pathologic conditions. J Orthop Sports Phys.Ther. 2001;31:291–303. doi: 10.2519/jospt.2001.31.6.291. [DOI] [PubMed] [Google Scholar]
- 12.Guiot BH, Fessler RG. Molecular biology of degenerative disc disease. Neurosurgery. 2000;47:1034–40. doi: 10.1097/00006123-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Hickey DS, Hukins DWL. Relation between the structure of the anulus fibrosus and the function and failure of the intervertebral disc. Spine. 1980;5:106–16. doi: 10.1097/00007632-198003000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402–10. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 16.Urban JPG, Holms S, Maroudas A, et al. Nutrition of the intervertebral disc: An in vivo study of solute transport. Clin Orthop. 1977;129:101–14. [PubMed] [Google Scholar]
- 17.Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: as in vivo study. Biorheology. 1978;15:203–21. doi: 10.3233/bir-1978-153-409. [DOI] [PubMed] [Google Scholar]
- 18.Ogata K, Whiteside LA. 1980 Volvo award winner in basic science. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine. 1981;6:211–6. [PubMed] [Google Scholar]
- 19.Holm S, Maroudas A, Urban JP, et al. Nutrition of the intervertebral disc: solute transport and metabolism. Connect.Tissue Res. 1981;8:101–19. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 20.Crock HV, Goldwasser M. Anatomic studies of the circulation in the region of the vertebral end-plate in adult Greyhound dogs. Spine. 1984;9:702–6. doi: 10.1097/00007632-198410000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Moore RJ, Osti OL, Vernon-Roberts B, et al. Changes in endplate vascularity after an outer anulus tear in the sheep. Spine. 1992;17:874–8. doi: 10.1097/00007632-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Roberts S, Urban JP, Evans H, et al. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21:415–20. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Brown MD, Tsaltas TT. Studies on the permeability of the intervertebral disc during skeletal maturation. Spine. 1976;1:240–4. [Google Scholar]
- 24.Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233–48. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- 25.Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disc: Effect of fluid flow on solute transport. Clin Orthop. 1982;170:296–302. [PubMed] [Google Scholar]
- 26.Gu WY, Yao H, Vega AL, et al. Diffusivity of ions in agarose gels and intervertebral disc: Effect of porosity. Annals of Biomed Engng. 2004;32:1710–7. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 27.Hsu EW, Setton LA. Diffusion tensor microscopy of the intervertebral disc anulus fibrosus. Magn Reson.Med. 1999;41:992–9. doi: 10.1002/(sici)1522-2594(199905)41:5<992::aid-mrm19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Chiu EJ, Newitt DC, Segal MR, et al. Magnetic resonance imaging measurement of relaxation and water diffusion in the human lumbar intervertebral disc under compression in vitro. Spine. 2001;26:E437–E444. doi: 10.1097/00007632-200110010-00017. [DOI] [PubMed] [Google Scholar]
- 29.Jackson A, Yao H, Brown MD, et al. Anisotropic ion diffusivity in intervertebral disc: an electrical conductivity approach. Spine. 2006;31:2783–9. doi: 10.1097/01.brs.0000245842.02717.1b. [DOI] [PubMed] [Google Scholar]
- 30.Adams MA, Hutton WC. The effect of posture on diffusion into lumbar intervertebral discs. J Anat. 1986;147:121–34. [PMC free article] [PubMed] [Google Scholar]
- 31.Kraemer J, Kolditz D, Gowin R. Water and electrolyte content of human intervertebral discs under variable load. Spine. 1985;10:69–71. doi: 10.1097/00007632-198501000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Ohshima H, Tsuji H, Hiarano N, et al. Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine. 1989;14:1234–44. doi: 10.1097/00007632-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Adams MA, Hutton WC. The effect of posture on the fluid content of lumbar intervertebral discs. Spine. 1983;8:665–71. doi: 10.1097/00007632-198309000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Drew SC, Silva P, Crozier S, et al. A diffusion and T2 relaxation MRI study of the ovine lumbar intervertebral disc under compression in vitro. Phys Med Biol. 2004;49:3585–92. doi: 10.1088/0031-9155/49/16/006. [DOI] [PubMed] [Google Scholar]
- 35.Burstein D, Gray ML, Hartman AL, et al. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res. 1993;11:465–78. doi: 10.1002/jor.1100110402. [DOI] [PubMed] [Google Scholar]
- 36.Quinn TM, Kocian P, Meister JJ. Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch Biochem Biophys. 2000;384:327–34. doi: 10.1006/abbi.2000.2077. [DOI] [PubMed] [Google Scholar]
- 37.Quinn TM, Morel V, Meister JJ. Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J Biomech. 2001;34:1463–9. doi: 10.1016/s0021-9290(01)00112-9. [DOI] [PubMed] [Google Scholar]
- 38.Ngwa W, Geier O, Stallmach F, et al. Cation diffusion in cartilage measured by pulsed field gradient NMR. Eur Biophys J. 2002;31:73–80. doi: 10.1007/s002490100184. [DOI] [PubMed] [Google Scholar]
- 39.Maroudas A, Bullough P, Swanson SA, et al. The permeability of articular cartilage. J Bone Joint Surg Br. 1968;50:166–77. [PubMed] [Google Scholar]
- 40.Gu WY, Justiz MA. Apparatus for measuring the swelling dependent electrical conductivity of charged hydrated soft tissues. J Biomech Engng. 2002;124:790–3. doi: 10.1115/1.1516571. [DOI] [PubMed] [Google Scholar]
- 41.Gu WY, Justiz MA, Yao H. Electrical conductivity of lumbar annulus fibrosis: Effects of porosity and fixed charge density. Spine. 2002;27:2390–5. doi: 10.1097/00007632-200211010-00014. [DOI] [PubMed] [Google Scholar]
- 42.Yao H, Justiz MA, Flagler D, et al. Effects of swelling pressure and hydraulic permeability on dynamic compressive behavior of lumbar annulus fibrosus. Annals of Biomed Engng. 2002;30:1234–41. doi: 10.1114/1.1523920. [DOI] [PubMed] [Google Scholar]
- 43.Hayat MA. Fixation for electron microscope. Academic Press ed.; New York: 1982. p. 501. [Google Scholar]
- 44.Iatridis JC, ap Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37:1165–75. doi: 10.1016/j.jbiomech.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Travascio F, Gu WY. Anisotropic Diffusive Transport in Annulus Fibrosus: Experimental Determination of the Diffusion Tensor By FRAP Technique. Annals of Biomed Engng. 2007 doi: 10.1007/s10439-007-9346-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nimer E, Schneiderman R, Maroudas A. Diffusion and partition of solutes in cartilage under static load. Biophycal Chemistry. 2003;106:125–46. doi: 10.1016/s0301-4622(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 47.Oshima H, Ishihara H, Urban JP, et al. The use of coccygeal discs to study intervertebral disc metabolism. J Orthop Res. 1993;11:332–8. doi: 10.1002/jor.1100110304. [DOI] [PubMed] [Google Scholar]