Abstract
OBJECTIVE
Interleukin (IL)-21 is a type 1 cytokine that has been implicated in the pathogenesis of type 1 diabetes via the unique biology of the nonobese diabetic (NOD) mouse strain. The aim of this study was to investigate a causal role for IL-21 in type 1 diabetes.
RESEARCH DESIGN AND METHODS
We generated IL-21R–deficient NOD mice and C57Bl/6 mice expressing IL-21 in pancreatic β-cells, allowing the determination of the role of insufficient and excessive IL-21 signaling in type 1 diabetes.
RESULTS
Deficiency in IL-21R expression renders NOD mice resistant to insulitis, production of insulin autoantibodies, and onset of type 1 diabetes. The lymphoid compartment in IL-21R−/− NOD is normal and does not contain an increased regulatory T-cell fraction or diminished effector cytokine responses. However, we observed a clear defect in autoreactive effector T-cells in IL-21R−/− NOD by transfer experiments. Conversely, overexpression of IL-21 in pancreatic β-cells induced inflammatory cytokine and chemokines, including IL-17A, IL17F, IFN-γ, monocyte chemoattractant protein (MCP)-1, MCP-2, and interferon-inducible protein-10 in the pancreas. The ensuing leukocytic infiltration in the islets resulted in destruction of β-cells and spontaneous type 1 diabetes in the normally diabetes-resistant C57Bl/6 and NOD × C57Bl/6 backgrounds.
CONCLUSIONS
This work provides demonstration of the essential prodiabetogenic activities of IL-21 on diverse genetic backgrounds (NOD and C57BL/6) and indicates that IL-21 blockade could be a promising strategy for interventions in human type 1 diabetes.
The nonobese diabetic (NOD) mouse model is the most well-characterized animal model of human type 1 diabetes and has provided important insights into the etiology and pathogenesis of this increasingly prevalent autoimmune disease (1). Rigorous genetic analysis of the NOD background has revealed the existence of multiple defined chromosomal regions known as insulin-dependent diabetes (idd) loci that confer susceptibility to or protection from the development of type 1 diabetes (2). Of the ∼15 regions identified, idd3 is of particular importance, because congenic NOD lines containing alleles from protected strains at this locus are significantly less susceptible to diabetes. To date, idd3 is the most potent disease modifying the non–major histocompatibility complex (MHC) locus (3). Therefore, some of the genes within the idd3 interval must play a crucial role in regulating immune destruction of pancreatic β-cells.
Among the several candidate genes within the idd3 locus, interleukin (IL)-21 is of particular interest, because dysregulated IL-21 production and signaling has been found in the NOD mouse (4). IL-21 belongs to the type 1 cytokine family, which includes potent immune modulators such as IL-2, IL-4, IL-7, and IL-15, whose high-affinity receptor complexes all use the common γc receptor subunit (5,6). The specificity of IL-21 signaling is achieved through its specific interaction with the IL-21 receptor subunit, which forms a heterodimer with the γc subunit (7). This receptor complex delivers IL-21 signals to a variety of immune cells including CD4+ and CD8+ T-cells, B-cells, NK cells, NKT cells, and dendritic cells (8–13), all of which can play some role in the pathogenesis of type 1 diabetes in the NOD mouse (14–20). Therefore, the aim of our present study was to better understand the role of IL-21 in type 1 diabetes. We demonstrate that loss of IL-21 signaling, via knockout of the IL-21 receptor, completely abrogates diabetes development on the NOD background. In addition, we demonstrate that overexpression of IL-21 in pancreatic β-cells induces a high incidence of spontaneous type 1 diabetes on the normally diabetes-resistant C57Bl/6 genetic background. Together, these findings clearly underline the potent prodiabetogenic activity of IL-21.
RESEARCH DESIGN AND METHODS
Mice.
All mice were housed in microisolator cages under specific pathogen-free conditions at the Harvard School of Public Health and the La Jolla Institute for Allergy and Immunology. All animal studies were performed according to institutional and National Institutes of Health guidelines for animal use and care. Blood glucose levels were monitored weekly using OneTouch Ultra (LifeScan) or Ascensia Contour glucometers (Bayer). Diabetes in NOD mice was defined as two consecutive blood glucose values >250 mg/dl. IL-21 receptor knockout mice were generated by homologous recombination as previously described (11). NOD/Ltj mice were purchased from The Jackson Laboratories, and the IL-21 receptor–null allele was backcrossed to the NOD background for 10 generations. The IL-21 transgenic (IL-21Tg) construct was generated by cloning the full-length murine IL-21 cDNA into a transgenic expression vector between the 5′ human insulin promoter and 3′ hepatitis virus B terminator sequence (21). The purified plasmid was linearized using the SacI and HindIII restriction sites, injected into C57Bl/6 fertilized embryos and implanted into pseudopregnant females. Founder lines were identified by Southern blot and maintained as heterozygotes for experimentation.
Tissue isolation, fixation, and immunohistochemical staining.
Pancreata were harvested from IL-21R+/+ and IL-21R−/− NOD mice, immersed in OCT Compound (Tissue-Tek, Sakura) and quick-frozen on dry ice. The 6-μm sections were cut at three nonoverlapping levels (200 μm apart) and fixed in acetone for 10 min at room temperature. Sections were incubated for 1 h at room temperature with guinea pig anti-swine insulin (Dako, 1:500), biotin–anti-mouse CD8 (BD clone 53–6.7, 1:50), and biotin–anti-mouse CD4 (BD, clone RM4-5, 1:50). Next, goat anti–guinea pig alkaline phosphatase (Sigma, 1:50) and Avidin-HRP (Vector, 1:2,000) were incubated for 45 min at room temperature. Alkaline phosphatase or horseradish peroxidase (HRP) activity was visualized using Vector Blue Alkaline Phosphatase III (blue signal) and AEC substrate (red precipitates). Slides were mounted without hematoxylin counterstain (Dako Faramount Aqueous Mounting Medium). Islets were scored visually by light microscopy and categorized as no insulitis, peri-insulitis, mild infiltration (<25%), and heavy infiltration and scars.
Pancreata were harvested from IL-21Tg and littermate controls and fixed overnight with 4% paraformaldehyde (Sigma-Aldrich) before routine paraffin embedding. After dewaxing, 6-μm sections were cut and treated with 3% H2O2 in MeOH (20 min at room temperature) to quench endogenous peroxidase activity. Antigen retrieval was performed using trypsin or proteinase K digestion. Next, slides were blocked in 1% BSA and 3% normal serum in PBS for 30 min at room temperature. Primary antibodies were incubated overnight at 4°C at the following concentrations: insulin 1:100 (#A0564, Dako), IL-21 1:1,200 (#AF594 R&D Systems), CD4 1:100 (BioLegend), B220 1:100 (#550286, BD Biosciences), F4/80 1:100 (BD Biosciences), CD11c 1:100 (#553800, BD Biosciences), and LGL-1 1:50 (#555314, BD Biosciences). Primary antibodies were visualized using sequential detection with HRP-conjugated secondary antibodies (Jackson ImmunoResearch), tyramide signal amplification (Perkin-Elmer), streptavidin-HRP (Jackson ImmunoResearch), and diaminobenzidine (Sigma-Aldrich). Slides were counterstained with hematoxylin before mounting. Cell type–specific islet infiltration was scored using an arbitrary scale ranging from 0 to 4: 0, no islet inflammation; 1, scattered cells surrounding islets; 2, foci of cells surrounding islets; 3, foci of cellular infiltrates surrounding and cells within islets; and 4, dense foci of cellular infiltrates surrounding and within islets.
Lymphocyte preparations, transfers, and flow cytometry.
Single-cell suspensions were prepared from spleen and peripheral lymph nodes by mechanical disruption, filtration through a 70-μm cell strainer (BD Biosciences), erythrocyte lysis using ACK buffer, and two washes inFACS buffer (PBS/0.5% BSA/0.01% NaN3). Pancreatic lymphocytes were prepared as previously described (22).
Splenocyte transfers were performed from newly diabetic IL-21R+/+NOD mice or age-matched IL-21R−/−NOD mice. The 2 × 107 splenocytes were transferred intravenously into 6-week-old female NOD/scid mice. Blood glucose levels were monitored twice weekly for 4 weeks and then once weekly. Insulitis was scored as above. Diabetic animals or nondiabetic animals at 7 weeks after transfer were killed.
FITC-, PE-, PerCP-, APC-, cascade blue–, and cascade orange–conjugated monoclonal antibodies to CD3, CD4, CD8, CD21, CD23, B220, IgM, and IgD (all BD) were used according to the manufacturer's instructions. Cells were analyzed using an LSRII flow cytometer (BD Biosciences).
Tissue collection and quantitative RT-PCR.
Mice were killed using CO2 narcosis, and harvested tissues were snap-frozen in liquid nitrogen. Total RNA was purified using Trizol reagents, and 2 μg was used for cDNA synthesis. Real-time quantitative PCR was performed using a Stratagene M×3005P QPCR system using β-actin as an internal reference control.
Insulin autoantibody assay.
Levels of autoantibodies to murine insulin (mIAA) were determined by a radioactive assay as described (23). The limit of normal (0.010) was chosen based on historical data (23).
Statistical analysis.
For diabetes incidence, significance was calculated using the log-rank test or one-way ANOVA followed by a Bonferroni's post test. For all other parameters, significance was calculated by Student's t test, indicated as follows in the figures: *P < 0.05, **P < 0.01, and ***P < 0.005.
RESULTS
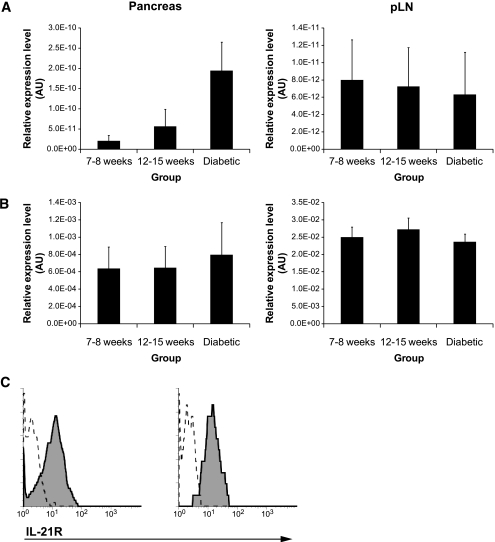
Genetic and cellular studies have suggested that IL-21 could be important for the pathogenesis of type 1 diabetes in the NOD mouse model (3,4). To begin, we defined the expression patterns of IL-21 and IL-21R mRNA in the pancreas and pancreatic draining lymph nodes in pre-diabetic and diabetic NOD mice. IL-21 mRNA levels, essentially unaltered in pancreas draining lymph nodes, showed an upward trend in the pancreas as diabetes developed in the NOD (Fig. 1A, P = 0.057, pre-diabetic vs. diabetic NOD). Levels of IL-21R mRNA remained unchanged in both pancreas and associated draining lymph nodes as diabetes develops (Fig. 1B). IL-21R is clearly detectable on the surface of pancreatic CD4+ and CD8+ T-cells (Fig. 1C), including diabetogenic nrp-V7 tetramer+ CD8+ T-cells (data not shown), at levels comparable to splenic CD4+ and CD8+ T-cells from NOD (data not shown) and other strains (24). These data indicate that increased pancreatic IL-21 production correlates with diabetes onset in NOD mice and that T-cells infiltrating the pancreas express IL-21 receptor (IL-21R).
FIG. 1.
Expression of IL-21 and IL-21R in pancreas and pancreatic lymph nodes of NOD mice. A: IL-21 mRNA analyzed in pancreas and pancreatic lymph node at indicated ages by quantitative PCR (n = 6 per group). B: IL-21R mRNA analyzed in pancreas and pancreatic lymph node at indicated ages by quantitative PCR (n = 6 per group). C: IL-21R expression on CD4+ (left panel) and CD8+ T-cells (right panel) from pancreas of pre-diabetic NOD mice was determined by flow cytometry using the three-step staining protocol described before (24). Specific and control staining are represented by the solid line and tinted area, respectively. AU, arbitrary units.
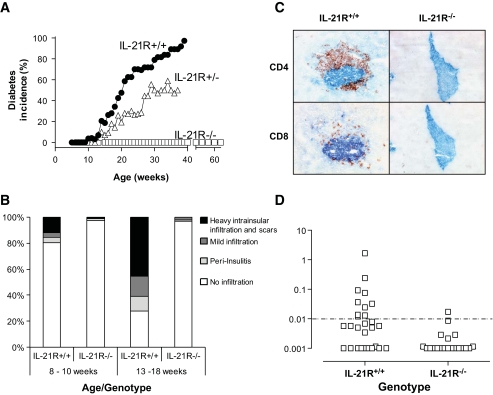
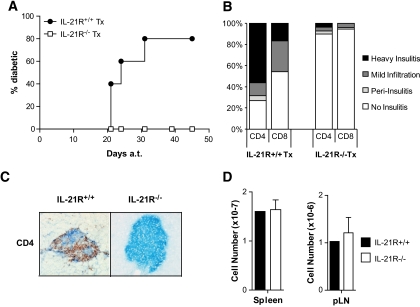
To assess the importance of signaling through the IL-21R during spontaneous type 1 diabetes development, we generated a colony of IL-21R–deficient NOD mice and compared disease parameters with littermate control animals. The IL-21R+/+NOD littermates developed type 1 diabetes beginning at week 11, with a median onset at 19 weeks and >90% penetrance of disease (Fig. 2A), comparable to our NOD colony (2,3). In contrast, IL-21R−/− NOD animals were completely protected from type 1 diabetes development up to 60 weeks of age (Fig. 2A). Heterozygotes displayed an intermediate phenotype with delayed onset (median onset 29 weeks) and reduced penetrance of disease (∼50%). The effect of IL-21R deficiency on mononuclear cell infiltration in the pancreas was determined by immunohistochemistry on pancreatic tissue sections. The severity of insulitis in IL-21R+/+NOD littermates increased with age. At 13–18 weeks, IL-21R+/+NOD islets were highly infiltrated or destroyed, before diabetes onset (Fig. 2B). The observed infiltrate was composed predominantly of CD4+ T-cells that preferentially resided in the islet zones where β-cell destruction had occurred (Fig. 2C). CD8+ T-cells were found scattered throughout the islet (Fig. 2C). In contrast, we observed minimal mononuclear cell infiltration in islets of IL-21R−/−NOD mice up to 40 weeks of age. In keeping with the lack of insulitis, autoimmunity to islet antigens was reduced in IL-21R−/−NOD. Quantitation of serum insulin autoantibodies revealed seropositivity in 10/27 IL-21R+/+NOD mice (8–12 weeks old), in contrast to only 1/20 IL-21R−/−NOD mice (Fig. 2D). Thus, loss of IL-21 signaling protects NOD mice from diabetes, islet inflammation, and the generation of islet autoantibodies.
FIG. 2.
IL-21R–deficient NOD mice are protected from type 1 diabetes. A: Diabetes incidence per group per week was determined from blood glucose levels of IL-21R+/+ (n = 35), IL-21R+/− (n = 17), and IL-21R−/−NOD (n = 16 until week 40, n = 8 until week 60). Onset was dated on the first of two consecutive readings of blood glucose levels >250 mg/dl. Data shown are significantly different in one-way ANOVA analysis (P < 0.0001) and Bonferonni's multiple comparison post-test (P < 0.001 for all comparisons). B: Mononuclear infiltration was scored in pancreatic islets from IL-21R+/+ and IL-21R−/−NOD mice at the indicated ages. At least six mice and 200–450 islets per group were used. C: Absence of islet infiltration by CD4+ and CD8+ T-cells in IL-21R−/−NOD mice. Frozen tissue sections (6 microns) from 15-week-old female IL-21R+/+ or IL-21R−/−NOD mice were stained for insulin (blue) and cellular infiltration by CD4+ or CD8+ T-cells (red). Original magnification: ×20. D: Insulin autoantibodies (IAA) in serum of IL-21R+/+NOD and IL-21R−/−NOD mice at 8–12 weeks of age. (A high-quality digital representation of this figure is available in the online issue.)
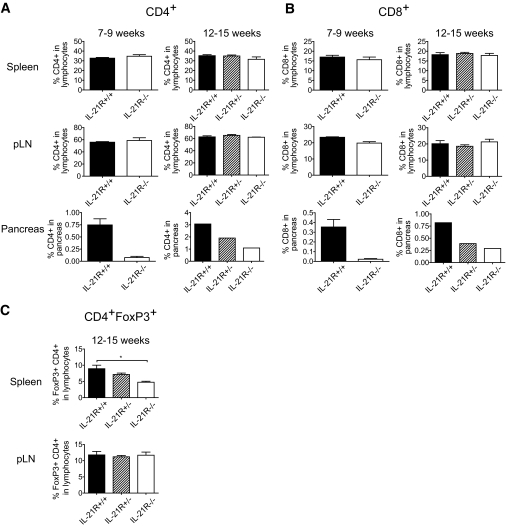
We next analyzed the constitution of the lymphoid compartment of various IL-21RNOD genotypes. We found roughly equal splenocyte numbers in IL-21R+/+NOD, IL-21R+/−NOD, and IL-21R−/−NOD at both early (7–9 weeks) and late pre-diabetic stages (12–15 weeks) (data not shown). The proportion of CD4+ and CD8+ T-cells within the lymphocyte population in spleen and the pancreas draining lymph node was not significantly influenced by IL-21R deficiency (Fig. 3A and B; supplementary Fig. 2, found in an online appendix at http://care.diabetesjournals/cgi/content/full/db08-0882/DC1). Moreover, the fraction of B-cells and NK cells at 12–15 weeks of age was similar between all genotypes (data not shown). Enumeration of pancreatic CD4+ and CD8+ T-cells corroborated the insulitis index scores (Fig. 2B) as CD4+ and CD8+ T-cell numbers increased from early to late pre-diabetic stage in IL-21R+/+NOD but were significantly reduced in IL-21R−/−NOD pancreata (Fig. 3A and B, lower panels). We hypothesized that an increased regulatory compartment could explain the observed diabetes resistance of IL-21R−/−NOD mice. Whereas no significant differences in CD4+FoxP3+ Tregs were observed in the spleen of late-stage pre-diabetic mice (Fig. 3C), the Treg fraction in the pancreatic lymph nodes of IL-21R−/−NOD mice was reduced (∼50%) compared with controls. This may represent a relative reduction of Tregs in IL-21R−/−NOD mice or an increase in IL-21R+/+NOD related to disease onset (25). Regardless, we conclude that the peripheral lymphoid compartment in IL-21R−/−NOD is essentially normal, with the unexpected exception of reduced Treg numbers in the pancreatic lymph nodes, which suggests that diabetes resistance is not due to an increased regulatory compartment.
FIG. 3.
IL-21R−/−NOD mice have a normal peripheral lymphoid compartment but less T-cells in the pancreas. CD4+ (A) and CD8+ (B) T-cells were measured in spleen, pancreas-draining lymph node, and pancreas of IL-21R+/+, IL-21R+/−, and IL-21R−/−NOD mice at 7–9 weeks (early pre-diabetic) or 12–15 weeks (late pre-diabetic) of age. Indicated percentages are fractions of CD4+ or CD8+ T-cells in the live lymphocyte gate. C: Flow cytometric determination of the proportion of CD4+FoxP3+ T-cells in the lymphocyte gate of IL-21R+/+, IL-21R+/−, and IL-21R−/−NOD spleens (left panel) and pancreatic lymph nodes (right panel) at 12–15 weeks of age.
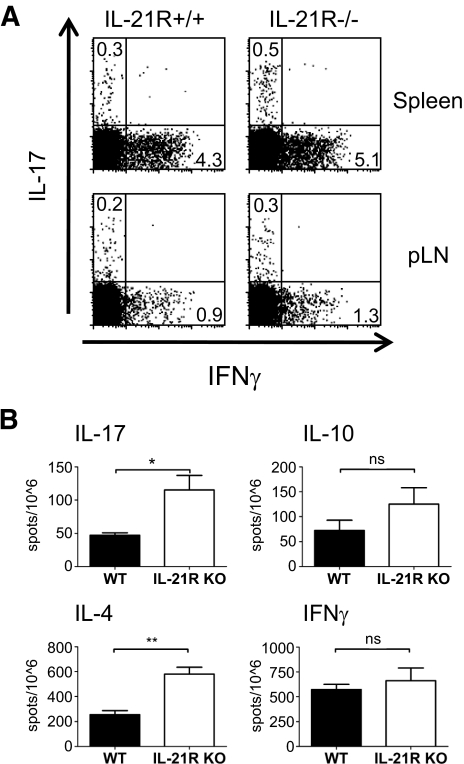
Given the absence of obvious cellular defects, we reasoned that modulation of Th effector responses from pathogenic (Th1, Th17) to protective (Th2) may account for diabetes protection in IL-21R−/−NOD mice. Lymphocytes from spleens and pancreatic lymph nodes of 8- to 9-week-old IL-21R+/+NOD and IL-21R−/−NOD mice were restimulated in vitro with phorbol 12-myristate 13-acetate/ionomycin for 3 h for intracellular cytokine detection by flow cytometry. We found a slight increase in the proportion of CD4+ T-cells that produce IL-17 or interferon (IFN)-γ in the splenic and pancreatic lymph node cells of IL-21R−/−NOD mice (Fig. 4A). We next used enzyme-linked immunosorbent spot assays to confirm these data. Splenocytes from IL-21R+/+NOD and IL-21R−/−NOD mice were stimulated for 72 h with anti-CD3/anti-CD28 under nonpolarizing conditions. We observed significantly increased numbers of IL-17– and IL-4–producing cells in IL-21R−/−NOD splenocytes compared with controls. We also found a trend toward increased IFN-γ– and IL-10–producing cells that failed to reach statistical significance. Thus, whereas there are increases in IL-4 production, concomitant increases in IL-17 and possibly IFN-γ make it unlikely that skewing toward protective Th2 response explains the diabetes resistance in IL-21R−/−NOD mice.
FIG. 4.
IL-21R−/−NOD mice display altered cytokine production profiles. A: IFN-γ and IL-17 production by CD4+ T-cells upon 3 h phorbol 12-myristate 13-acetate/ionomycin stimulation of freshly isolated IL-21R+/+ and IL-21R−/−NOD splenocytes. B: enzyme-linked immunosorbent spot analysis of IFN-γ, IL-17, IL-4, and IL-10 production upon 72 h of in vitro unpolarized anti-CD3/28 stimulation of freshly isolated IL-21R+/+ and IL-21R−/−NOD splenocytes.
To decipher whether an IL-21R+/+NOD environment was sufficient to restore the diabetogenic potential of IL-21R−/−NOD lymphocytes, we performed parallel transfers of IL-21R+/+NOD and IL-21R−/−NOD splenocytes into lymphopenic NOD/scid recipients. As previously published, splenocytes from recently diabetic IL-21R+/+NOD mice induced diabetes upon transfer to NOD/scid mice starting at 3 weeks post-transfer (Fig. 5A). In contrast, transfer of age-matched IL-21R−/−NOD splenocytes could not induce diabetes in NOD/scid mice (Fig. 5A). Immunohistochemistry on pancreatic sections revealed limited islet infiltration by CD4+ and CD8+ T-cells in NOD/scid recipients of IL-21R−/−NOD splenocytes, but abundant infiltration by transferred IL-21R+/+NOD splenocytes. Defective reconstitution of lymphoid space by IL-21R−/−NOD lymphocytes could not explain these observations, as we found equivalent numbers of lymphoid cells in spleen or pancreatic lymph nodes of NOD/scid mice receiving either IL-21R+/+NOD or IL-21R−/−NOD splenocytes (Fig. 5D). These observations indicate that IL-21R−/− NOD mice lack auto-aggressive splenocytes compared with their wild-type littermates and that lymphopenia-induced proliferation of IL-21R−/−NOD lymphocytes does not confer them with diabetogenic properties.
FIG. 5.
IL-21R−/−NOD splenocytes do not induce diabetes in NOD/scid. A: Splenocytes (2 × 107) from diabetic IL-21R+/+NOD mice (●) or age-matched IL-21R−/−NOD mice (□) were transferred into 6-week-old NOD/scid mice. The diabetes incidence in recipient mice is shown (IL-21R+/+NOD, n = 5; IL-21R−/−NOD, n = 6). B: Insulitis scores for CD4+ and CD8+ infiltration in NOD/scid recipients of IL-21R+/+NOD (left) and IL-21R−/−NOD (right) splenocytes. C: Representative picture of CD4+ (red) and insulin (blue) staining of pancreas sections from NOD/scid after transfer of IL-21R+/+NOD (left) or IL-21R−/−NOD (right) splenocytes. D: Splenic and pancreatic lymph nodes cell numbers in NOD/scid after transfer of IL-21R+/+NOD (■) or IL-21R−/−NOD (□) splenocytes. (A high-quality digital representation of this figure is available in the online issue.)
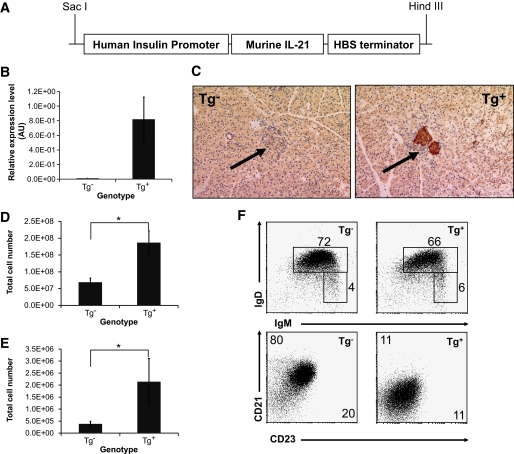
We showed that pancreatic levels of IL-21 increase during diabetes development in NOD (Fig. 1A) and that loss of IL-21 signaling protects NOD mice from islet infiltration and diabetes development (Fig. 2). We therefore hypothesized that elevated levels of IL-21 would exacerbate disease pathogenesis. To test this, we generated transgenic C57Bl/6 mice in which IL-21 is under the control of the human insulin promoter, resulting in pancreatic β-cell–specific overexpression of IL-21 (Fig. 6A). Next, we measured IL-21 levels by quantitative RT-PCR (Fig. 6B) and by immunohistochemistry using a polyclonal anti-mouse IL-21 antibody (Fig. 6C). These data revealed distinct overexpression of IL-21 mRNA and protein in pancreatic islets of IL-21 transgenic animals.
FIG. 6.
Expression of IL-21 in pancreatic islets through an insulin promoter transgenic construct leads to increased cellularity of lymphoid organs and altered expression of B-cell maturation markers in IL-21Tg mice. A: Murine IL-21 was cloned into the transgenic construct under the control of the human insulin promoter and HBS (hepatitis B virus) terminator sequences. B: IL-21 mRNA levels were measured in pancreatic tissue from IL-21Tg and littermate controls by quantitative RT-PCR. C: Immunohistochemical staining for paraffin embedded pancreatic tissue with an IL-21–specific polyclonal antibody (left panel Tg−, right panel Tg+, a representative islet is marked with an arrow). Original magnification: ×20. Total cell numbers in spleen (D) and pancreatic draining lymph nodes (E) (*P < 0.05) and IgD vs. IgM staining (F) of B220+ cells (top panels) and frequencies of mature (IgM+ IgD+) and marginal zone/transitional (IgM+ IgD−) B-cells are shown. CD23 vs. CD21 staining of IgM+ cells (bottom panels) and mean fluorescence intensity for each marker is shown on the relevant axis (n = 4 for all experiments shown). (A high-quality digital representation of this figure is available in the online issue.)
Analysis of lymphoid compartments revealed splenomegaly and lymphadenopathy in IL-21Tg mice. We identified an ∼2.5-fold increase in total cell numbers in spleen (Fig. 6D) and pancreatic draining lymph nodes (Fig. 6E) resultant from expansion of both the T-cell (CD3+) and B-cell (B220+) compartments (data not shown). Most B-cells in our IL21Tg mice displayed a mature phenotype, while expressing reduced levels of CD21 and CD23 (IgD+, IgM+, CD21lo, CD23lo) (Fig. 6F). Other studies have shown that IL-21 can downregulate surface CD21 and CD23 on B-cells, and expansion of IgD+IgM+CD21loCD23lo B-cells was also observed in other IL-21Tg mouse lines driven by ubiquitous promoters (10). Thus, these data suggest that bioactive IL-21, expressed specifically by pancreatic β-cells, is released systemically from the endocrine pancreas to mediate effects in peripheral lymphoid compartments.
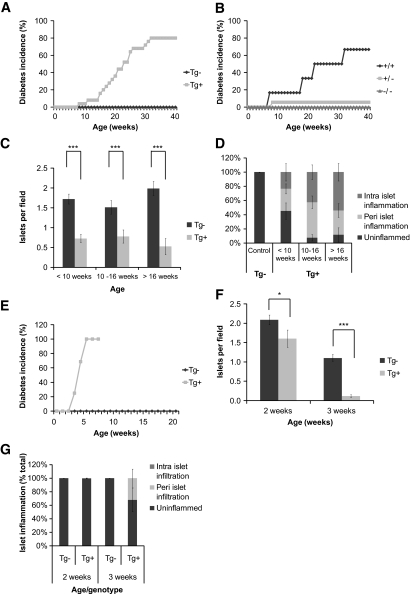
To determine whether IL-21 overexpression resulted in diabetes onset, we monitored blood glucose levels of IL-21Tg mice and wild-type littermate controls weekly from the time of weaning until ∼40 weeks of age. As expected, wild-type animals (C57BL/6) remained normoglycemic over the duration of the study (Fig. 7A). In contrast, IL-21Tg animals started developing diabetes from ∼8 weeks of age (Fig. 7A), with a median onset at ∼22 weeks and ∼80% penetrance in both sexes of the experimental population. To prove specificity of the IL-21 effect and to exclude a transgenesis artifact, we crossed IL-21TgB6 to IL-21R−/−B6 mice. IL-21TgxIL-21R−/− animals were completely protected from diabetes onset (Fig. 7B). Taken together, these data show that β-cell–specific overexpression can induce type 1 diabetes on the diabetes-resistant C57Bl/6 background.
FIG. 7.
IL-21Tg mice develop spontaneous type 1 diabetes on the C57Bl/6 background, the severity of which is increased in the context of NOD alleles. A: IL-21Tg mice and wild-type littermate controls. Diabetic incidence was calculated per group as two consecutive readings over 250 mg/dl and displayed as percentage of diabetic mice per group (Tg−, n = 24; Tg+, n = 25). Diabetes incidence was scored as survival curve data and is different by log-rank test (P < 0.0001). B: Weekly measurements of blood glucose were performed on IL-21Tg mice after crossing to the IL-21R knockout (C57BL/6), and diabetes incidence was calculated (all mice Tg+, IL-21R+/+, n = 7; IL-21R+/−, n = 25; IL-21R−/−, n = 8). Paraffin-embedded pancreatic tissue from wild-type and IL-21Tg mice was sectioned, stained with an anti-insulin polyclonal antibody, and counterstained with hematoxylin. C: Total number of insulin-positive islets per visual field. D: Individual islets were scored for the presence of peri- and intra-islet infiltration and displayed as percentage of infiltrated islets per group (<10 weeks, Tg−, n = 3; Tg+, n = 8; 10–16 weeks, Tg−, n = 12; Tg+, n = 14; >16 weeks, Tg−, n = 7; Tg+, n = 12). Original magnification: ×10. E: IL-21Tg mice on the C57Bl/6 background were crossed with the NOD strain to generate a B6×NOD F1 mixed background. Weekly measurements of blood glucose were performed on IL-21Tg littermate controls, and diabetes incidence was calculated (Tg−, n = 19; Tg+, n = 16). Diabetes incidence was scored as survival curve data and is different by logrank test (P < 0.0001). Paraffin-embedded pancreatic tissue from this cross was quantitated for total number of insulin-positive islets per visual field (F) and individual islets scored for the presence of peri- and intra-islet infiltration and displayed as percentage of infiltrated islets per group (G) (2 weeks, Tg−, n = 11; Tg+, n = 12; 3 weeks, Tg−, n = 15; Tg+, n = 7).
Next, we determined β-cell mass and pancreatic islet infiltration by immunohistochemistry. Pancreatic sections stained for insulin and insulin-positive islets were quantified per visual field. Islet inflammation was scored based on the presence of peri- and intra-islet cellular infiltration. The number of islets in IL-21Tg mice was significantly reduced at all time points compared with controls (Fig. 7C). In addition, ∼50% of the islets in pre-diabetic (10 weeks of age) IL-21Tg mice were infiltrated (Fig. 7D). The severity of islet inflammation increased with age and, at 16 weeks of age, ∼90% of the islets revealed some level of cellular infiltration.
β-Cell–specific IL-21 overexpression precipitates diabetes in diabetes-resistant C57BL/6 (Fig. 7A). To test whether the presence of diabetes susceptibility alleles from NOD influences disease onset, we crossed IL-21Tg mice (C57BL/6) to NOD mice. We found that IL-21Tg F1 (B6xNOD) mice developed diabetes as early as 3 weeks of age, with a median onset at ∼4 weeks and 100% penetrance of disease at 6 weeks (Fig. 7E). This represents a striking acceleration of diabetes onset in IL-21Tg on the mixed B6×NOD versus the B6 background (median onset 4 vs. 22 weeks, respectively; Fig. 7A vs. E). We determined β-cell mass and pancreatic islet infiltration by immunohistochemistry and found a reduced amount of islets and distinct infiltration of the remaining islets between 2 and 3 weeks of age in the IL-21Tg B6×NOD F1 compared with wild-type B6×NOD littermates (Fig. 7F and G). Our data show that one “dose” of NOD-derived alleles exacerbates diabetes in IL-21Tg C57Bl/6 mice.
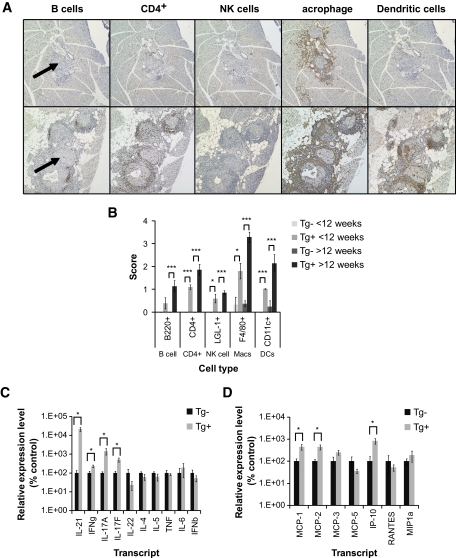
Next, we used immunohistochemistry to determine which cell subsets infiltrate the islets in IL-21Tg C57Bl/6 mice. We analyzed the presence of B-cells (B220+), CD4+ cells (CD4+), NK cells (LGL-1+), macrophages (F4/80+), and dendritic cells (CD11c+) in islet infiltrates from pre-diabetic (8–10 weeks; Fig. 8A, top panel) and diabetic IL-21Tg cohorts (24 weeks; Fig. 8A, bottom panel). We observed more severe infiltration by all cell types in IL-21Tg versus littermate controls, and in diabetic versus pre-diabetic mice (Fig. 8B), corroborating our data in Fig. 7. The infiltrates in the pre-diabetic IL-21Tg cohort predominantly contained F4/80+ macrophages but also CD4+ and dendritic cells. In diabetic IL-21Tg mice, the infiltrates consisted mostly of macrophages and contained focal accumulation of CD4+ cells, B-cells, dendritic cells, and NK cells. We reasoned that the distinct pattern of infiltration could result in part from the production of cytokines and chemokines. Therefore, we performed quantitative RT-PCR on pancreatic tissue from IL-21Tg and littermate controls, which revealed significantly increased production of IFN-γ, IL-17A, and IL-17F in the pancreas of IL-21Tg mice (Fig. 8C). In addition, we found a significant increase in monocyte chemoattractant protein (MCP)-1, MCP-2, and IFN-inducible protein (IP)-10 production (Fig. 8D). Thus, pancreatic β-cell–specific overexpression of IL-21 results in the production of inflammatory cytokines and chemokines and predominant infiltration of the islets by macrophages and CD4+ T-cells.
FIG. 8.
Spontaneous type 1 diabetes in IL-21Tg mice is associated with a macrophage-rich islet infiltrate and the expression of inflammatory cytokines and chemokines in the pancreas. A: Paraffin-embedded pancreatic tissue was sectioned and stained with anti-B220, CD4, LGL-1, F4/80, and CD11c antibodies. Representative sections from 8-week-old (top panels) and 24-week-old mice (bottom panels) are shown (a representative islet is marked with an arrow). All sections were scored by visual inspection on an arbitary scale of 0–4 (no infiltrate to dense, severe infiltrate) and plotted in B (<12 weeks, Tg−, n = 3; Tg+, n = 5; >12 weeks, Tg−, n = 4; Tg+, n = 7). Original magnification: ×10. Total RNA was extracted from pancreatic tissue harvested from a cohort of IL-21Tg mice and wild-type controls. Levels of transcripts for a panel of cytokines (C) and chemokines (D) was measured using quantitative RT-PCR (24–30 weeks, Tg−, n = 3; Tg+, n = 5, *P < 0.05). (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
In this study, we demonstrate a causal relationship between IL-21 production and type 1 diabetes. First, IL-21 production increases as spontaneous diabetes develops in the NOD model. Second, IL-21R–deficient NOD mice are protected from type 1 diabetes. Third, β-cell–specific overexpression of IL-21 precipitates diabetes in diabetes-resistant C57Bl/6 mice.
Type 1 diabetes pathogenesis in the NOD model consists of a sequence of stages. Initially, islet antigens are released during postnatal remodeling of the pancreas and captured by migratory and resident antigen-presenting cell that prime anti-islet T-cells in the pancreatic draining lymph nodes (20,26–28). At an early stage, macrophages are recruited to the islets (29) and are a necessary cellular component of diabetes pathogenesis (30). Next, chemotactic factors, produced by β-cells in response to inflammatory stimuli, attract mononuclear cells to the islets, particularly CD4+ and CD8+ effector T-cells. The transition from nondestructive islet inflammation to a β-cell–destructive state is a key event that precipitates type 1 diabetes (18,31). Because IL-21R is broadly expressed throughout the immune system and other nonhematopoeitic lineages (6,32–35), there are multiple time points and sites of action for IL-21 during the pathogenesis of type 1 diabetes.
We show here that IL-21 levels are increased in the pancreas as NOD mice develop diabetes and that CD4+ and CD8+ T-cells infiltrating the pancreas can respond to local IL-21 as they express IL-21R. Our data are consistent with recent studies by the labs of Leonard and Sarvetnick (36,37) showing that IL-21R−/−NOD mice are protected from insulitis and type 1 diabetes. Similar to Spolski et al. (37), we find unaltered numbers of T-cells, B-cells, and NK cells in IL-21R−/−NOD lymphoid organs (Fig. 3 and data not shown). In contrast to ours and other studies, Datta and Sarvetnick detected higher lymphocyte numbers in IL-21R−/−NOD mice, interpreting this as a normalization of IL-21–induced, type 1 diabetes–promoting lymphopenia (4,36). We see no differences in the expansion of IL-21R+/+NOD and IL-21R−/−NOD splenocytes when transferred to lymphopenic NOD/scid recipients (Fig. 5D), yet IL-21R−/−NOD splenocytes still fail to induce diabetes. We therefore think it unlikely that IL-21 catalyzes diabetes development by regulating homeostatic proliferation.
Given that T-cell numbers and responses are intact in IL-21R−/− mice (8,11), we hypothesized that altered cytokine production may partially account for the protection from type 1 diabetes. Our analyses show that production of various effector cytokines was not impaired in IL-21R−/−NOD mice (Fig. 4A and B). One of these cytokines, IL-17, has recently been shown to modulate some aspects of the type 1 diabetes pathogenesis in NOD (38), and recent studies identify IL-21 as an amplifying factor for Th17 responses (39,40). Spolski et al. (37) identify defective polarization toward the Th17 lineage in IL-21R−/−NOD lymphocytes and reason that defective IL-17 production may explain diabetes resistance in IL-21R−/−NOD mice. We (data not shown) and others (39) find similarly defective in vitro Th17 polarization using IL-21R−/− T-cells. Moreover, our data show increased IL-17 production in the pancreas of β-cell–specific IL-21 overexpressing mice (Fig. 8D). However, we show increased numbers of IL-17–producing cells in IL-21R−/−NOD mice when cells are restimulated directly ex vivo, which is likely to be more reflective of the in vivo context. Thus, we conclude that reduced IL-17 production in IL-21R−/−NOD mice is unlikely to be the mechanism for the protection from type 1 diabetes.
The reduced frequency of insulin autoantibodies and insulitis in IL-21R−/−NOD mice (Fig. 2B and D) shows that the anti-islet response is impaired at multiple levels. Reduced autoantibody levels may reflect impairments in CD4+ T helper cell function or antibody production in the absence of IL-21R (9,41,42). Anti-islet IL-21R−/− T-cells may be primed ineffectively or possess inherent defects in migration to islet tissue. Since IL-21R−/−NOD mice have normal or fewer numbers of regulatory T-cells (Fig. 3C), and the function of these cells is not altered (37), it is unlikely that increased regulatory function explains the reductions in autoimmunity. Transfer experiments using diabetogenic T-cell receptor–transgenic T-cells may elucidate the existence of defects in priming or trafficking and are the subject of ongoing studies.
Although IL-21R deficiency protects diabetes-prone NOD mice from type 1 diabetes, β-cell–specific overexpression of IL-21 causes severe diabetes in otherwise diabetes-resistant C57Bl/6 mice. Few other models of cytokine overexpression in pancreatic islets cause diabetes of similar severity (43,44). The phenotype of IL-21Tg mice most closely resembles that of IFN-γTg mice in terms of onset and severity of disease. The IFN-γTg model is both T-cell and macrophage dependent (21,45,46). Similar to the IFN-γTg model, the high numbers of macrophages in the islet infiltrates of IL-21Tg mice suggest an important role for macrophages, since macrophage-derived inflammatory cytokines and reactive oxygen species are directly toxic to β-cells (47). In vitro stimulation of macrophages with IL-21 enhances their T-cell priming capacity (48); thus, phagocytosis of damaged islets and presentation of β-cell antigens to CD4+ T-cells may cause enhanced killing of islets in the IL-21Tg model. In IL-21Tg pancreatic tissue, we showed upregulation of chemokine transcripts such as MCP-1, MCP-2, and IP-10, which recruit inflammatory cells such as macrophages and CXCR3+ T-cells (Fig. 8E) (49). Previous studies identified β-cells as an important source of chemokines during diabetes pathogenesis, but our experiments have failed to identify IL-21R expression on β-cells (supplementary Fig. 1). Regardless, we believe that IL-21–dependent inflammatory chemokine production could be an important element of the pathogenesis of type 1 diabetes and partially explain the protection afforded by IL-21R deficiency in the NOD model (33,50,51).
In conclusion, we demonstrate a critical role of IL-21 for diabetes pathogenesis in animal models. The disease-promoting activities of IL-21 involve the recruitment of CD4+ cells and macrophages to inflamed islets and may reflect events that occur in response to IL-21 production by infiltrating cells. In addition, the partial protection from diabetes in IL-21R+/−NOD mice shows the sensitivity of the diabetogenic response to alterations in IL-21 signaling and, by inference, IL-21 levels. Thus, both of our experimental models suggest that the use of IL-21 blocking agents, antibodies, or IL-21R-Fc fusion proteins has potential therapeutic value for the prevention or treatment of human type 1 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
A.P.R.S. is a CJ Martin Fellow of the National Health and Medical Research Council of Australia. T.V.B. is a fellow of the Belgian American Educational Foundation and funded by The Brehm Center for Type 1 Diabetes Research and Analysis.
No potential conflicts of interest relevant to this article were reported.
We thank Kirsten Sigrist, Jennifer Donovan, Diana Pascual, Therese Juntti, Jeanette Liao, and the staff at the Harvard School of Public Health Animal Testing Facility for assistance with animal care.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson MS, Bluestone JA: The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005; 23: 447– 385 [DOI] [PubMed] [Google Scholar]
- 2.Wicker LS, Todd JA, Peterson LB: Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol 1995; 13: 179– 200 [DOI] [PubMed] [Google Scholar]
- 3.Wicker LS, Todd JA, Prins JB, Podolin PL, Renjilian RG, Peterson LB: Resistance alleles at two non-major histocompatibility complex-linked insulin-dependent diabetes loci on chromosome 3, Idd3 and Idd10, protect nonobese diabetic mice from diabetes. J Exp Med 1994; 180: 1705– 1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King C, Ilic A, Koelsch K, Sarvetnick N: Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 2004; 117: 265– 277 [DOI] [PubMed] [Google Scholar]
- 5.Mehta DS, Wurster AL, Grusby MJ: Biology of IL-21 and the IL-21 receptor. Immunol Rev 2004; 202: 84– 95 [DOI] [PubMed] [Google Scholar]
- 6.Leonard WJ, Spolski R: Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol 2005; 5: 688– 698 [DOI] [PubMed] [Google Scholar]
- 7.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushandsky K, Holly RD, Foster D: Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408: 57– 63 [DOI] [PubMed] [Google Scholar]
- 8.Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ: Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med 2002; 196: 969– 977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ: A critical role for IL-21 in regulating immunoglobulin production. Science 2002; 298: 1630– 1634 [DOI] [PubMed] [Google Scholar]
- 10.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ: Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004; 173: 5361– 5371 [DOI] [PubMed] [Google Scholar]
- 11.Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, Wurster AL, Donaldson DD, Collins M, Young DA, Grusby MJ: IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 2002; 16: 559– 569 [DOI] [PubMed] [Google Scholar]
- 12.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI: IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 2007; 178: 2827– 2834 [DOI] [PubMed] [Google Scholar]
- 13.Brandt K, Bulfone-Paus S, Foster DC, Ruckert R: Interleukin-21 inhibits dendritic cell activation and maturation. Blood 2003; 102: 4090– 4098 [DOI] [PubMed] [Google Scholar]
- 14.Christianson SW, Shultz LD, Leiter EH: Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice: relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes 1993; 42: 44– 55 [DOI] [PubMed] [Google Scholar]
- 15.Herbelin A, Gombert JM, Lepault F, Bach JF, Chatenoud L: Mature mainstream TCR alpha beta+CD4+ thymocytes expressing L-selectin mediate “active tolerance” in the nonobese diabetic mouse. J Immunol 1998; 161: 2620– 2628 [PubMed] [Google Scholar]
- 16.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC: Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes 1994; 43: 505– 509 [DOI] [PubMed] [Google Scholar]
- 17.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM: B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol 1998; 161: 3912– 3918 [PubMed] [Google Scholar]
- 18.Poirot L, Benoist C, Mathis D: Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc Natl Acad Sci U S A 2004; 101: 8102– 8107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cain JA, Smith JA, Ondr JK, Wang B, Katz JD: NKT cells and IFN-gamma establish the regulatory environment for the control of diabetogenic T cells in the nonobese diabetic mouse. J Immunol 2006; 176: 1645– 1654 [DOI] [PubMed] [Google Scholar]
- 20.Turley S, Poirot L, Hattori M, Benoist C, Mathis D: Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med 2003; 198: 1527– 1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarvetnick N, Liggitt D, Pitts SL, Hansen SE, Stewart TA: Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell 1988; 52: 773– 782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seewaldt S, Thomas HE, Ejrnaes M, Christen U, Wolfe T, Rodrigo E, Coon B, Michelsen B, Kay TW, von Herrath MG: Virus-induced autoimmune diabetes: most beta-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) cytotoxic T-lymphocytes. Diabetes 2000; 49: 1801– 1809 [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Robles DR, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS: Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 2000; 97: 1701– 1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H, Carrio R, Yu A, Malek TR: Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol 2004; 173: 657– 665 [DOI] [PubMed] [Google Scholar]
- 25.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA: Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 2008; 28: 687– 697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DR: Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes 2000; 49: 1– 7 [DOI] [PubMed] [Google Scholar]
- 27.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D: Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med 1999; 189: 331– 339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, O'Brien B, Trudeau J, Tan R, Santamaria P, Dutz JP: In situ beta cell death promotes priming of diabetogenic CD8 T lymphocytes. J Immunol 2002; 168: 1466– 1472 [DOI] [PubMed] [Google Scholar]
- 29.Yoon JW, Jun HS, Santamaria P: Cellular and molecular mechanisms for the initiation and progression of beta cell destruction resulting from the collaboration between macrophages and T cells. Autoimmunity 1998; 27: 109– 122 [DOI] [PubMed] [Google Scholar]
- 30.Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW: The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med 1999; 189: 347– 358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andre-Schmutz I, Hindelang C, Benoist C, Mathis D: Cellular and molecular changes accompanying the progression from insulitis to diabetes. Eur J Immunol 1999; 29: 245– 255 [DOI] [PubMed] [Google Scholar]
- 32.Caruso R, Fina D, Peluso I, Fantini MN, Tosti C, Del Vecchio Blanco G, Paoluzi OA, Caprioli F, Andrei F, Stolfi C, Romano M, Ricci V, MacDonald TT, Pallone F, Monteleone G: IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. J Immunol 2007; 178: 5957– 5965 [DOI] [PubMed] [Google Scholar]
- 33.Caruso R, Fina D, Peluso I, Stolfi C, Fantini MC, Gioia V, Caprioli F, Del Vecchio Blanco G, Paoluzi OA, Macdonald TT, Pallone F, Monteleone G: A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells. Gastroenterology 2007; 132: 166– 175 [DOI] [PubMed] [Google Scholar]
- 34.Monteleone G, Caruso R, Fina D, Peluso I, Gioia V, Stolfi C, Fantini MC, Caprioli F, Tersigni R, Alessandroni L, MacDonald TT, Pallone F: Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut 2006; 55: 1774– 1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonard WJ, Zeng R, Spolski R: Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol 2008; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta S, Sarvetnick NE: IL-21 limits peripheral lymphocyte numbers through T cell homeostatic mechanisms. PLoS ONE 2008; 3: e3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ: IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A 2008; 105: 14028– 14033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain R, Tartar DM, Gregg RD, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H: Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med 2008; 205: 207– 218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK: IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007; 448: 484– 487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bettelli E, Korn T, Kuchroo VK: Th17: the third member of the effector T cell trilogy. Curr Opin Immunol 2007; 19: 652– 657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King CVogelzang A, et al. : A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 2008; 29: 127– 137 [DOI] [PubMed] [Google Scholar]
- 42.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C: Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008; 29: 138– 149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabinovitch A: An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab Rev 1998; 14: 129– 151 [DOI] [PubMed] [Google Scholar]
- 44.Rabinovitch A, Suarez-Pinzon WL: Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol 1998; 55: 1139– 1149 [DOI] [PubMed] [Google Scholar]
- 45.Sarvetnick N, Shizuru J, Liggitt D, Martin L, McIntyre B, Gregory A, Parslow T, Stewart T: Loss of pancreatic islet tolerance induced by beta-cell expression of interferon-gamma. Nature 1990; 346: 844– 847 [DOI] [PubMed] [Google Scholar]
- 46.Gu D, O'Reilly L, Molony L, Cooke A, Sarvetnick N: The role of infiltrating macrophages in islet destruction and regrowth in a transgenic model. J Autoimmun 1995; 8: 483– 492 [DOI] [PubMed] [Google Scholar]
- 47.Dahlen E, Dawe K, Ohlsson L, Hedlund G: Dendritic cells and macrophages are the first and major producers of TNF-alpha in pancreatic islets in the nonobese diabetic mouse. J Immunol 1998; 160: 3585– 3593 [PubMed] [Google Scholar]
- 48.Ruckert R, Bulfone-Paus S, Brandt K: Interleukin-21 stimulates antigen uptake, protease activity, survival and induction of CD4+ T cell proliferation by murine macrophages. Clin Exp Immunol 2008; 151: 487– 495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada S, Oikawa Y, Sakai G, Atsumi Y, Maruyama T, Shimada A: Expression levels of CXC chemokine receptors 3 are associated with clinical phenotype of type 1 diabetes. Ann N Y Acad Sci 2006; 1079: 186– 189 [DOI] [PubMed] [Google Scholar]
- 50.Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA: Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes 2008; 57: 3025– 3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christen U, Von Herrath MG: IP-10 and type 1 diabetes: a question of time and location. Autoimmunity 2004; 37: 273– 282 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.