Abstract
Chronic chagasic cardiomyopathy, which is caused by the protozoan Trypanosoma cruzi, is a major cause of heart failure in Latin America. It is a disease for which effective treatment in its advanced clinical forms is lacking. We have previously shown that bone marrow mononuclear cell (BMC) transplantation is effective in reducing inflammation and fibrosis in the mouse model of Chagas disease. The present study used magnetic resonance imaging to assess changes in the cardiac morphology of infected mice after therapy with BMCs. Serial imaging of the BMC-treated mice revealed regression of the right ventricular dilatation typically observed in the chagasic mouse model.
Infection with the protozoan Trypanosoma cruzi causes Chagas disease, which can result in acute myocarditis and a dilated cardiomyopathy. Although treatment with antiparasitic drugs may be beneficial in cases of acute infection, once chronic cardiomyopathy has been established these drugs are no longer of value. No specific treatment exists at this stage of the disease, and management involves drugs to treat intractable congestive heart failure (CHF). In the chronic phase, patients can remain asymptomatic for decades (in the so-called indeterminate period of the disease). Approximately 30% of infected individuals develop syndromes affecting mainly the gastrointestinal system and the heart.
Chagasic cardiomyopathy is characterized by diffuse inflammation and widespread fibrosis of the myocardium, leading to a dilated cardiomyopathy that progresses to CHF. At this stage, patients have limited therapeutic options. For example, therapy with standard drugs used in the treatment of CHF may not be efficacious, and heart transplantation may be the only option. However, heart transplantation may result in reactivation of the infection due to immunosuppression caused by drugs used to maintain the transplant. This has led to the search for new therapies, and the use of stem cells in various models of cardiac diseases has generated new hope for therapy of chronic chagasic cardiomyopathy [1-6]. In this regard, a pioneering study by Soares et al. [7] showed that, in a mouse model of chronic chagasic cardiomyopathy, intravenous injection of bone marrow–derived mononuclear cells significantly reduced inflammation and fibrosis for prolonged periods of time. We now report that the use of mononuclear cell fraction from the bone marrow not only prevents but also reverses right ventricular (RV) dilatation in a murine model of chagasic cardiomyopathy.
Methods
All mice were obtained from Jackson Laboratory. Trypomastigotes of T. cruzi (Brazil strain) were maintained in C3H mice. Female C-129 mice were infected with 5 × 104 trypomastigotes in saline, by the intraperitoneal route. All mice were infected at 8−10 weeks of age. Infected mice were monitored by parasitemia evaluation at various time points after infection, by counting the number of trypomastigotes in peripheral blood. All infected mice had a parasitemia that peaked by day 35−40 after infection. All animal protocols were approved by our institutional animal care and use committee.
Bone marrow mononuclear cells (BMCs) were prepared as described elsewhere [7]. Briefly, as donors we used uninfected C-129 mice. Femurs and tibias were harvested and thoroughly cleaned of all muscle tissue. Bone marrow was flushed with RPMI 1640, and the cell suspension was centrifuged in histopaque 1083. Mononuclear cells were collected at the histopaque medium interface and passed through a nylon filter. Cells were washed in RPMI 1640 twice, counted in a hemocytometer, and checked for viability using trypan blue. Cells were then diluted in 0.2 mL of saline and injected into the tail vein of the mice (1 × 107 cells/mouse). Mice were treated with the cells and monitored for 1 to 4 months after treatment, depending on the experimental protocol used.
For magnetic resonance imaging (MRI), mice were anesthetized with isoflurane inhalation anesthesia (1%−1.5% in medical air administered via a nose cone). A set of Gould electrocardiogram (ECG) leads with custom silver electrodes were attached to the limbs for monitoring the ECG signal. The ECG signal was transmitted to a personal computer running Ponemah Physiology Suite software (version 3.12; Data Sciences International). The ECG signal was used to monitor the status of the mice and permitted us to adjust anesthesia levels to maintain a stable physiologic heart rate (500−550 bpm). Mice were positioned in a 40-mm homebuilt bird-cage MRI coil in a 9.4-T GE Omega vertical-bore imaging system. Body temperature was maintained by a water-heating system. Heart rate and ECG were monitored continuously. MRI experiments typically lasted ∼1 h per mouse, after which time the mice were allowed to recover and then returned to their cages in the animal institute. The data were analyzed using SPSS; statistically significant differences (P < .05) between groups are indicated by asterisks in the figures.
Results
We performed serial in vivo MRI experiments on infected mice treated with BMCs. Figure 1 demonstrates images obtained from age-matched control mice (panel A), chronically infected mice that were left untreated (panel B), and chronically infected mice that were treated with BMCs (panel C). Infection resulted in marked dilatation of the RV cavity (compare panels A and B), a characteristic of this particular combination of mouse and parasite strain previously described by us [8, 9]. Treatment with the BMCs resulted in reduction of the RV dilation (3 months after treatment), as illustrated in panel C. In this study of chronic infection, infected mice were treated with BMCs 6 months after infection and were evaluated monthly by MRI to assess the dilatation of the RV (RV inner dimension [RVID], measured in millimeters), our marker for chagasic cardiomyopathy. The results are shown in the graph in figure 1D. Most notably, the dilation of the RV was significantly reduced after 3 months of treatment with BMCs, compared with that in untreated infected mice. These results demonstrate that BMCs can cause regression of RV dilation in infected mice that are treated during the chronic stage, when significant infection-associated cardiac remodeling has already taken place. BMC treatment of uninfected mice did not alter RV dimension (data not shown). The left ventricular dimension and wall thickness were also measured; however, there were no significant differences between uninfected, untreated infected, and treated infected mice (data not shown).
Figure 1.
Bone marrow mononuclear cell (BMC) treatment of chronically infected mice. Shown are magnetic resonance images of an uninfected control mouse (A), an untreated chronically infected mouse (B), and a chronically infected mouse after BMC treatment (C) (note the enlarged right ventricle [RV] in panel B and the regression in panel C; white arrows and lines indicate the RV) as well as a graph (D) of the RV inner dimension (RVID) in control mice, untreated chronically infected mice (6 months after infection [MAI]), and chronically infected mice treated with BMCs for 1 (BMC-1M) through 4 (BMC-4M) months. Graphical data are presented as mean ± SE values. *P < .05 (statistically significant difference) for the comparison between the indicated groups.
In a second MRI experiment, mice were infected and BMC treatment was initiated 1 month after infection to determine whether BMCs prevent dilatation of the RV in treated infected mice. RV dilation was already significant 1 month after infection and increased up to 3 months after infection in untreated infected mice, at which time it stabilized. BMC treatment 1 month after infection prevented RV dilation in the treated infected mice, as shown in figure 2. This effect persisted for as long as 5 months after infection, the longest time interval examined in the present study.
Figure 2.
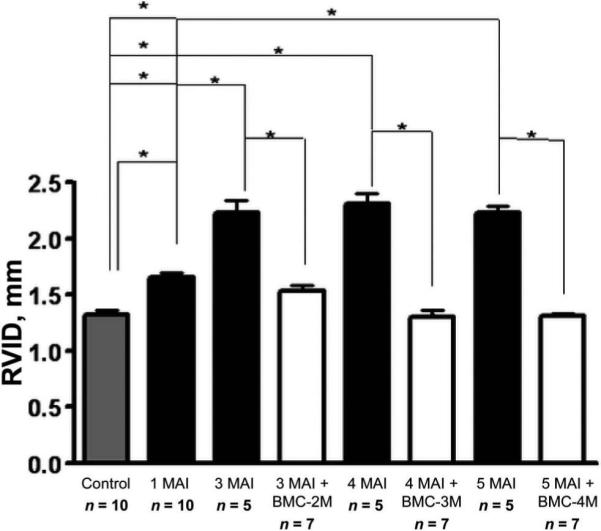
Bone marrow mononuclear cell (BMC) treatment of acutely infected mice. Shown is a graph of the right ventricular inner dimension (RVID) in control mice, untreated infected mice (1, 3, 4, and 5 months after infection [MAI]), and infected mice treated with BMCs at 2 months (BMC-2M), 3 months (BMC-3M), and 4 months (BMC-4M). RV dilation was prevented by BMCs and the RVID remained close to normal control values in the treated infected mice, whereas the RVID in untreated infected mice had increased significantly by 3 months after infection and remained high throughout the study. Data are presented as mean ± SE values. *P < .05 (statistically significant difference) for the comparison between the indicated groups.
Discussion
The potential use of bone marrow stem cells in therapies for ischemic heart diseases has been widely investigated, but few studies of their use in experimental chronic chagasic cardiomyopathy have been published [7, 10]. We previously demonstrated that treatment with bone marrow mononuclear cells in a mouse model of chronic chagasic cardiomyopathy significantly reduced cardiac inflammation and fibrosis [7]. This was the same in our present study (data not shown); however, in the previous report no structural evaluation was conducted. Recently, Guarita-Souza et al. [10] reported improvement of cardiac function in a rat model of Chagas disease. In that model, skeletal myoblasts and mesenchymal stem cells were cotransplanted directly into the left ventricle of the rats. The ejection fraction (as measured by echocardiography) was significantly higher in the infected rats that received the cotransplanted cells than in sham-treated infected control rats.
The present study is the first report of a serial in vivo MRI evaluation of BMC therapy in a mouse model of Chagas disease. It also reiterates that noninvasive cardiac testing can provide complementary information when the pathogenesis of dilated cardiomyopathy in experimental models of chronic Chagas disease is being studied. Previous MRI studies of mice infected with the Brazil strain of T. cruzi demonstrated remodeling of the RV. RVID increased during the acute phase, during the early stages of the chronic phase, and during the chronic phase of infection, thus indicating that enlargement of this chamber can be a marker for murine experimental chagasic cardiomyopathy [8, 9]. Similarly, a recent report by Nunes et al. [11] demonstrated for the first time that RV dysfunction predicts mortality in humans with chagasic cardiomyopathy.
In the present study, chronically infected mice underwent cardiac MRI 6 months after infection and then monthly (1−4 months) after BMC treatment. We compared the RVID of control, untreated infected, and BMC-treated infected mice (figure 1D). There were significant increases in the RVID in all infected mice, compared with that in the control mice. The dilation was significantly reduced 3 months after BMC treatment, compared with that in untreated infected mice. The regression of the dilatation observed in the chagasic mouse model is rather striking, and we do not have a mechanistic explanation at the moment. We speculate that injected cells home to the heart and may decrease wall stress, thus contributing to a “reverse” remodeling of the heart. Recent simulations by Wall et al. [12] have suggested that addition of noncontractile material to damaged hearts may have effects on cardiac mechanics, with the possible maintenance of stroke volume and reduction in end diastolic volumes after cell injection. We also treated mice that were infected for 1 month with BMCs. At this time point during the acute stage of infection there is a robust inflammatory response [8], which may provide an environment attractive for stem cell homing and adhesion.
In recent years, the idea of using stem cells for the repair of injured myocardium has been explored primarily in the setting of acute myocardial infarction. In that regard, chagasic cardiomyopathy represents an unusual challenge in that the damage—unlike the damage secondary to acute myocardial infarction—is diffuse and not focal. Our results indicate that BMCs prevents RV dilation (figure 2). Although the underlying mechanisms are not well understood, this observation suggests that BMC therapy can prevent the cardiac remodeling process that leads to dilated cardiomyopathy. Similar findings have been reported with the use of BMCs in models of myocardial infarction, but again, mechanistic insights are not yet available [13, 14].
Our present observations are novel and are particularly important to the treatment of dilated cardiomyopathies, for which prevention or reversion of cardiac dilatation is a major therapeutic goal. Moreover, our findings also provide further evidence for the beneficial effects of BMC therapies in chagasic cardiomyopathy. The results of a pilot human study using BMCs in chagasic cardiomyopathy have been reported [15]. A controversy regarding stem cell therapy for heart disease in general is whether the stem cells actually transform into functioning myocytes or serve as a delivery vehicle for certain growth factors and cytokines. We are currently using BMCs from transgenic mice under the control of a cardiac-specific promoter to investigate this question.
Acknowledgments
Financial support: National Institutes of Health (grant R01- HL073732); Fogarty International Training Grant (grant D43TW007129).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res. 2002;91:1092–102. doi: 10.1161/01.res.0000046045.00846.b0. [DOI] [PubMed] [Google Scholar]
- 3.Min JY, Yang Y, Converso KL, et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol. 2002;92:288–96. doi: 10.1152/jappl.2002.92.1.288. [DOI] [PubMed] [Google Scholar]
- 4.Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002;74:19–24. doi: 10.1016/s0003-4975(02)03591-9. [DOI] [PubMed] [Google Scholar]
- 5.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares MB, Lima RS, Rocha LL, et al. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am J Pathol. 2004;164:441–7. doi: 10.1016/s0002-9440(10)63134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang H, Chan J, Wittner M, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 9.de Souza AP, Tang B, Tanowitz HB, Araujo-Jorge TC, Jelicks LA. Magnetic resonance imaging in experimental Chagas disease: a brief review of the utility of the method for monitoring right ventricular chamber dilatation. Parasitol Res. 2005;97:87–90. doi: 10.1007/s00436-005-1409-4. [DOI] [PubMed] [Google Scholar]
- 10.Guarita-Souza LC, Carvalho KA, Woitowicz V, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation. 2006;114:I120–4. doi: 10.1161/CIRCULATIONAHA.105.000646. [DOI] [PubMed] [Google Scholar]
- 11.Nunes MD, Rocha MO, Ribeiro AL, et al. Right ventricular dysfunction is an independent predictor of survival in patients with dilated chronic Chagas’ cardiomyopathy. Int J Cardiol. 2007 August 7; doi: 10.1016/j.ijcard.2007.06.012. (electronically published ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Guo J, Zhang P, et al. Long-term effects of bone marrow mononuclear cell transplantation on left ventricular function and remodeling in rats. Life Sci. 2004;74:2853–64. doi: 10.1016/j.lfs.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Ge J, Sun A, et al. Comparison of various kinds of bone marrow stem cells for the repair of infracted myocardium: single clonally purified non-hematopoietic mesenchymal stem cells serve as a superior source. J Cell Biochem. 2006;99:1132–47. doi: 10.1002/jcb.20949. [DOI] [PubMed] [Google Scholar]
- 15.Vilas-Boas F, Feitosa GS, Soares MB, et al. Early results of bone marrow cell transplantation to the myocardium of patients with heart failure due to Chagas disease. Arq Bras Cardiol. 2006;87:159–66. doi: 10.1590/s0066-782x2006001500014. [DOI] [PubMed] [Google Scholar]



