Abstract
Oncolytic herpes simplex viruses (HSV) possess direct oncolytic and antiangiogenic activities and are promising anti-cancer agents, but efficacy as single agents needs to be improved. We investigated whether combination therapy with Trichostatin A (TSA), an agent that also targets cancer cells and tumor vasculature, can result in enhanced efficacy. In vitro, TSA and G47Δ showed strong synergy in proliferating endothelial cells, varying degrees of synergy in most cancer cell lines, but no effect in quiescent normal endothelial and prostate epithelial cells. Synergy is dependent on viral replication, but not on dosing sequence between TSA and G47Δ, viral genetic alterations, infectivity, or replication kinetics of G47Δ. Using an isogenic cell system, we found that high cellular cyclin D1 level is also critical to interaction. Normal cells with low cyclin D1 levels were not subjected to toxicity by either agent. In tumor and proliferating endothelial cells, combination treatment enhanced cyclin D1 and VEGF inhibition. Concurrent systemic TSA and intratumoral G47Δ administration resulted in enhanced antiangiogenesis and enhanced antitumoral efficacy in animal models. Combination treatment with TSA and oncolytic HSV thus provides a novel approach for cancer therapy.
Keywords: oncolytic virus, antiangiogenesis, cyclin D1, trichostatin A, herpes simplex virus
Introduction
Recent advances in cancer genetics and molecular biology have led to the development of several novel cancer therapeutics, including oncolytic viruses [1, 2]. Oncolytic herpes simplex virus (HSV) can be engineered to infect and lyse a wide variety of cancer cells without damaging normal cells. Safety has been demonstrated in humans and therapeutic benefits are being explored [3]. Importantly, oncolytic HSV lacks cross-resistance with existing therapies and has the potential to synergize with other treatment modalities [1, 4].
Trichostatin A (TSA) belongs to a group of compounds termed histone deacetylase (HDAC) inhibitors (HDACIs). HDACs and histone acetylases (HATs) are enzymes responsible for deacetylating and acetylating the amino-termini of histones, respectively, and disruption of the HAT-HDAC balance is associated with development of cancer [5]. HDACIs are being tested as anti-cancer agents [6–9]. They have relatively low toxicity compared to most chemotherapy [10, 11]. Responses as monotherapy range between 5 to 57%, but they can potentiate the effect of chemotherapy as well as other therapeutics [12–14]. Mechanisms for synergistic tumor killing include Fas and TRAIL sensitization, induction of p21WAF, inhibition of Akt and ERK, among others [15–17].
TSA and other HDACIs have also been tested in conjunction with genetic therapies. By upregulating coxsackie-adenovirus receptor (CAR) and αvintegrin [18–20], they enhance the infectivity of adenovirus and adeno-associated virus vectors. However, antitumoral activities of HDACIs per se were not addressed in those studies, which need to be distinguished from enhanced efficacy seen with combination treatment [21]. Interestingly, in addition to their antitumoral effect, certain HDACIs have been shown to exert antiangiogenic activities [9, 22, 23], and both characters are shared by oncolytic HSVs [24].
Here we report the effect of TSA and oncolytic HSV combination therapy on tumor cells and tumor vasculatures, and examine mechanism using an isogenic cell system.
Results
TSA synergizes with oncolytic HSV in proliferating endothelial and tumor cells
We tested the potency of TSA and various HSVs (including wildtype strain F, oncolytic G47Δ, and replication-deficient d120) in a panel of tumor and normal cells, including both proliferating and quiescent endothelial cells. Strong synergy was seen in proliferating endothelial cells HUVEC and Py-4-1 with G47Δ and TSA combination treatment (Table 1). Various degrees of synergy were also seen in all but one tumor cell line (MCF-7). In contrast, no effect was seen in normal primary cells PrEC and quiescent HUVEC (neither TSA nor G47Δ showed toxicity). Of note, the interaction was not dependent on dosing schedule; treatment starting with either agent achieved similar results (Table 1).
Table 1. Interaction of TSA and HSV.
Interaction of TSA and HSV analyzed using Chou-Talalay methods. Cells were treated with virus and/or TSA (Sigma) at different doses. +/−: additive (0.8 < CI < 1.2); +: weak synergy (0.5 ≤ CI ≤ 0.8); ++: moderate synergy (0.2 ≤ CI < 0.5); +++: strong synergy (CI < 0.2). N: no effect (one or both of the agents did not show cytotoxicity).
| TSA →G47 Δ | G47Δ →TSA | TSA → F | TSA→d120 | |
|---|---|---|---|---|
| Endothelial cells | ||||
| HUVEC (human; proliferating) | +++ | +++ | + | N |
| HUVEC (quiescent) | N | N | N | N |
| Py-4-1 (murine) | +++ | ++ − +++ | ++ − +++ | N |
| Tumor cells | ||||
| U87 (brain) | ++ − +++ | ++ − +++ | ++ | N |
| U87 (brain) pretreated with cyclin D1 Ab | +/− | +/− | N | N |
| T98 (brain) | + | + | + | N |
| SW480 (colon) | + − ++ | + − +++ | ++ | N |
| HeLa (cervical) | + − ++ | + | + | N |
| MCF-7 (breast) | +/− | +/− | +/− | N |
| Normal primary cells | ||||
| PrEC (prostate) | N | N | N | N |
Synergy is independent of HSV mutations but viral replication is required
Similar interactions were seen with wildtype HSV, although in some cell lines the magnitude was weaker than with G47Δ (Table 1). Therefore, the genetic alterations of oncolytic G47Δ play little role in the interaction with TSA. Although wildtype HSV kills quiescent HUVEC and PrEC efficiently, combination treatment did not show any additional toxicity. In contrast, replication-deficient d120 only causes cytotoxicity at high doses (eg, 50 pfu/cell) in most tumor cells, and did not enhance the effect of TSA (Table 1). Therefore, viral replication is necessary for synergy.
TSA does not affect infectivity or virus replication
HSV infectivity in the presence of TSA was tested using d120-GFP. Among the 4 cell lines tested (U87, A549, T98, and SW480), there was no significant difference in the percentage of GFP-positive cells treated with or without TSA (within 8% change in GFP-positive percentage in the presence of TSA).
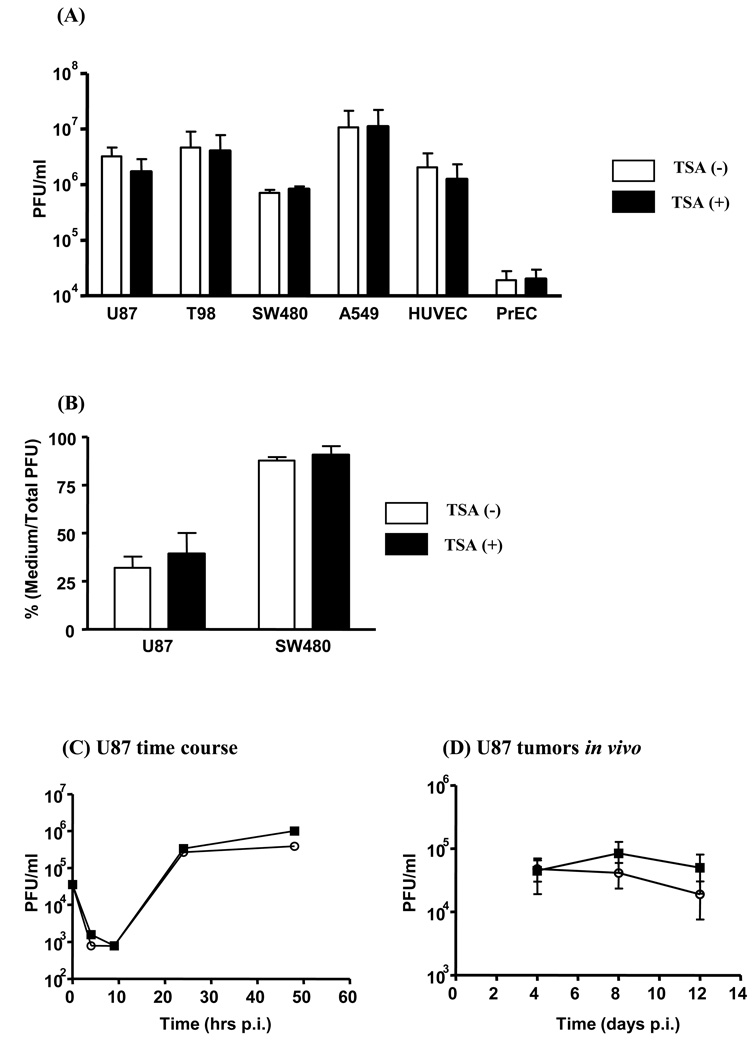
The burst size of G47Δ (pfu/cell) ranged from 7.1 to 100 in tumor cells (SW480, U87, T98, and A549), 20 in proliferating HUVEC, and significantly lower in normal PrEC and quiescent HUVEC (0.02 to 0.15). No significant difference was noted in the presence of TSA (Fig. 1a; p > 0.05). With TSA, the cell-free virus ratio (supernatant/total pfu) in U87 and SW480 cells was slightly, but not significantly, increased (Fig. 1b; p > 0.05). Detailed time course experiments in U87 cells also failed to show significant changes in replication kinetics (Fig. 1c; p > 0.05 at all time points).
Figure 1.
TSA does not alter viral replication and release in vitro or in vivo. (A) Tumor or normal cells were infected with G47Δ and harvested at 48 hours p.i.. TSA did not significantly alter virus yield (n = 3; p > 0.05). (B) Virus yield in culture medium was assessed and compared that with total viral yield at 24 hours p.i.. TSA did not significantly alter viral release (n = 3; p > 0.05). (C) U87 cells were infected with G47Δ at 0.01 pfu/cell and samples harvested at different time points. TSA did not alter viral replication kinetics. Circles: TSA(−); boxes: TSA(+). (D) U87 tumors received intratumoral G47Δ ± TSA and tumors were analyzed for infectious pfu at different time points. TSA did not significantly alter viral replication in vivo (p > 0.05).
Viral replication was also assessed in vivo. U87 tumors injected with intratumoral G47Δ were treated with/without systemic TSA administration. The average infectious G47Δ virus recovery (× 104 pfu) from tumors not treated with TSA was 4.5 (day 4), 8.4 (day 8), and 5.0 (day 12), and 4.8 (day 4), 4.1 (day 8), and 3.9 (day 12) from tumors treated with TSA (Fig. 1d; p > 0.05 at all time points). Therefore, TSA does not significantly impact G47Δ replication and spread in vitro and in vivo.
Enhanced inhibition of cyclin D1 and VEGF with combination therapy
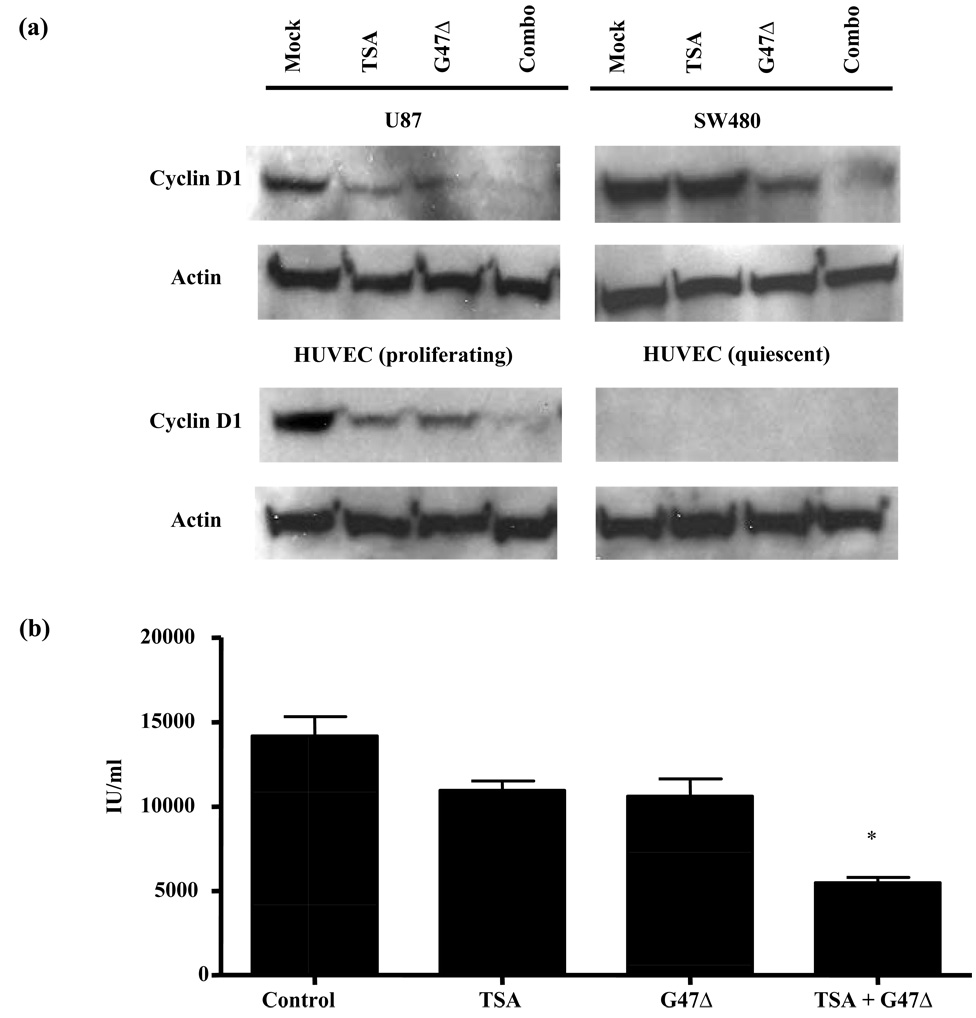
We next explored molecular mechanism of synergistic interaction. None of the signaling pathways that TSA is known to involve (including Akt, ERK, NF-κB, etc) were significantly altered under combination treatment (data not shown). However, in tumor cells (U87 and SW480) and proliferating HUVEC, reduced cyclin D1 expression levels was seen with TSA/G47Δ combination treatment compared to untreated controls and single agent treatment (Fig. 2a). In contrast, quiescent HUVEC had undetectable cyclin D1 levels, which was not affected by any treatment (Fig. 2a). Therefore, endogenous cyclin D1 levels correlates with therapeutic efficacy and synergism of TSA/G47Δ treatment.
Figure 2.
(A) Enhanced cyclin D1 inhibition by TSA and G47Δ only in tumor and proliferating endothelial cells. In U87, SW480, and proliferating HUVEC, TSA and G47Δ combination treatment strongly reduces cyclin D1 compared to single agents or untreated controls. In quiescent HUVEC, no detectable level of cyclin D1 was noted in all treatment groups. (B) TSA and G47Δ combination enhanced VEGF inhibition in U87 cells. *: p < 0.05 compared to all other groups.
To further study the role of cyclin D1 in the interaction, we pretreated U87 cells, which showed high cyclin D1 expression level and strong synergy between oncolytic HSV and TSA, with a polyarginated cyclin D1 monoclonal antibody [25]. This approach has been demonstrated to successfully block intracellular cyclin D1 [25]. As seen in Table 1, blocking of intracellular cyclin D1 largely diminished the synergistic effect of combination therapy. Therefore, cyclin D1 is critical in mediating the synergistic interaction between the two agents.
We next explored if TSA/G47Δ combination treatment can lead to enhanced antiangiogenesis in addition to synergistic killing. U87 cells were tested for VEGF secretion, and samples were analyzed for VEGF levels at early time point (12 hours pi) before there was significant changes in viable cell numbers between control and different treatment groups (data not shown). U87 cells treated with TSA or G47Δ showed reduced, albeit insignificantly, VEGF secretion (Fig. 2b; p > 0.05 between PBS and TSA or G47Δ groups), whereas the combination treatment significantly inhibited VEGF secretion to 39% (Fig. 2b; p < 0.01).
Enhanced antitumoral efficacy by combining TSA with G47Δ
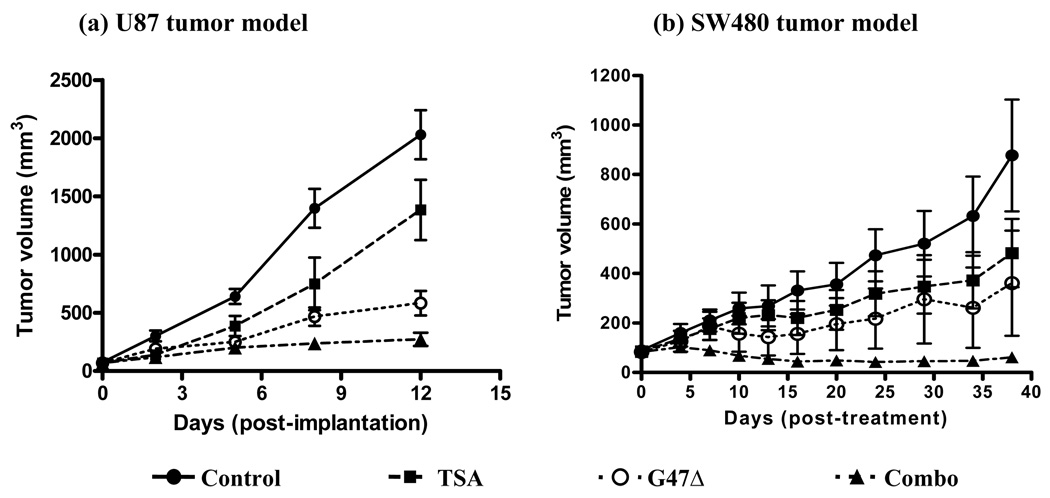
Two human tumor mice xenograft models were tested for the efficacy of combination treatment. In the U87 tumor model, both TSA and G47Δ treatment groups significantly inhibited tumor growth, whereas combination group showed further inhibition in tumor growth (Fig. 3a; p < 0.001 between PBS and other groups; p < 0.001 between G47Δ and combination groups).
Figure 3.
TSA enhances the antitumoral efficacy of G47Δ. Tumor growth were compared for U87 (a; n = 10/group) and SW480 (b; n = 7/group) models. Black circles: control; black squares: TSA; white circles: G47Δ; black triangles: combination. In both models, TSA and G47Δ treatment groups showed significantly smaller tumor volumes compared to the mock treatment group, whereas the combination group showed a further significant tumor volume reduction.
Similar results were observed in a second tumor model with SW480 tumors. Both agents significantly inhibited tumor growth, and combination treatment further significantly reduced tumor growth (Fig. 3b; p < 0.01 between PBS and other groups; p < 0.005 between G47Δ and combination groups). Therefore, enhanced antitumoral efficacy with TSA/G47Δ combination therapy was confirmed in two tumor models. In addition, no signs of physical toxicity were noted in TSA-treated animals.
Enhanced antiangiogenic activity in vivo
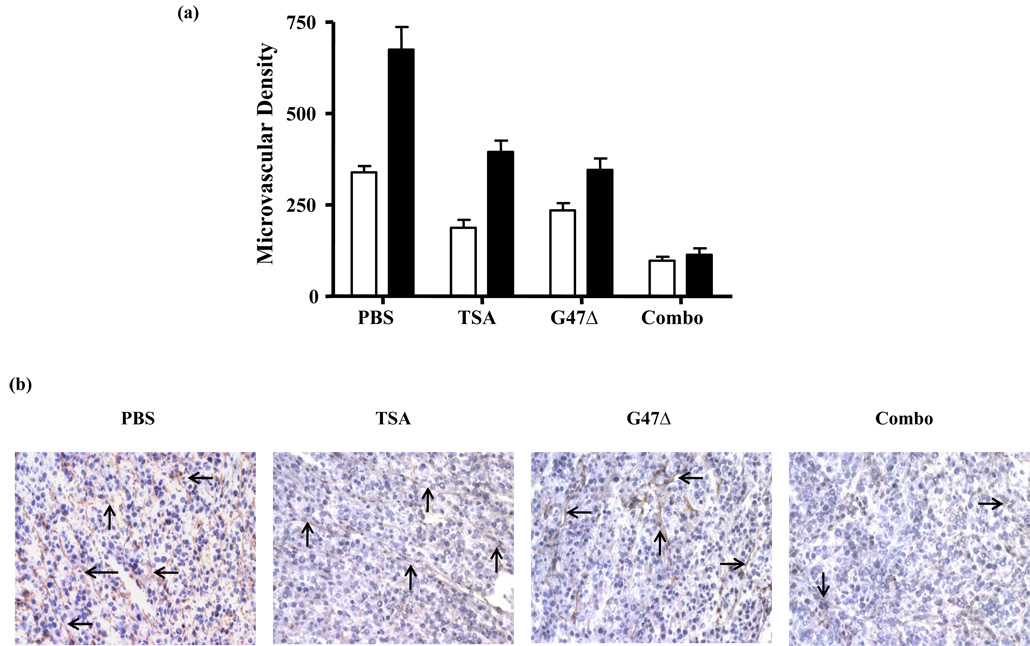
Consistent with our data on VEGF secretion and the literature, TSA and G47Δ both reduced the vascularity of U87 tumors (Fig. 4a; p < 0.001 between PBS and TSA or G47Δ). Combination treatment further reduced tumor vascularity compared to single agents (Fig. 4a; p < 0.01). Representative photos are shown in Fig. 4b.
Figure 4.
TSA and G47Δ synergistically inhibited tumor vasculature formation in vivo. (A) While both TSA and G47Δ significantly inhibited MVD, combination therapy showed further significant inhibition. Open bars: 4 days post-treatment; closed bars: 12 days post-treatment. (B) Representative photos of each group (day 12; 10 × 20). Arrows: typical VWF-positive vascular structures.
Discussion
Here we report a novel approach by which antitumoral and antiangiogenic efficacy of oncolytic HSV can be enhanced. G47Δ and TSA combination showed synergistic cell killing in most of the tumor cell lines and proliferating vascular endothelial cells, but not in quiescent normal primary cells. The synergistic killing was not due to altered viral entry, replication kinetics, virus spread, virus gene mutation, or dosing sequence. Viral replication was, however, critical. Synergistic interaction correlates with enhanced degradation of cyclin D1 and VEGF inhibition. Finally, enhanced antitumoral efficacy and antiangiogenesis were confirmed in vivo.
These are important findings with significant impact to the field. Recent advances with oncolytic HSV include the demonstration of enhanced viral replication by co-administration of standard chemotherapeutic agents through cellular ribonucleotide reductase and/or GADD34 upregulation [26]. However, this approach is restricted to viruses with specific mutations (i.e. ICP6 and γ34.5), and those viruses are severely attenuated in tumor cells (up to 100-fold). It is possible that combination treatments that solely increase replication of those viruses will not be potent enough to achieve a clinical response. Our study indicates that enhanced efficacy with TSA combination can be applicable to other oncolytic HSV designs. Previous studies have also shown synergistic interaction between oncolytic adenovirus and TSA treatment through a distinct mechanism [20]. TSA enhances infectivity of adenovirus by upregulating CAR expression [18, 20]. Although this is of interest, it is unclear whether CAR expression, and subsequent viral infection, will also be enhanced in normal tissues, and thus poses a safety concern when translating into clinic. In addition, retargeted adenoviruses might not benefit from co-administration with TSA, as the infectivity of those viruses is CAR-independent. Our strategy therefore allows a broader application for the use of oncolytic HSV without compromising safety.
Recent studies have also highlighted the importance of cyclin D1 regulation and overexpression in tumorigenesis [27, 28]. Cyclin D1 associates with CDK4/CDK6, and the CDK/cyclin D1 complex facilitates cell cycle progression through E2F activation. Agents inducing cyclin D1 degradation (including all-trans retinoic acid, resveratrol, cyclohexamide, rapamycin, and TSA) are being pursued as novel cancer treatments [27–29]. Coincidently, various HSV gene products reduce cyclin D1 levels in HSV-infected cells, including ICP0, ICP4, ICP22, and VHS [30]. Cyclin D1 inhibition in oncolytic HSV-infected cells is a distinct mechanism from oncolysis, which has not been explored for cancer therapy previously. The way HSV controls cell cycle is also in sharp contrast to that of adenovirus, widely-used oncolytic virus species, which induces transition from G0 or G1 into the S phase of the cell cycle and allows cellular proliferation [31].
Quiescent HUVEC were tested here as an isogenic cell counterpart to proliferating HUVEC and as a model reflecting normal vasculature. Tumor and proliferating endothelial cells are susceptible to combination therapy due to their active cyclin D1 status, whereas cells in low cell cycling status (quiescent HUVEC and PrEC) are not subject to toxicity to either agent. Although disruption in certain tumorigenic signaling (e.g. Akt, p38, ERK, NF-κB, etc) has been shown by TSA, we failed to identify any specific signaling pathway that is responsible for synergistic interaction. Of note, TSA is known to have positive and negative impacts on the expression of multiple proteins, and that certain HSV gene products (e.g. viral host shutoff protein) is known to deactivate multiple host proteins, it is possible that there are other targets that are jointly affected by TSA and oncolytic HSV that are yet identified.
It is unclear whether cyclin D1 blockade and VEGF inhibition are sequential or independent events. Nonetheless, our data are consistent with a previous report that cyclin D1 blockade leads to a reduction in VEGF secretion and inhibition of VEGF-induced endothelial cell growth [32]. Oncolytic HSVs are antiangiogenic through direct endothelial cell killing and paracrine angiogenic factor(s) inhibition [33–35]. Recent studies with oncolytic viruses have also shown that inflammation induced by oncolytic virotherapy can compromise tumor blood flow [36]. Given the immunomodulatory activities of TSA [37–39], the effect of combination treatment on tumor vasculatures might be more profound in immunocompetent animal models.
Interestingly, although we did not observe significant changes in infectivity, viral replication, and viral spread in the presence of TSA, several publications have explored the impact of HDACIs on viral gene expression, protein production, and anti-viral response induction [40–42]. TSA treatment has been shown to enhance the gene expression of ICP0(−) HSV but not ICP0(+) HSV [40]. TSA is also able to reactivate HSV after quiescent infection (under acyclovir treatment) [41]. This effect, however, is strain-specific. Our study is consistent with these results; the viruses used in our study were all ICP0 intact, and the strain used in our study, F, did not show significant reactivation after TSA treatment [41]. It remains to be seen whether oncolytic HSV constructed on different strains will show different results. Of note, in our study we did not observe any toxicity due to TSA or TSA/G47Δ treatment. Other studies exploring chemotherapy-oncolytic virus combination or viruses encoding therapeutic transgene (as distinct antitumoral mechanisms) have demonstrated that synergistic tumor killing can be either replication-dependent, in which enhanced viral replication results in enhanced potency [26, 43], or replication-independent, in which enhanced potency is due to mechanisms other than enhanced viral replication [24, 44].
HDAC activities are also required for interferon (IFN) regulatory factor 3-mediated gene expression activated by virus infection, and it plays an important role in antiviral response against certain viral species (e.g. RNA viruses) [42]. TSA treatment can lead to global impairment of IFN-stimulated gene expression. Treatment with other HDACIs also rescued the IFN-mediated HCV suppression [42]. This implies a possible enhancement in viral replication in the presence of TSA, as IFN signaling pathway is now blocked. Our study shows that this is not the case with oncolytic HSV with the following possible explanations: first of all, the replication of the HSV vectors in these tumor cells in vitro is not affected by IFN, even at high dose (Liu et al, unpublished); secondly, it is possible that different MOIs used in these studies contributed to different results. It is also possible that different virus species have different IFN signaling responses with TSA. For instance, in the same report, TSA did not inhibit Newcastle disease virus (NDV) viral RNA and protein production [42].
Strategies combining virotherapy and antiangiogenic approach has shown promise in various preclinical models. For instance, we have previously shown that oncolytic HSV armed with an antiangiogenic factor (platelet factor 4) enhanced antiangiogenic activities of oncolytic virus G47Δ (the same virus used in this study), which leads to enhanced efficacy [45]. Others have shown that oncolytic virus encoding antiangiogenic transgenes achieve significantly enhanced tumor inhibition [46, 47]. Combination therapy with oncolytic HSV and antiangiogenic agent also resulted in enhanced antitumoral efficacy [35, 48]. It will be interesting to test these strategies in future clinical trials.
In summary, we demonstrated that oncolytic HSV and TSA combination therapy enhances antitumoral efficacy, antiangiogenesis, and widens the therapeutic index. We also identified cyclin D1 blockade and VEGF inhibition as main mechanisms for antitumoral and antiangiogenic activities. Concurrent TSA-oncolytic HSV treatment is therefore expected to enhance clinical responses. Future directions include revealing the molecular details of enhanced cyclin D1 blockade, and determining if other agents that block cyclin D1 show similar interactions with oncolytic HSV.
Materials and Methods
Cells, viruses, and reagents
Human cancer cell lines U87 and T98 (both glioblastoma), SW480 (colorectal), MCF-7 (breast), HeLa (cervical), and African green monkey kidney cell line Vero were obtained from ATCC and grown in DMEM + 10% calf serum (CS). Normal human umbilical vascular endothelial cells (HUVEC) and human prostate epithelial cells (PrEC) were obtained from Cambrex (Walkersville, MD) and maintained as instructed. HUVEC were made quiescent by contact inhibition followed by fluid shear stress induction. Murine endothelial cell line Py-4-1 was provided by Victoria Bautch (University of North Carolina) and maintained in DMEM + 10% CS. Wildtype HSV (Strain F) and its derivatives G47Δ and d120 (provided by Neil DeLuca; University of Pittsburgh) were described before. G47Δ contains deletions in ICP6, γ34.5, and α47, and the promoter region of Us11, placing the late US11 gene under control of the immediate-early alpha47 promoter, which suppresses the reduced growth properties of gamma34.5-deficient mutants [49]. d120 contains 4.1 kilobases deletion in both copies of ICP4 [50]. d120-GFP, provided by Toshihiko Kuroda (Massachusetts General Hospital), has GFP inserted into ICP6 in d120. TSA was obtained from Sigma (St. Louis, MO) and dissolved in DMSO. Polyarginated monoclonal cyclin D1 antibody was kindly provided by Bernard Erlanger (Columbia University) [25].
Cell survival assay and Chou-Talalay analysis
Cells were seeded into 96-well plates at 5,000 – 10,000 cells/well. After 24 hours, cells were treated with virus or TSA at different doses. The doses of TSA tested in these cell lines ranged from 100,000 – 0.0169 ng/mL, and the doses of HSV tested in these cell lines ranged from 30 – 0.005 pfu/cell. 3-fold serial dilutions were used for all agents. The cells were incubated for a further 72 hrs, when a MTT assay (Sigma) was performed, and dose-response curves and ED50 (dose effective to achieve 50% cell killing) values for both agents were obtained. Experiments were repeated at least three times with each condition in triplicate. After ED50 values of both agents were obtained for each cell line, both agents were then added to cells in combinations in a ratio equaling the ratio of their ED50 values. Combined dose-response curves were fit to Chou-Talalay lines, and Chou-Talalay combination indices (CIs) were calculated for each fraction affected (Fa) using the equation CI = (D1/Dx1) + (D2/Dx2) + (D1)(D2)/[(Dx1)(Dx2)], where Dx1 and Dx2 are the TSA and HSV doses required to achieve a particular Fa, respectively, and D1 and D2 are the doses of the two agents combined required to achieve the same Fa [26]. For each Fa, CI values of 0.8 and 1.2 as cutoffs for synergy and antagonism, respectively. Synergistic interaction was further stratified into strong (CI < 0.2), moderate (0.2 ≤ CI < 0.5), or weak (0.5 ≤ CI < 0.8), and simplified as +++, ++, +, respectively. Antagonism was simplified as −, and additive interaction was simplified as +/−. No effect was defined when any of the agents did not show cytotoxicity at any of the tested doses. For experiments with cyclin D1 antibody, U87 cells were plated, treated with polyarginated monoclonal cyclin D1 antibody (0.03 mg/ml) overnight, followed by treatment with HSV and TSA as described above.
Infectivity assay
Tumor cells (U87, A549, T98, and SW480) were seeded into 12-well plates (1 × 105 cells/well). 24 hours later, cells were treated with TSA (100 ng/ml) for 6 hours, followed by infection with d120-GFP at 3 pfu/cell for 2 hours. 24 hours post-infection (pi), cells were observed for GFP-expression under a fluorescent microscope. Percentages of GFP-expressing cells were calculated for each condition by randomly selecting 3 high power fields.
Virus replication assays
Cells were seeded and treated with TSA as above, followed by virus infection at 1 pfu/cell for 2 hours. TSA concentration are nontoxic to cells when treatment is for 54 hours (data not shown). Cells and medium were harvested at 48 hours pi, processed and titered as described before [24]. Experiments were repeated at least three times with each condition in duplicate.
Western blotting
U87 and SW480 cells were seeded into 6-well plates (3 × 105 cells/well). 24 hours later, cells were treated with TSA (100 ng/ml) for 6 hours, followed by infection with different HSVs at 3 pfu/cell. Samples were harvested at 4 hours pi and subjected to SDS-PAGE, transferred to PVDF Plus membrane, and blotted overnight with antibody to cyclin D1 (Cell Signaling; Danver, MA; diluted 1: 1000) or anti-actin (Sigma; diluted 1: 1500). The membrane was then processed and developed as described before [24].
VEGF detection
U87 cells were treated with TSA and/or G47Δ as described above. Culture medium was collected at 12 hours pi and analyzed for VEGF concentration by ELISA (R&D Systems; Minneapolis, MN).
Efficacy studies
U87 cells were implanted into the flanks of 6–8 week-old nu/nu mice (1 × 106 cells/implantation). Once tumor size reached 50 – 100 mm3, the animals were randomized into 4 groups (n = 10/group): PBS, TSA, G47Δ, or combination. TSA (20 µg/kg) was given intraperitoneally daily for 12 days (the first day of injection counted as day 0), and intratumoral injections of G47Δ (3 × 105 pfu/injection) were given on days 1 and 4. Tumor volumes and survival were monitored 2–3 times a week. Animals were sacrificed according to institutional regulations.
A similar experiment was performed with SW480 tumors. Tumor-bearing mice were randomized into 4 groups (n = 7/group). TSA was given as above, and intratumoral injections of G47Δ were given on days 1, 4, and 7.
Intratumoral biological endpoint studies
U87 cells were implanted into the flanks of 6–8 week-old nu/nu mice (1 × 106 cells/implantation). Once tumor size reached 50 – 100 mm3, the animals were randomized as above (n = 10/group): G47Δ alone or G47Δ + TSA groups. Intratumoral injection of G47Δ (3 × 105 pfu) was given on day 1, and TSA was given as above. Tumors were harvested on days 4, 8, and 12.
Immunohistochemistry and microvessel counting
Snap-frozen tumors were subjected to sectioning and staining for von Willebrand factor (VWF) and microvessel density (MVD) obtained as described previously [24].
Statistical analysis
One-way ANOVA was used for statistical analysis.
Acknowledgement
Supported in part by NIH grant NS32677 (to RLM).
We thank Victoria Bautch for providing Py-4-1 cells, Neil DeLuca for providing virus d120, Toshihiko Kuroda for providing d120-GFP, and Bernard Erlanger for providing polyarginated monoclonal cyclin D1 antibody.
References
- 1.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 2.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 3.Liu TC, Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429–432. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- 4.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 5.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 6.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 7.He LZ, Tolentino T, Grayson P, Zhong S, Warrell RP, Jr, Rifkind RA, et al. Histone deacetylase inhibitors induce remission in transgenic models of therapy-resistant acute promyelocytic leukemia. J Clin Invest. 2001;108:1321–1330. doi: 10.1172/JCI11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 10.Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors--development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 11.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 12.Dowdy SC, Jiang S, Zhou XC, Hou X, Jin F, Podratz KC, et al. Histone deacetylase inhibitors and paclitaxel cause synergistic effects on apoptosis and microtubule stabilization in papillary serous endometrial cancer cells. Mol Cancer Ther. 2006;5:2767–2776. doi: 10.1158/1535-7163.MCT-06-0209. [DOI] [PubMed] [Google Scholar]
- 13.Qi H, Ratnam M. Synergistic induction of folate receptor beta by all-trans retinoic acid and histone deacetylase inhibitors in acute myelogenous leukemia cells: mechanism and utility in enhancing selective growth inhibition by antifolates. Cancer Res. 2006;66:5875–5882. doi: 10.1158/0008-5472.CAN-05-4048. [DOI] [PubMed] [Google Scholar]
- 14.Piacentini P, Donadelli M, Costanzo C, Moore PS, Palmieri M, Scarpa A. Trichostatin A enhances the response of chemotherapeutic agents in inhibiting pancreatic cancer cell proliferation. Virchows Arch. 2006;448:797–804. doi: 10.1007/s00428-006-0173-x. [DOI] [PubMed] [Google Scholar]
- 15.Archer SY, Meng S, Shei A, Hodin RA. p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci U S A. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakata S, Yoshida T, Horinaka M, Shiraishi T, Wakada M, Sakai T. Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene. 2004;23:6261–6271. doi: 10.1038/sj.onc.1207830. [DOI] [PubMed] [Google Scholar]
- 17.Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- 18.Kitazono M, Goldsmith ME, Aikou T, Bates S, Fojo T. Enhanced adenovirus transgene expression in malignant cells treated with the histone deacetylase inhibitor FR901228. Cancer Res. 2001;61:6328–6330. [PubMed] [Google Scholar]
- 19.Okada T, Uchibori R, Iwata-Okada M, Takahashi M, Nomoto T, Nonaka-Sarukawa M, et al. A Histone Deacetylase Inhibitor Enhances Recombinant Adeno-associated Virus-Mediated Gene Expression in Tumor Cells. Mol Ther. 2006;13:738–746. doi: 10.1016/j.ymthe.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Bieler A, Mantwill K, Dravits T, Bernshausen A, Glockzin G, Kohler-Vargas N, et al. Novel three-pronged strategy to enhance cancer cell killing in glioblastoma cell lines: histone deacetylase inhibitor, chemotherapy, and oncolytic adenovirus dl520. Hum Gene Ther. 2006;17:55–70. doi: 10.1089/hum.2006.17.55. [DOI] [PubMed] [Google Scholar]
- 21.Vigushin DM, Ali S, Pace PE, Mirsaidi N, Ito K, Adcock I, et al. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin Cancer Res. 2001;7:971–976. [PubMed] [Google Scholar]
- 22.Deroanne CF, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21:427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 23.Qian DZ, Kato Y, Shabbeer S, Wei Y, Verheul HM, Salumbides B, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12:634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 24.Liu TC, Zhang T, Fukuhara H, Kuroda T, Todo T, Canron X, et al. Dominant-negative fibroblast growth factor receptor expression enhances antitumoral potency of oncolytic herpes simplex virus in neural tumors. Clin Cancer Res. 2006;12:6791–6799. doi: 10.1158/1078-0432.CCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 25.Chen BX, Erlanger BF. Cell cycle inhibition by an anti-cyclin D1 antibody chemically modified for intracellular delivery. Cancer Lett. 2006;244:71–75. doi: 10.1016/j.canlet.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 27.Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene. 2006;25:1620–1628. doi: 10.1038/sj.onc.1209371. [DOI] [PubMed] [Google Scholar]
- 28.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu J, Colburn NH. Histone deacetylase inhibition down-regulates cyclin D1 transcription by inhibiting nuclear factor-kappaB/p65 DNA binding. Mol Cancer Res. 2005;3:100–109. doi: 10.1158/1541-7786.MCR-04-0070. [DOI] [PubMed] [Google Scholar]
- 30.Song B, Yeh KC, Liu J, Knipe DM. Herpes simplex virus gene products required for viral inhibition of expression of G1-phase functions. Virology. 2001;290:320–328. doi: 10.1006/viro.2001.1175. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Israel H, Kleinberger T. Adenovirus and cell cycle control. Front Biosci. 2002;7:d1369–d1395. doi: 10.2741/ben. [DOI] [PubMed] [Google Scholar]
- 32.Yasui M, Yamamoto H, Ngan CY, Damdinsuren B, Sugita Y, Fukunaga H, et al. Antisense to cyclin D1 inhibits vascular endothelial growth factor-stimulated growth of vascular endothelial cells: implication of tumor vascularization. Clin Cancer Res. 2006;12:4720–4729. doi: 10.1158/1078-0432.CCR-05-1213. [DOI] [PubMed] [Google Scholar]
- 33.Cinatl J, Jr, Michaelis M, Driever PH, Cinatl J, Hrabeta J, Suhan T, et al. Multimutated herpes simplex virus g207 is a potent inhibitor of angiogenesis. Neoplasia. 2004;6:725–735. doi: 10.1593/neo.04265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benencia F, Courreges MC, Conejo-Garcia JR, Buckanovich RJ, Zhang L, Carroll RH, et al. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther. 2005;16:765–778. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 35.Aghi M, Rabkin SD, Martuza RL. Angiogenic response caused by oncolytic herpes simplex virus-induced reduced thrombospondin expression can be prevented by specific viral mutations or by administering a thrombospondin-derived peptide. Cancer Res. 2007;67:440–444. doi: 10.1158/0008-5472.CAN-06-3145. [DOI] [PubMed] [Google Scholar]
- 36.Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 37.Maecker HL, Yun Z, Maecker HT, Giaccia AJ. Epigenetic changes in tumor Fas levels determine immune escape and response to therapy. Cancer Cell. 2002;2:139–148. doi: 10.1016/s1535-6108(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 38.Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer. 2003;3:30. doi: 10.1186/1471-2407-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magner WJ, Tomasi TB. Apoptotic and necrotic cells induced by different agents vary in their expression of MHC and costimulatory genes. Mol Immunol. 2005;42:1033–1042. doi: 10.1016/j.molimm.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Poon AP, Liang Y, Roizman B. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J Virol. 2003;77:12671–12678. doi: 10.1128/JVI.77.23.12671-12678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller CS, Danaher RJ, Jacob RJ. ICP0 is not required for efficient stress-induced reactivation of herpes simplex virus type 1 from cultured quiescently infected neuronal cells. J Virol. 2006;80:3360–3368. doi: 10.1128/JVI.80.7.3360-3368.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang HM, Paulson M, Holko M, Rice CM, Williams BR, Marie I, et al. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:9578–9583. doi: 10.1073/pnas.0400567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adusumilli PS, Chan MK, Chun YS, Hezel M, Chou TC, Rusch VW, et al. Cisplatin-Induced GADD34 Upregulation Potentiates Oncolytic Viral Therapy in the Treatment of Malignant Pleural Mesothelioma. Cancer Biol Ther. 2006;5 doi: 10.4161/cbt.5.1.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raki M, Kanerva A, Ristimaki A, Desmond RA, Chen DT, Ranki T, et al. Combination of gemcitabine and Ad5/3-Delta24, a tropism modified conditionally replicating adenovirus, for the treatment of ovarian cancer. Gene Ther. 2005;12:1198–1205. doi: 10.1038/sj.gt.3302517. [DOI] [PubMed] [Google Scholar]
- 45.Liu TC, Zhang T, Fukuhara H, Kuroda T, Todo T, Martuza RL, et al. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther. 2006;14:789–797. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Wong RJ, Chan MK, Yu Z, Ghossein RA, Ngai I, Adusumilli PS, et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res. 2004;10:4509–4516. doi: 10.1158/1078-0432.CCR-04-0081. [DOI] [PubMed] [Google Scholar]
- 47.Yoo JY, Kim JH, Kwon YG, Kim EC, Kim NK, Choi HJ, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15:295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 48.Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, et al. Effect of Tumor Microenvironment Modulation on the Efficacy of Oncolytic Virus Therapy. J Natl Cancer Inst. 2007 doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 49.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeLuca NA, McCarthy AM, Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]