Abstract
The pathophysiology of spinal cord injury (SCI) is characterized by the initial, primary injury followed by secondary injury processes in which oxidative stress is a critical component. Secondary injury processes not only exacerbate pathology at the site of primary injury, but also result in spreading of injuries to the adjacent, otherwise healthy tissue. The lipid peroxidation byproduct acrolein has been implicated as one potential mediator of secondary injury. In order to further and rigorously elucidate the role of acrolein in secondary injury, a unique ex vivo model is utilized to isolate the detrimental effects of mechanical injury from toxins such as acrolein that are produced endogenously following SCI. We demonstrate that: 1) acrolein-lys adducts are capable of diffusing from compressed tissue to adjacent, otherwise uninjured tissue; 2) secondary injury by itself produces significant membrane damage and increased superoxide production; and 3) these injuries are significantly attenuated by the acrolein scavenger hydralazine. Furthermore, hydralazine treatment results in significantly less membrane damage 2 hours following compression injury, but not immediately after. These findings support our hypothesis that, following SCI, acrolein is increased to pathologic concentrations, contributes significantly to secondary injury, and thus represents a novel target for scavenging to promote improved recovery.
Keywords: Acrolein, Hydralazine, Aldehyde, Lipid Peroxidation, Oxidative, Spinal Cord
INTRODUCTION
Following traumatic SCI, the initial, primary insult is only a portion of the total pathology. Mechanical injury sets into play a number of secondary injury processes that occur not only at the site of the initial primary injury, but also result in spreading of the injury to adjacent, otherwise uninjured tissues in the hours, days, and weeks following the initial insult. Therefore, inhibition of secondary injury processes may be one of the most important means of therapeutic intervention to prevent further degeneration of damaged tissue, preserve healthy tissue, and thus promote functional recovery. In vivo, secondary injury mechanisms are mediated by a number of factors, including ischemia-reperfusion injury, inflammation, glutamate excitotoxicity, elevation of intracellular calcium, activation of proteases and caspases, and oxidative stress (Braughler and Hall 1989; Hall 1996; Lucas et al. 2002; Kumar et al. 2004; Park et al. 2004). Mechanisms of oxidative stress include production of free radicals, depletion of glutathione by reactive aldehydes, and lipid peroxidation (LPO). In particular, oxidative stress is well-established to play a significant role in the pathophysiology of SCI and is a hallmark of secondary injury (Braughler and Hall 1989; Hall 1996; Kumar et al. 2004). However, conventional strategies targeting free radicals have largely failed to yield any effective treatment. Thus, a novel target for alleviating oxidative stress is highly warranted.
Acrolein is a highly reactive α,β-unsaturated aldehyde that is produced as a byproduct of both peroxidation of polyunsaturated fatty acids in cell membranes (Witz 1989; Esterbauer et al. 1991; Uchida 1999; O'Brien et al. 2005) and intracellular enzymatic oxidation of polyamine metabolites (Esterbauer, 1991; Seiler, 2000). Acrolein is also capable of stimulating the generation of free radicals and subsequent LPO, and its formation may thus be thought of as a bioamplification step (Adams and Klaidman 1993). In addition, acrolein readily forms conjugates with glutathione that are subsequently excreted, resulting in glutathione depletion and thus further impairing the endogenous antioxidant system (Witz 1989; Esterbauer et al. 1991; Ghilarducci and Tjeerdema 1995; Uchida 1999; Kehrer and Biswal 2000). Previous studies suggest that the reaction of acrolein with glutathione may represent bioactivation rather than detoxification, because the reaction product, GS-propionaldehyde, reacted with xanthine oxidase to produce superoxide more readily than did free acrolein (Adams and Klaidman 1993). Acrolein also reacts with proteins to form toxic adducts (Burcham et al. 2004; Kaminskas et al. 2004a; Burcham and Pyke 2006). Previous studies have demonstrated that acrolein is significantly increased following SCI ex vivo and in vivo (Luo et al. 2005a; Hamann et al. 2008) and is highly toxic to spinal cord tissue (Lovell et al. 2001; Shi et al. 2002; Peasley and Shi 2003; Luo and Shi 2004; Luo et al. 2005b; Luo and Shi 2005; Luo et al. 2005a). Luo et al. (2005a) detected increases in acrolein following SCI in vivo that began as early as 4 hours and persisted for at least seven days after injury, and acrolein may remain increased for even longer. In addition, it's half-life (hours to days) (Ghilarducci and Tjeerdema 1995) is significantly longer than the transient free radicals, which makes it a potentially better target for therapeutic intervention than free radicals. Previous studies have demonstrated that the antihypertensive drug hydralazine is capable of binding to and neutralizing acrolein (Burcham et al. 2000; Burcham et al. 2002; Kaminskas et al. 2004a, b) and acrolein-protein adducts (Burcham et al. 2004; Kaminskas et al. 2004a, b; Burcham and Pyke 2006) (Figure 1). Furthermore, hydralazine significantly attenuated acrolein-mediated cell death in cultured hepatocytes (Burcham et al. 2000; Burcham et al. 2004), in mice in vivo (Kaminskas et al. 2004b), and in PC12 cells (Liu-Snyder et al. 2006), as well as acrolein- and compression-induced injuries in spinal cord ex vivo (Hamann et al. 2008).
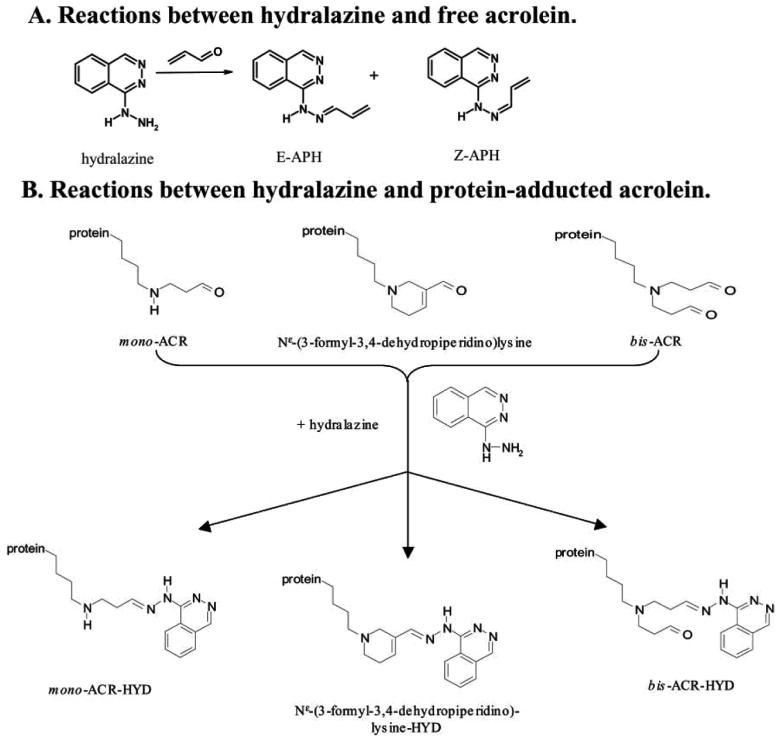
Figure 1. Chemistry of Reaction of Hydralazine and Acrolein.
This illustrates the chemistry of reaction of hydralazine with free acrolein (A) and protein-adducted acrolein (B). This figure is reproduced with permission from the original manuscript (Kaminskas et al, 2004b).
However, due to the limitations of previous experimental settings, it is still not clear whether endogenously generated acrolein reaches physiologically relevant concentrations. As a result, the relative contribution of acrolein to the pathophysiology of SCI has yet to be established. In order to address this issue, we developed a unique model to isolate the endogenous secondary injury processes from mechanical injury, and thus elucidate their relative contribution to the pathophysiology of spinal cord injury. This model allows us to evaluate the ability of endogenously generated compounds such as acrolein to damage the adjacent, otherwise healthy tissue. Therapeutic benefits of the acrolein scavenger hydralazine were also evaluated following injury. We have also presented evidence that endogenously generated compounds such as acrolein play a role in exacerbating tissue damage at the site of primary injury. This data strongly suggests that acrolein is one of the key factors in the secondary injury mechanisms, and thus represents a novel target for scavenging to promote functional recovery following traumatic SCI.
EXPERIMENTAL PROCEDURES
Isolation of Spinal Cord
The experimental protocols have been reviewed and approved by the Purdue University Animal Care and Use Committee (PACUC). Guinea pigs were housed and handled in accordance with PACUC guidelines. All efforts were made to minimize the number of animals used and their discomfort. Guinea pigs were sedated with an IP or IM injection of acepromazine (0.6 mg/kg). Anesthesia was induced by IM injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). Once animals were very deeply anesthetized (assessed by pinching the toe and/or abdominal musculature), they were perfused transcardially with approximately 500 ml of cold, oxygenated Kreb's solution (Concentration in mmol/L: 124 NaCl, 2 KCl, 1.24 KH2PO4, 1.3 MgSO4, 1.2 CaCl2, 10 glucose, 26 NaHCO3, and 10 ascorbic acid), prepared fresh daily. The vertebral column was then rapidly removed and a complete dorsal laminectomy was performed along the length of the vertebral column, exposing the spinal cord. The spinal cord was carefully removed and divided into 1 cm segments for each experiment. Modified Kreb's solution (same as above with ascorbic acid omitted and warmed to 37°C) was used for preparing all other solutions unless otherwise specified. Control spinal cord segments were incubated in modified Kreb's solution only. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
Isolating Secondary Injury from Mechanical Injury
In this model, a segment of spinal cord was compressed approximately 95% with forceps and then incubated in 2 ml of modified Kreb's solution (with or without hydralazine added). A compressed and an uncompressed cord were incubated together in the same solution to allow toxic compounds that are produced as a result of compression injury to diffuse to the uncompressed cord (Figure 2A). Thus, in the uncompressed cord, there is no mechanical injury, and the only injury is the result of endogenous toxins that are released into solution from the compressed cord. The uncompressed cord was used for analysis unless otherwise specified. For controls, identical segments of spinal cord were incubated in the same volume of modified Kreb's solution (with or without hydralazine added) for the same length of time. Thus, there were 4 different treatment groups: control (modified Kreb's solution), hydralazine (modified Kreb's solution with hydralazine added at a concentration of 500 μmol/L 15 minutes after the start of incubation), secondary injury (modified Kreb's solution), and secondary injury plus hydralazine (modified Kreb's solution with hydralazine added at a concentration of 500 μmol/L 15 minutes after the start of incubation).
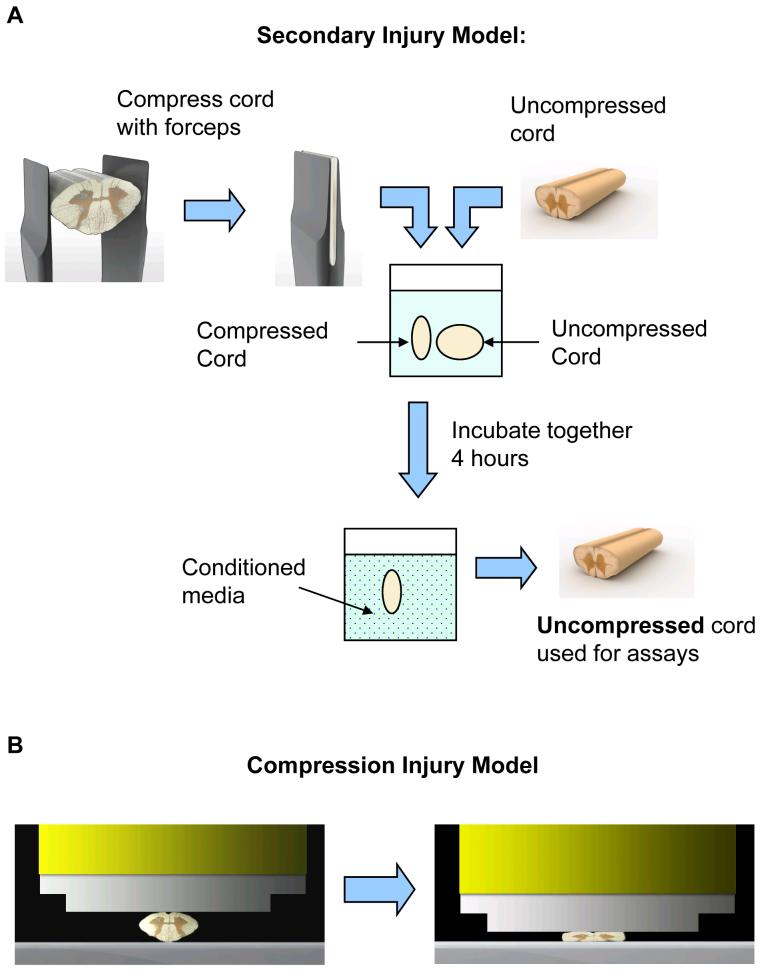
Figure 2. Injury models.
A. In the unique model of secondary injury, this illustrates the methods that were used to isolate secondary injury from mechanical injury. In this model, endogenous toxins diffuse from the compressed form, forming a “conditioned media,” in which an otherwise uninjured spinal cord segment is being bathed.
B. In the compression injury model, this illustrates the compression rod that was used to induce compression injury in isolated spinal cord. The ventral side of the spinal cord was on top, and the spinal cord was compressed 70% (i.e., to 30% its original diameter) at rate of 5 mm/s.
Lactate Dehydrogenase Release
Membrane damage was assessed by lactate dehydrogenase (LDH, 140 kD) release. LDH is an intracellular enzyme that is normally present at low concentrations in the extracellular fluid and is only released from cells whose membranes have been injured. LDH release from spinal cord was measured similarly to previously described techniques (Luo et al. 2002; Luo and Shi 2004). Segments were incubated for three hours at 37°C in each treatment group. The compressed cords were then removed, samples rinsed three times, and then incubated in fresh solution for one additional hour at 37°C in modified Kreb's solution to allow LDH to leak out of any membrane breaches that were produced in each of the treatment groups. A small volume (200 μl) of the solution bathing the segments was then removed, and levels of LDH released were assayed using the TOX-7 kit (Sigma-Aldrich, St. Louis, MO, USA).
Superoxide Production
Superoxide production was detected by dihydroethidium (HE) (Invitrogen, Carlsbad, CA, USA) (Bindokas et al. 1996; Tarpey et al. 2004). Segments of spinal cord were incubated for 4 hours at 37°C in each treatment group. After 4 hours, spinal cord segments were incubated in 10 μmol/L HE (prepared fresh daily in modified Kreb's from 5 mmol/L stock in DMSO) for 30 minutes. Spinal cord segments were then fixed for 2 hours in 4% paraformaldehyde in phosphate buffer (PB), prepared fresh daily. Segments were then embedded in Tissue-Tek OCT compound (VWR, Batavia, IL, USA), frozen in liquid nitrogen, and stored at −80°C for up to one week. Sections were cut at 50 μm on a cryostat and coverslipped with Immu-Mount (Thermo Electron, Waltham, MA, USA). Sections were visualized by epi-fluorescence on an Olympus BX61 microscope with a standard rhodamine cube (excitation filter: BP545, emission filter: LP590, Olympus, Center Valley, PA, USA), fluorescence intensity of grey matter quantified using Image J (NIH, Bethesda, MD, USA), and averaged for 5 sections randomly selected from the center of each spinal cord segment.
Detection of Acrolein-Lys Adducts by Immunohistochemistry
Acrolein-lys adducts were detected by immunohistochemistry, similarly to previously described methods (Calingasan et al. 1999; Luo et al. 2005a; Shen et al. 2005; Hamann et al. 2008). Briefly, spinal cord segments were incubated for 4 hours at 37°C in each treatment group. Segments of spinal cord were then fixed for 18 hours in 4% paraformaldehyde in PB, prepared fresh daily. Segments were cryoprotected in 10% sucrose (in PB) for 18 hours, followed by 20% sucrose (in PB) for 24 hours. Segments were then embedded in Tissue-Tek OCT compound (VWR, Batavia, IL, USA), frozen in liquid nitrogen, and stored at −80°C for up to one month. Sections were cut at 15 μm on a cryostat, mounted on gelatin-coated slides, and then post-fixed in a 50/50 (v/v) solution of methanol/acetone at −20°C for 15 minutes. Sections were permeabilized in 0.3% Triton X-100 (in PBS) for 30 minutes. Antigen retrieval was performed in citrate buffer (10 mM trisodium citrate, 0.05% Tween 20, pH 6.0) heated to 95°C for 20 minutes. Citrate buffer was then allowed to cool for 10 minutes at room temperature. Endogenous peroxidase activity was quenched for 5 minutes in 3% hydrogen peroxide (in PBS). Sections were blocked in 5% goat serum (in PBS) for 1 hour and transferred to 1:250 polyclonal rabbit anti-acrolein (in PBS with 2% goat serum, 0.1% sodium azide) (Novus Biologicals, Littleton, CO, USA) for 18 hours. Slides were then incubated for 2 hours in 1:400 biotinylated goat anti-rabbit IgG (in PBS) (Vector Laboratories, Burlingame, CA, USA) followed by 2 hours in 1:5000 peroxidase-labeled streptavidin (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA). Sections were transferred to 0.024% diaminobenzidine and 0.006% hydrogen peroxide (in 0.05 M Tris-HCl, pH 7.6) for approximately 12 minutes. The reaction was stopped by transferring slides to 0.05 M Tris-HCl. Slides were dehydrated in a graded series of ethanol followed by xylene and then coverslipped with DPX. Sections were visualized on an Olympus BX61 microscope. Density of staining was quantified using Image J (NIH, Bethesda, MD, USA) and averaged for 3 sections randomly selected from the center of each spinal cord segment. Measurements are expressed as % control values ± SD. For negative controls, anti-acrolein antibody was omitted (data not shown).
Detection of Acrolein-Lysine Adducts by Immunoblotting
Acrolein-lys adducts in the solution bathing tissue was measured using a Bio-Dot SF Microfiltration Apparatus (Bio-Rad, Hercules, CA, USA), similarly to previously described methods (Li et al. 2004; Luo et al. 2005a; Shao et al. 2006). Briefly, the solution bathing tissue was collected, and the following anti-proteases were added: 2 mmol/L pefabloc, 15 μmol/L pepstatin A, 20 μg/ml aprotinin, and 25 μg/ml leupeptin. The solution was then centrifuged to pellet any large pieces of tissue and the supernatant stored at −80°C. This supernatant (200 μl) was transferred to a nitrocellulose membrane. The membrane was blocked for 1 hour in blocking buffer (0.2% casein and 0.1% Tween 20 in PBS). The membrane was then transferred to 1:1000 polyclonal rabbit anti-acrolein (in blocking buffer with 2% goat serum, 0.025% sodium azide) (Novus Biologicals, Littleton, CO, USA) for 18 hours at 4°C. The membrane was washed in blocking buffer and then transferred to 1:10,000 alkaline phosphatase conjugated goat anti-rabbit IgG. The membrane was washed in blocking buffer followed by 0.1% Tween 20 in tris-buffered saline. The membrane was then exposed to Bio-Rad Immuno-Star Substrate (Bio-Rad, Hercules, CA, USA) and visualized by chemiluminescence. Density of bands was evaluated using Image J (NIH, Bethesda, MD, USA) and is expressed as % control values ± SD.
Typically in immunoblots, the total protein of samples is quantified, and a known quantity of total protein is then loaded into the wells. This model is unique however, and as such it is not necessary or meaningful to quantify the total protein in the samples. In this model, the actual concentration of acrolein-modified protein in the fluid bathing the tissue is meaningful, because that is the concentration of acrolein-modified protein to which the tissue is exposed. Thus, the total protein in the fluid is irrelevant.
Compression Injury
In addition to the secondary injury model, the effects of mechanical injury were also evaluated (Figure 2B). One cm segments were isolated as described and incubated in Kreb's solution for at least one hour to allow for recovery of injury resulting from tissue isolation (Shi and Blight 1996; Shi and Pryor 2000). Compression injury was produced by 70% strain at a constant rate of 5 mm/s with the ventral side of the spinal cord on top. Applied force and vertical displacement were simultaneously measured to ensure a uniform, standardized, and repeatable injury.
Exclusion of Tetramethyl Rhodamine Dextran
Membrane permeability was measured by exclusion of the hydrophilic dye tetramethyl rhodamine dextran (TMR, 10 kD) (Invitrogen, Carlsbad, CA). Briefly, segments of spinal cord were incubated for 2 hours at 37°C in one of the following groups: control (modified Kreb's solution), hydralazine (modified Kreb's solution with hydralazine added at a concentration of 500 μmol/L 15 minutes after the start of incubation), compression (modified Kreb's solution), and compression plus hydralazine (modified Kreb's solution with hydralazine added at a concentration of 500 μmol/L 15 minutes after the start of incubation). At the end of 2 hours, spinal cord segments were transferred to 0.01% lysine fixable TMR and incubated for 15 minutes. Spinal cord segments were then fixed in 4% paraformaldehyde in PB, prepared fresh daily, for 2 hours. Segments were then embedded in Tissue-Tek OCT compound (VWR, Batavia, IL, USA), frozen in liquid nitrogen, and stored at −80°C for up to one month. Sections were cut at 50 μm on a cryostat and coverslipped with Immu-Mount (Thermo Electron, Waltham, MA, USA). Sections were visualized by epi-fluorescence on an Olympus BX61 microscope with a standard rhodamine cube (excitation filter: BP545, emission filter: LP590, Olympus, Center Valley, PA, USA), grey matter fluorescence quantified using Image J (NIH, Bethesda, MD, USA), and averaged for 5 sections randomly selected from the center of each spinal cord segment.
Statistical Analysis
Unless otherwise specified, one-way ANOVA and Post Hoc Newman Keul's test were used for statistical analyses (InStat). Normal distribution was tested by the Kolmogorov-Smirnov Test, and equality of variances was tested by the method of Bartlett. Results are expressed as mean ± SD. P<0.05 was considered statistically significant.
RESULTS
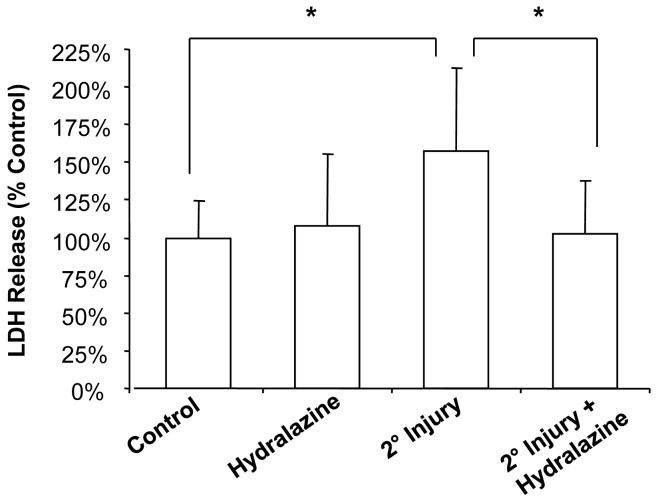
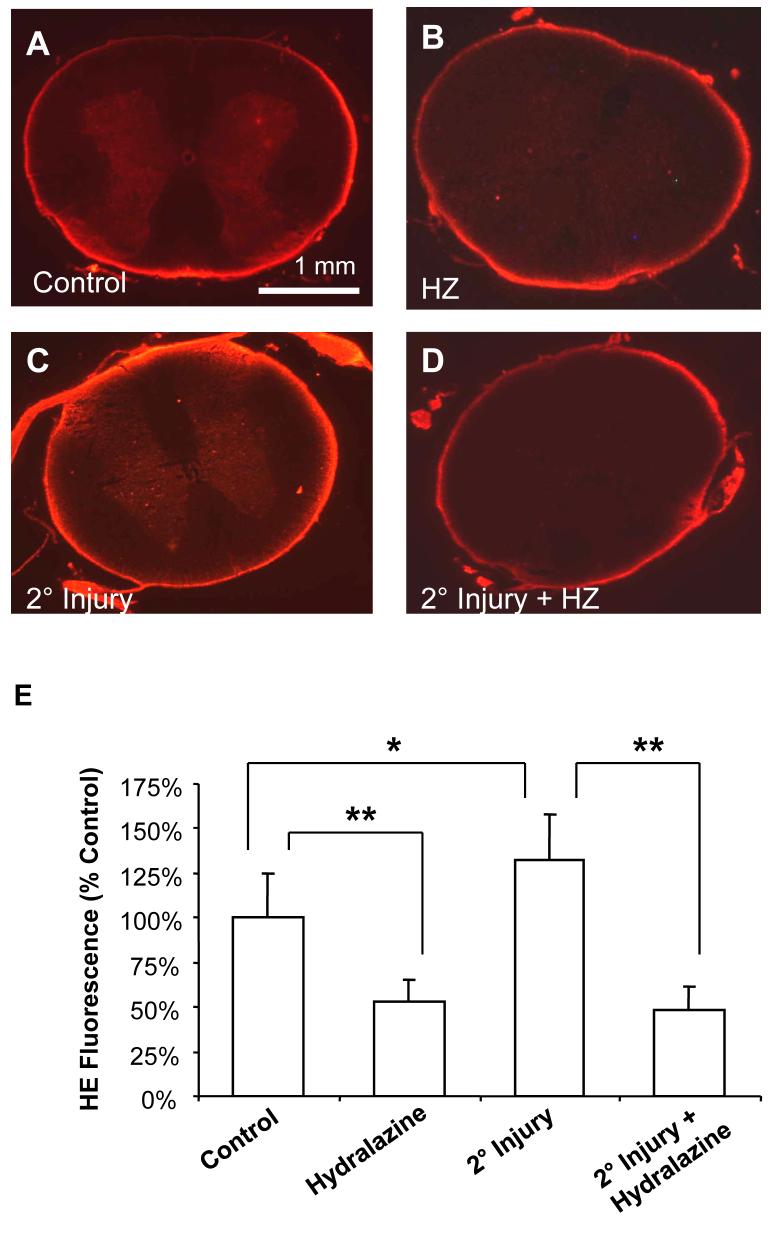
In the secondary injury model described in the methods (Figure 2A), incubation with the compressed cord resulted in significant membrane damage and increased superoxide production in spinal cord that did not receive mechanical injury (i.e., the only injury was from incubation with toxic compounds that were produced by the compressed cord). Specifically, LDH release was increased from 100 ± 24.8% to 157.2 ± 55.3% of controls (P < 0.05) as a result of secondary injury (Figure 3). Treatment with the acrolein scavenger hydralazine decreased LDH release in injured cords to 102.6 ± 35.2% of controls (P < 0.05 compared to injury only) (Figure 3). Hydralazine treatment did not have a significant effect on uninjured spinal cord (108.1 ± 47.3% of controls, P > 0.05) (Figure 3). Similarly, following secondary injury, superoxide production was increased from 100 ± 24.9% to 137.2 ± 25.5% of controls (P < 0.05) (Figure 4). Hydralazine treatment significantly decreased superoxide production in both uninjured (53.0 ± 12.5% of controls, P < 0.001) and injured (47.8 ± 13.6% of controls, P < 0.001) spinal cord (Figure 4).
Figure 3. Membrane permeability to LDH following secondary injury.
Membrane permeability was assessed by release of LDH (140 kD), an intracellular enzyme that leaks out of damaged cells. Spinal cord segments were incubated for 3 hours in each of the treatment groups. The compressed cords were removed and the remaining segments rinsed and placed in fresh medium. After one additional hour, samples were taken from the medium bathing spinal cord and assayed for LDH using the TOX-7 kit. Results are expressed as % control values ± SD (n = 6). Secondary injury significantly increased permeability to LDH, an effect that was attenuated by hydralazine. One-way repeated measures ANOVA and Post Hoc Newman Keul's test were used for statistical analysis. *P<0.05.
Figure 4. Superoxide production following secondary injury.
Superoxide production was detected by increased fluorescence following staining with HE. Representative images are shown for A) Control, B) 500 μM hydralazine (HZ), C) secondary injury, or D) secondary injury plus 500 μM hydralazine. Notice the increased fluorescence intensity following secondary injury (C). This effect is most obvious in the grey matter, and is reduced by treatment with hydralazine (B, D). Sub-pial fluorescence can probably be attributed to glial cells as well as artifactual oxidation of HE. E) Fluorescence was quantified using Image J (NIH), and is expressed as percent control values ± SD (n = 6). One-way ANOVA and Post Hoc Newman Keul's test were used for statistical analysis. *P<0.05, ** P<0.001 (compared to control).
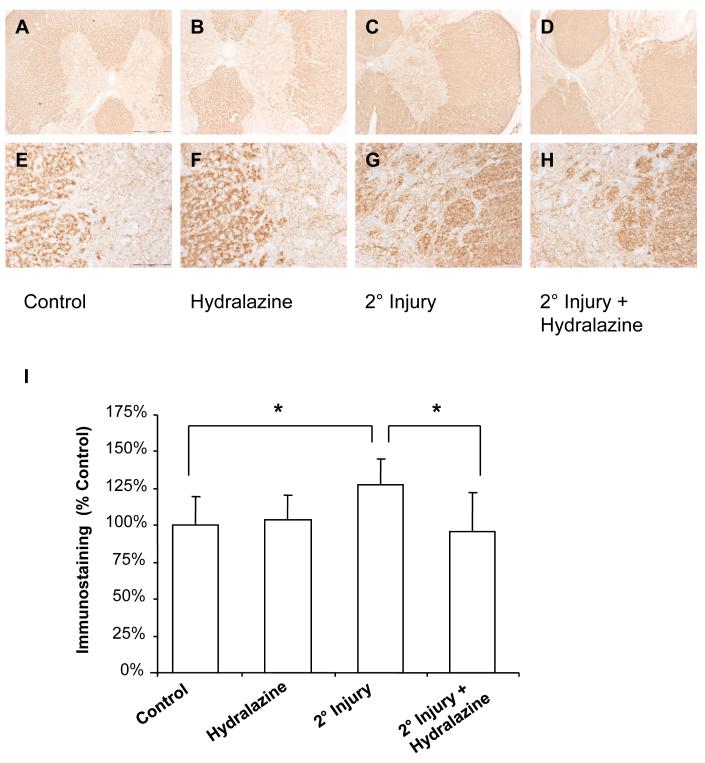
In order to demonstrate an increase in acrolein-lys adducts following secondary injury, immunohistochemistry was performed in the tissue that did not receive mechanical injury, but was incubated with compressed tissue (Figure 5). Histological examination revealed diffuse staining of tissue for acrolein-lys adducts, especially in the white matter. Specifically, secondary injury increased immunostaining for acrolein-lys adducts from 100.0 ± 19.4% to 127.6 ± 17.8%% of controls (P < 0.05). Hydralazine treatment in injured cords resulted in anti-acrolein immunostaining of 95.9 ± 26.2% of controls (P < 0.05 compared to injury only). Hydralazine did not have a significant effect in uninjured tissue (103.5 ± 17.4% of controls, P > 0.05).
Figure 5. IHC for acrolein-lys adducts.
Acrolein-lys adducts were detected by immunohistochemistry. Representative images are shown (A-H). Scale bar = 500 μm for A-D, and 100 μm for E-H. Staining intensity was quantified using Image J (NIH) and is expressed as % control values ± SD (n = 4) (I). Secondary injury resulted in a slight, but statistically significant, increase in immunostaining. One-way repeated measures ANOVA and Post Hoc Newman Keul's test were used for statistical analysis. *P<0.05.
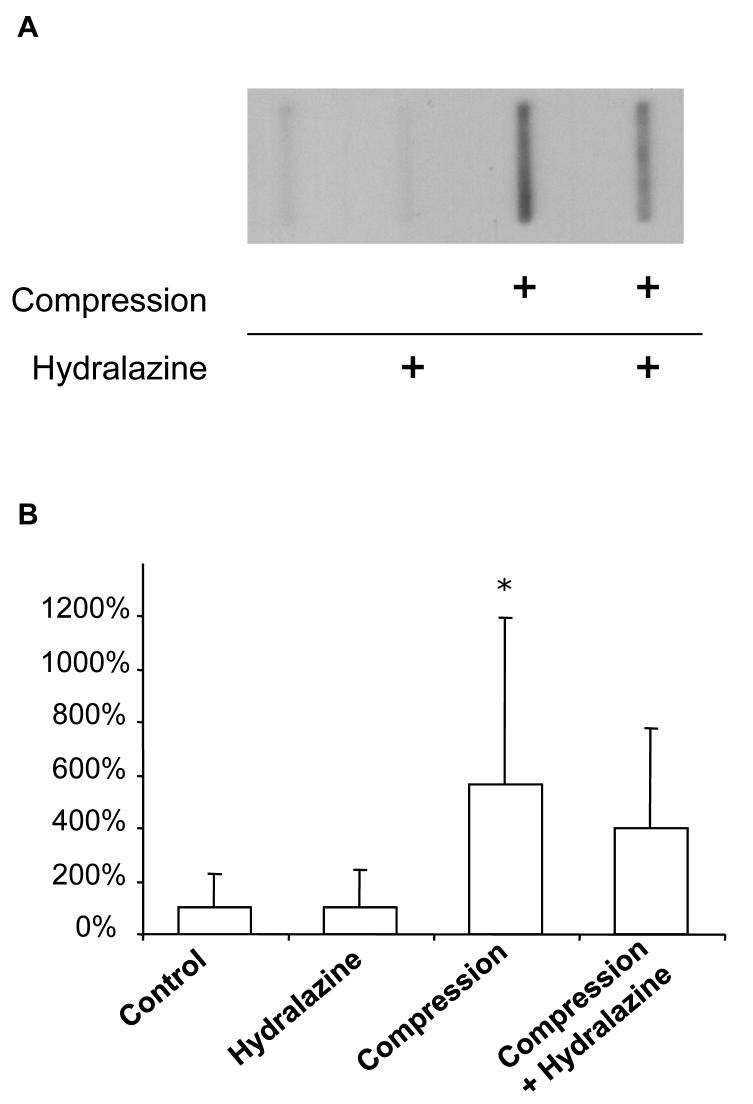
We also evaluated the concentration of acrolein-lys adducts in the medium bathing the tissue (Figure 6), the only route by which acrolein could have diffused from compressed tissue to uncompressed tissue in this model. As expected, acrolein-lys adducts were also significantly increased in the solution bathing the tissue. Specifically, relative density of bands from compressed tissue was 564.1% of controls (P < 0.05). Hydralazine treatment tended to decrease acrolein-lys adducts in compressed cords (399.6% controls), an effect that did not reach statistical significance (P > 0.05, compared to compression only).
Figure 6. Immunoblotting for acrolein-lys adducts.
Acrolein-lys adducts were detected in the Kreb's solution bathing tissue using a Bio-Dot SF Microfiltration Apparatus. A representative blot is shown (A). Relative densities were quantified using Image J (NIH) and expressed as % control values ± SD (n = 8) (B). Acrolein-lys adducts were significantly increased in Kreb's solution following compression injury, an effect that tended to be reduced by hydralazine treatment. Oneway repeated measures ANOVA and Post Hoc Newman Keul's test were used for statistical analysis. *P<0.05.
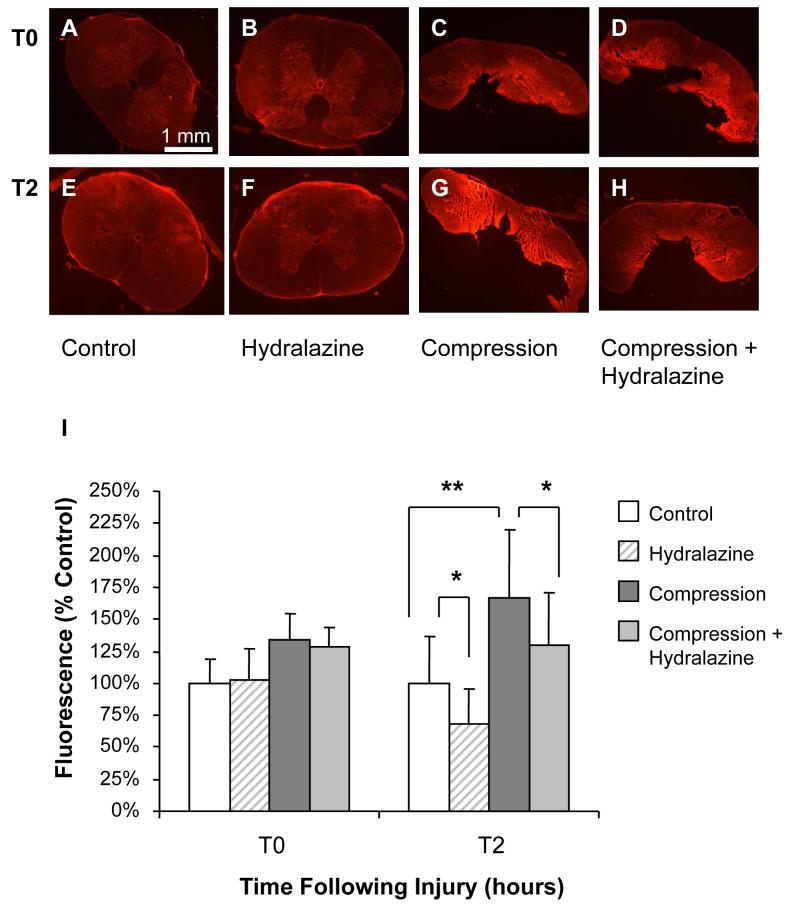
Compression resulted in a significant increase in membrane permeability to TMR 2 hours after injury (Figure 7). Specifically, fluorescence intensity increased from 100.0 ± 37.1% to 166.2 ± 53.8% of controls (P < 0.001). Hydralazine treatment in compressed spinal cord significantly reduced fluorescence intensity to 129.2 ± 41.0% of controls (P < 0.05 compared to compression only) 2 hours following compression. Fluorescence intensity of uninjured spinal cord was also reduced to 67.9 ± 28.0% of controls following 2 hours in hydralazine (P < 0.05). However, immediately after injury, hydralazine treatment had no effect on permeability to TMR in compressed or uncompressed cords (P > 0.05), which suggests hydralazine does not lessen the membrane damage as a result of primary injury nor directly interfere with dye uptake. This is consistent with our hypothesis that hydralazine acts to protect against acrolein-mediated secondary membrane damage.
Figure 7. Permeability to TMR following compression injury.
Membrane integrity following compression was assessed using tetramethyl rhodamine dextran (TMR, 10 kD), a hydrophilic dye that is excluded from cells with an intact membrane. Representative images are shown at 2 hours (E-H) and immediately after compression injury (A-D). Notice the increase in fluorescence intensity in compressed spinal cord compared to controls. Fluorescence was significantly reduced by treatment with hydralazine at 2 hours in both compressed and uninjured spinal cord. Immediately following injury, hydralazine had no effect on fluorescence. Fluorescence intensity was quantified using Image J (NIH) and is expressed as % control values ± SD (n = 5) (I). One-way repeated measures ANOVA and Post Hoc Newman Keul's test were used for statistical analysis. *P<0.05, ** P<0.001.
DISCUSSION
It is well established that the overall pathophysiology of spinal cord injury consists of the initial primary insult, which is characterized by mechanical injury, as well as a number of secondary injuries, which are largely mediated by biochemical mechanisms and oxidative stress. The observation that the spinal cord continues to degenerate following the initial insult, and that the lesion spreads to adjacent, otherwise uninjured tissue, supports the hypothesis that secondary injury contributes significantly to the pathophysiology of SCI. However, the mechanisms and lesions of primary and secondary injury are difficult to distinguish both spatially and temporally, and it is thus difficult to assess their relative contributions to the total pathology. In attempts to overcome this problem many studies focus on characterizing a particular mechanism of secondary injury and then evaluating its effect on healthy and injured tissues. However, one inherent limitation of models that introduce exogenous secondary injury mechanisms is the degree of relevance of the “artificial” secondary injury to the endogenous injury.
The current model is unique, however, in that it isolates endogenous secondary injury mechanisms from the primary injury, and demonstrates that secondary injury alone can create significant pathology that is distinct from the effects of mechanical injury. This model also provides evidence that endogenously generated acrolein is capable of diffusing to and injuring the otherwise healthy tissue. This is based on the findings that: 1) acrolein-lys adducts are significantly increased in mechanically-injured tissue (Luo et al. 2005a; Hamann et al. 2008); 2) acrolein-lys adducts are significantly increased in the solution that baths the traumatized tissue, as well as uncompressed tissue that is bathed in the same solution (Figures 5 and 6); and 3) acrolein generated in this model can create additional damage in the nearby healthy tissues (Figure 3 and 4) as well as the mechanically injured tissue (Figure 7). Based on the diffuse staining of tissue for acrolein-lys adducts (Figure 5) as well as the increase in acrolein-lys adducts in the medium (Figure 6), we also propose that acrolein generated as a result of primary injury is capable of diffusing through the extracellular fluid, a plausible avenue for acrolein to damage the adjacent healthy tissue in vivo. This provides an explanation for previous findings that, following SCI in vivo, acrolein was increased not only at the injury site, but in the adjacent tissue as well (Luo et al. 2005a).
One key evidence in demonstrating that acrolein contributes significantly to secondary injury is that treatment with the acrolein scavenger hydralazine significantly attenuates secondary injury (Figures 3 and 4). This correlates well with our findings that the acrolein scavenger hydralazine prevents increases in acrolein-protein adducts following secondary injury (Figures 5 and 6). We previously demonstrated that hydralazine was not an efficient scavenger of superoxide at the concentrations used in this study and had no direct effect on recovery of membrane potential following transection (Hamann et al. 2008). Thus, its mechanism of protection against compression-mediated superoxide production and membrane damage is likely through its acrolein scavenging activity.
Our data have also provided evidence that, in addition to damaging the adjacent healthy tissue, endogenous toxins such as acrolein begin to accumulate and reach concentrations which exacerbate the pathology at the site of the initial, primary injury. For example, we have shown that hydralazine-mediated protection against compression-induced membrane damage was evident at 2 hours after injury, but not immediately after (Figure 7). This is consistent with previous studies that found hydralazine treatment resulted in improved membrane integrity at 2 and 3 hours following compression; but not 1 hour after injury (Hamann et al. 2008). Based on this data, we suggest that the increase in membrane permeability at less than 2 hours after ex vivo injury can be largely attributed to the effects of the primary mechanical injury, while after 2 hours, secondary injury processes are probably producing additional damage. Thus, the proposed mechanism by which hydralazine protects compressed spinal cord is by targeting secondary injury processes and preventing acrolein-mediated injury. The available evidence also supports the hypothesis that hydralazine does not act to directly repair the primary, mechanical injury, for example by resealing damaged membranes. Previous studies have found that damaged neuronal membranes are capable of being repaired by endogenous mechanisms (Xie and Barrett 1991; Shi and Blight 1996; Eddleman et al. 1997; Shi and Pryor 2000; Shi and Pryor 2002; Shi and Whitebone 2006). Therefore, preventing oxidative stress and secondary degeneration likely creates a more favorable environment to allow these endogenous repair mechanisms to occur.
As detailed in the introduction, there are a number of secondary injury mechanisms following SCI, and it is most likely that other endogenous toxins, in addition to acrolein, are produced in this model. It fact, the combination of endogenous toxins produced may be significantly more toxic than any of those compounds by themselves. Other compounds that may contribute to the pathology in this model include reactive oxygen species, reactive nitrogen species, and other reactive aldehydes including malondialdehyde (MDA) and 4-hydroxynonenal (HNE). It is important to consider that many of these compounds, such as the hydroxyl and peroxynitrate radical, are extremely short lived and highly reactive, and thus unlikely to avoid reacting with other compounds and diffuse to the uncompressed tissue (Sayre et al. 2008). This possibility is more likely with less reactive compounds such as superoxide, hydrogen peroxide, and reactive aldehydes (Hall and Braughler 1989; Sayre et al. 2008). Among the reactive aldehydes, acrolein has been found to be far more reactive than MDA or HNE with biomolecules including proteins and glutathione. Acrolein's half-life, on the order of hours to days (Ghilarducci and Tjeerdema 1995), is also much longer than free radicals, which makes it a better candidate to be able to diffuse away from the injury site to damage adjacent tissue. It is for these reasons that aldehydes and acrolein in particular may have the potential to be more detrimental than free radicals, and this study focused on acrolein scavenging.
It is important to point out some limitations for use of hydralazine in vivo. Specifically, hydralazine is a vasodilator, which would be an undesirable effect following SCI, where the subject would likely be in neurogenic shock. In addition, it is unlikely to reach therapeutic concentrations in vivo (Hamann et al. 2008). A previous study by Wood, et.al. (Wood et al. 2006) provided evidence that phenelzine protected again ischemia-reperfusion brain injury in gerbils in vivo and was probably an acrolein scavenger. Phenelzine is not a vasodilator, can be safely administered at higher doses than hydralazine, and thus has greater potential for use following SCI in vivo. Our lab is also currently working to design a safer and more effective scavenger that does not have the vasodilatory effects of hydralazine. Hydralazine was selected for these studies because the nature of reaction with acrolein and acrolein-modified protein is efficient and well characterized (Burcham et al. 2000; Burcham et al. 2002; Burcham et al. 2004; Kaminskas et al. 2004a, b; Burcham and Pyke 2006) (Figure 1). In spite of hydralazine's limitations for in vivo use, these studies provide valuable proof-of-principle that acrolein scavenging may be a novel means to attenuate oxidative stress and thus promote improved recovery following SCI. In addition, the findings that hydralazine completely prevented the membrane damage and superoxide production induced by this secondary injury model suggests that acrolein plays a pivotal role in triggering secondary injury. In summary, this study supports the hypothesis that acrolein is increased to pathologic concentrations following compression-mediated injuries, and therefore constitutes a key factor in secondary injury mechanisms.
ACKNOWLEDGEMENTS
This research was supported by NIH T32 Training Grant #RR017502-01A2, Merck-Merial Summer Research Fellowship, the Center for Paralysis Research, and the Department of Basic Medical Sciences at Purdue University. Special thanks to Dr. Xun Wang, Dr. Louise Abbott, Gary Leung, Brandi Butler, and Sonya Eden for their invaluable assistance. Thanks also to Drs. Brad Duerstock, Richard Borgens, and Kevin Hannon for the generous use of their equipment. We also thank Michel Schweinsberg for his efforts with illustrations.
Abbreviations used
- HE
dihydroethidium
- HNE
4-hydroxynonenal
- LPO
lipid peroxidation
- LDH
lactate dehydrogenase
- MDA
malondialdehyde
- PB
phosphate buffer
- PBS
phosphate buffered saline
- SCI
Spinal cord injury
- TMR
tetramethyl rhodamine dextran
REFERENCES
- Adams JD, Jr., Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic. Biol. Med. 1993;15:187–193. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braughler JM, Hall ED. Central nervous system trauma and stroke I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic. Biol. Med. 1989;6:289–301. doi: 10.1016/0891-5849(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol. Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–49. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, Pyke SM. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology. 2002;181-182:229–236. doi: 10.1016/s0300-483x(02)00287-1. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Fontaine FR, Kaminskas LM, Petersen DR, Pyke SM. Protein adduct-trapping by hydrazinophthalazine drugs: mechanisms of cytoprotection against acrolein-mediated toxicity. Mol. Pharmacol. 2004;65:655–664. doi: 10.1124/mol.65.3.655. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer's disease. J. Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- Eddleman CS, Ballinger ML, Smyers ME, Godell CM, Fishman HM, Bittner GD. Repair of plasmalemmal lesions by vesicles. Proc. Natl. Acad. Sci. USA. 1997;94:4745–4750. doi: 10.1073/pnas.94.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev. Environ. Contam. Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- Hall ED. Lipid peroxidation. Adv. Neurol. 1996;71:247–257. discussion 257-248. [PubMed] [Google Scholar]
- Hall ED, Braughler JM. Central nervous system trauma and stroke II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic. Biol. Med. 1989;6:303–313. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- Hamann K, Nehrt G, Ouyang H, Duerstock B, Shi R. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J. Neurochem. 2008;104:708–718. doi: 10.1111/j.1471-4159.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, Pyke SM, Burcham PC. Reactivity of hydrazinophthalazine drugs with the lipid peroxidation products acrolein and crotonaldehyde. Org. Biomol. Chem. 2004a;2:2578–2584. doi: 10.1039/B408796H. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, Pyke SM, Burcham PC. Strong protein adduct trapping accompanies abolition of acrolein-mediated hepatotoxicity by hydralazine in mice. J. Pharmacol. Exp. Ther. 2004b;310:1003–1010. doi: 10.1124/jpet.104.067330. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol. Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N. Robbins & Cotran Pathologic Basic of Disease. Elsevier; Philadelphia: 2004. [Google Scholar]
- Li H, Wang J, Kaphalia B, Ansari GA, Khan MF. Quantitation of acrolein-protein adducts: potential biomarker of acrolein exposure. J. Toxicol. Environ. Health A. 2004;67:513–524. doi: 10.1080/15287390490276539. [DOI] [PubMed] [Google Scholar]
- Liu-Snyder P, Borgens RB, Shi R. Hydralazine rescues PC12 cells from acrolein-mediated death. J. Neurosci. Res. 2006;84:219–227. doi: 10.1002/jnr.20862. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Lucas JH, Wheeler DG, Guan Z, Suntres Z, Stokes BT. Effect of glutathione augmentation on lipid peroxidation after spinal cord injury. J. Neurotrauma. 2002;19:763–775. doi: 10.1089/08977150260139138. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem. Int. 2004;44:475–486. doi: 10.1016/j.neuint.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem. Int. 2005;46:243–252. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Luo J, Borgens RB, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J. Neurochem. 2002;83:471–480. doi: 10.1046/j.1471-4159.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem. Res. 2005a;30:291–295. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- Luo J, Robinson JP, Shi R. Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem. Int. 2005b;47:449–457. doi: 10.1016/j.neuint.2005.07.002. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J. Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Peasley MA, Shi R. Ischemic insult exacerbates acrolein-induced conduction loss and axonal membrane disruption in guinea pig spinal cord white matter. J. Neurol. Sci. 2003;216:23–32. doi: 10.1016/s0022-510x(03)00201-6. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Seiler N. Oxidation of Polyamines and Brain Injury. Neurochem. Res. 2000;25:471–490. doi: 10.1023/a:1007508008731. [DOI] [PubMed] [Google Scholar]
- Shao C, Roberts KN, Markesbery WR, Scheff SW, Lovell MA. Oxidative stress in head trauma in aging. Free Radic. Biol. Med. 2006;41:77–85. doi: 10.1016/j.freeradbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Shen J, Yang X, Dong A, Petters RM, Peng YW, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- Shi R, Blight AR. Compression injury of mammalian spinal cord in vitro and the dynamics of action potential conduction failure. J. Neurophysiol. 1996;76:1572–1580. doi: 10.1152/jn.1996.76.3.1572. [DOI] [PubMed] [Google Scholar]
- Shi R, Pryor JD. Temperature dependence of membrane sealing following transection in mammalian spinal cord axons. Neuroscince. 2000;98:157–166. doi: 10.1016/s0306-4522(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Shi R, Pryor JD. Pathological changes of isolated spinal cord axons in response to mechanical stretch. Neuroscience. 2002;110:765–777. doi: 10.1016/s0306-4522(01)00596-6. [DOI] [PubMed] [Google Scholar]
- Shi R, Whitebone J. Conduction deficits and membrane disruption of spinal cord axons as a function of magnitude and rate of strain. J. Neurophysiol. 2006;95:3384–3390. doi: 10.1152/jn.00350.2005. [DOI] [PubMed] [Google Scholar]
- Shi R, Luo J, Peasley MA. Acrolein inflicts axonal membrane disruption and conduction loss in isolated guinea pig spinal cord. Neuroscience. 2002;115:337–340. doi: 10.1016/s0306-4522(02)00457-8. [DOI] [PubMed] [Google Scholar]
- Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R431–444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc. Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Witz G. Biological interactions of alpha,beta-unsaturated aldehydes. Free Radic. Biol. Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- Wood PL, Khan MA, Moskal JR, Todd KG, Tanay VA, Baker G. Aldehyde load in ischemia-reperfusion brain injury: neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Res. 2006;1122:184–190. doi: 10.1016/j.brainres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Xie X, Barrett JN. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca2+-triggered protease activity and cytoskeletal disassembly. J. Neurosci. 1991;11:3257–3267. doi: 10.1523/JNEUROSCI.11-10-03257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]