Abstract
OBJECTIVE
The cytokine interleukin-6 (IL-6) stimulates AMP-activated protein kinase (AMPK) and insulin signaling in skeletal muscle, both of which result in the activation of endothelial nitric oxide synthase (eNOS). We hypothesized that IL-6 promotes endothelial cell signaling and capillary recruitment in vivo, contributing to increased glucose uptake.
RESEARCH DESIGN AND METHODS
The effect of IL-6 with and without insulin on AMPK, insulin, and eNOS signaling in and nitric oxide (NO) release from human aortic endothelial cells (HAECs) was examined. The physiological significance of these in vitro signaling events was assessed by measuring capillary recruitment in rats during control and euglycemic-hyperinsulinemic clamps with or without IL-6 infusion.
RESULTS
IL-6 blunted increases in insulin signaling, eNOS phosphorylation (Ser1177), and NO production and reduced phosphorylation of AMPK in HAEC in vitro and capillary recruitment in vivo. In contrast, IL-6 increased Akt phosphorylation (Ser473) in hindlimb skeletal muscle and enhanced whole-body glucose disappearance and glucose uptake during the clamp. The differences in endothelial cell and skeletal muscle signaling were mediated by the cell-specific, additive effects of IL-6 and insulin because this treatment markedly increased tumor necrosis factor (TNF)-α protein expression in HAECs without any effect on TNF-α in skeletal muscle. When HAECs were incubated with a TNF-α–neutralizing antibody, the negative effects of IL-6 on eNOS signaling were abolished.
CONCLUSIONS
In the presence of insulin, IL-6 contributes to aberrant endothelial cell signaling because of increased TNF-α expression.
It is now recognized that obesity promotes secretion of many proinflammatory cytokines including tumor necrosis factor (TNF)-α, resistin, interleukin (IL)-1β, and IL-6 from both adipocytes and macrophages within the adipose tissue bed (1). Given this proinflammatory response and the observation that systemic IL-6 plasma concentrations are elevated in obesity and in patients with type 2 diabetes (2–4), it is generally thought that IL-6 inhibits insulin action (5). This is consistent with epidemiological data associating IL-6 with increased risk of cardiovascular disease (6). Whether IL-6 has positive or negative effects on metabolic processes is the subject of continuing controversy (7,8). The notion that IL-6 induces insulin resistance has been challenged by the observations that IL-6 is both produced in (9,10), and subsequently released from (11), contracting skeletal muscle cells. It is well-known that physical exercise training increases insulin sensitivity (12), while in the immediate postexercise period, insulin action is enhanced (13).
Recent evidence suggests that IL-6, at least when administered acutely, enhances insulin action because of the upregulation of key signal transduction pathways. IL-6 activates AMP kinase (AMPK) in both skeletal muscle and adipose tissue (14). Activation of AMPK may increase glucose uptake (15) via insulin signal transduction–dependent and –independent pathways (16). We recently observed that acute treatment of muscle cells in vitro with IL-6 increased both basal glucose uptake and GLUT4 translocation from intracellular compartments to the plasma membrane (14). IL-6 increased basal and insulin-stimulated glucose uptake in vitro, whereas infusion of recombinant human IL-6 into healthy humans during a euglycemic-hyperinsulinemic clamp increased glucose infusion rate without affecting the total suppression of endogenous glucose production (14). The effects of IL-6 on glucose uptake in vitro appeared to be mediated by activation of AMPK because the results were abolished in cells infected with an AMPK-dominant, negative adenovirus (14). Apart from activating AMPK, signaling through the gp130 receptor complex results in activation of phosphoinositol 3-kinase (PI3-kinase), a key protein in the insulin signaling transduction cascade. Recent studies in vitro have demonstrated that acute IL-6 treatment can activate PI3-kinase and its downstream target Akt (17). It appears that IL-6 signaling through the gp130 receptor activates signal transduction pathways that favor enhanced insulin action (18).
In 1990, Laakso et al. (19) described a novel role for insulin in modulating skeletal muscle blood flow. The effect of insulin on total blood flow to skeletal muscle has produced conflicting results with some, but not all, studies demonstrating an increase (rev. in ref. 20). It is clear that insulin increases nutritive blood flow and capillary recruitment in skeletal muscle (21,22). Insulin signaling is thought to promote vasodilatation through enhanced endothelial nitric oxide synthase (eNOS) activity (23). It is likely that activation of AMPK may lead to vasodilatation because AMPK phosphorylates eNOS at serine residue 1177, thereby activating this enzyme (24). We have previously shown that the proinflammatory cytokine TNF-α not only reduces insulin signaling and AMPK activity (25) but also attenuates insulin-stimulated increases in capillary recruitment (26). Given that IL-6, in direct contrast with TNF-α, increases AMPK activation and augments insulin signaling in skeletal muscle and adipose tissue and because both AMPK and insulin result in phosphorylation of eNOS in endothelial cells, we hypothesized that IL-6 may increase endothelial cell signaling and capillary recruitment leading to enhanced insulin-stimulated glucose uptake.
RESEARCH DESIGN AND METHODS
Reagents.
See supplementary methods, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db08-0775/DC1.
In vitro experiments.
Human aortic endothelial cells (HAECs) (passage four to eight; Cell Application, San Diego, CA) were maintained in endothelial cell media (EGM-2 bullet kit; Lonza, Walkersville, MD) and seeded into experimental 10-cm flat-bottom tissue culture dishes, precoated with 0.2% gelatin. When cultures were 80% confluent, cells were serum deprived for 18 h before experiments were conducted. Cells were treated with (10 ng/ml) or without (PBS control) IL-6 for 2 h, followed by treatment with (100 nmol/l) or without (PBS control) insulin for 15 min. In separate experiments, cells were treated with (1, 10, and 100 ng/ml) or without (PBS control) TNF-α for 2 h, followed by treatment with (100 nmol/l) or without (PBS control) insulin for 15 min. For TNF-α–neutralizing antibody experiments, cells were pretreated for 1 h with 0.1 μg/ml of either mouse IgG1 isotype control antibody (BD Biosciences, North Ryde, NSW, Australia) or mouse IgG1 anti-human TNF-α neutralization antibody (R&D Systems, Gymea, NSW, Australia). Cells were then treated with (10 ng/ml) or without (PBS control) IL-6 for 2 h and coincubated with either isotype control or TNF-α neutralization antibody, followed by treatment with (100 nmol/l) or without (PBS control) insulin for 15 min.
Endothelial nitric oxide levels.
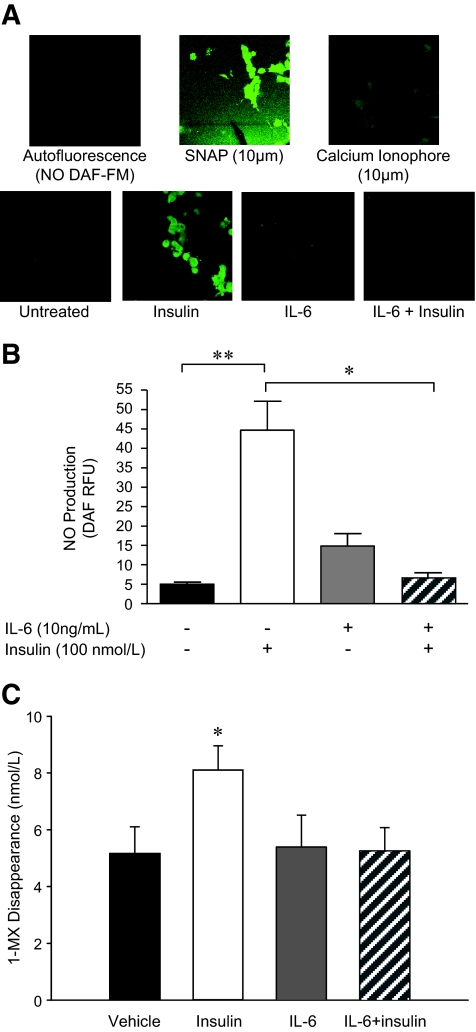
Nitric oxide (NO) release from human aortic endothelial cells (HAECs) was measured by using 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate fluorescence. HAECs were seeded into gelatin coated plastic eight-well chambers (BD Falcon, North Ryde, NSW, Australia). After serum starvation, HAECs were treated with or without IL-6 or TNF-α for 2 h in phenol red–free Dulbecco's modified Eagle's medium. After 1.5 h of treatment, DAF-FM diacetate (2.5 μmol/l final concentration) was spiked into the media. After 2 h of IL-6 treatment, insulin was spiked into the media, and cells were incubated for a further 15 min. The cell culture chamber was then placed in the humidified confocal microscope chamber (37°C; 5% CO2). Calcium ionophore (2 min of treatment) and the NO donor S-nitroso-N-acetylpenicilamine (1 min of treatment) were used as positive controls for eNOS. Cells were viewed for 10 min, and fluorescence intensity was recorded at 15-s intervals using confocal microscopy (Olympus IX70 inverted microscope using Perkin-Elmer Wallac Ultraview and Zeiss META 510 systems). Time-matched images were also quantified. Because the TNF-α experiment measured basal NO release alone, the detector gain was increased (compared with the initial experiment) to capture basal images.
In vivo experiments
Surgery, experimental procedure, and capillary recruitment.
For a description of the animals, see supplementary methods. All experiments were approved by the University of Tasmania Animal Ethics Committee and conducted using the anesthetized rat model (n = 6) as described previously (26–28). After a 45-min equilibration period, rats were infused intravenously with IL-6 (recombinant rat IL-6; R&D Systems, Minneapolis, MN) at 5 μg · h−1 · kg−1 for 3 h. To test the effect of IL-6 on insulin action, a euglycemic-hyperinsulinemic clamp, in which insulin (Humulin R; Eli Lilly, Indianapolis, IN) was infused into rats at 3 mU · min−1 · kg−1 for 2 h, was started after 1 h of IL-6 infusion. At the conclusion of the experiment, the muscles of the lower leg were freeze-clamped in liquid nitrogen and stored at −80°C until required for analysis. The surface area of the perfused capillary bed of muscle was measured by a previously established method involving the steady-state infusion of 1-methylxanthine (1-MX) and its metabolism by capillary endothelial xanthine oxidase (26,27).
Analytical techniques.
RESULTS
HAECs express the gp130Rβ/IL-6Rα receptor complex that is activated by IL-6.
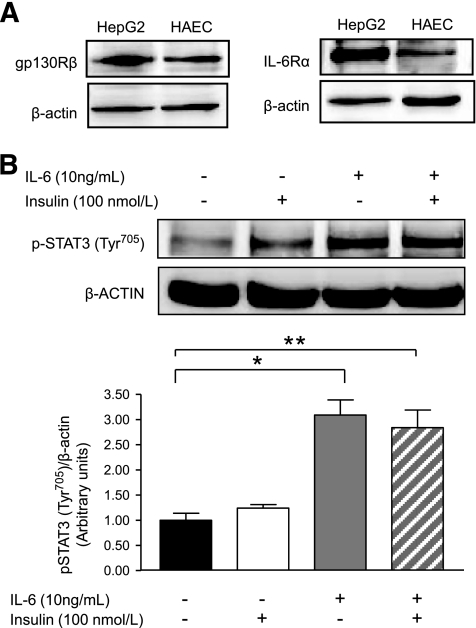
To first establish that IL-6 would transduce signals through its tripartite IL-6/IL-6Rα/gp130Rβ signaling complex, we determined the expression of both the IL-6Rα and gp130Rβ in HAECs. As seen in Fig. 1A, both components of the receptor complex were expressed in HAECs to a lesser extent than in HepG2 cells, which were used as a reference positive control. We treated the cells with insulin and/or IL-6 and measured the phosphorylation of signal transducer and activator of transcription (STAT) 3 at Tyr705 (p-STAT3) as a marker of IL-6 bioactivity in HAECs. As seen in Fig. 1B, IL-6, but not insulin, markedly increased p-STAT3.
FIG. 1.
HAECs express the gp130Rβ/IL-6Rα receptor complex that is activated by IL-6. A: Representative blots of gp130Rβ and IL-6Rα in HAECs and positive control human hepatoma cells (HepG2). B: Representative blots and quantification of phosphorylation of STAT3 in HAECs treated with or without (PBS control) IL-6 (10 ng/ml) for 2 h, followed by treatment with or without (PBS control) insulin (100 nmol/l) for 15 min. *Difference (P < 0.05) IL-6 versus control; **difference (P < 0.05) IL-6 + insulin versus control (data are means ± SE; n = 3–5 replicates from three different experiments).
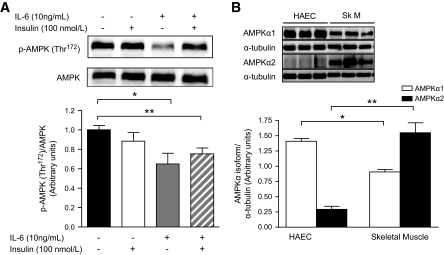
IL-6 decreases phosphorylation of AMPK in HAECs.
We previously demonstrated that IL-6 family cytokines activate AMPK in skeletal muscle and adipose tissue (14,32). Because AMPK is known to phosphorylate eNOS at Ser1177 that activates the enzyme (24), we examined whether IL-6 would phosphorylate AMPK in HAECs. Contrary to our hypothesis, IL-6 decreased p-AMPK in both the presence and absence of insulin (Fig. 2A; supplementary Fig. 1). The differences when comparing skeletal muscle with HAECs with respect to the role of IL-6 in activation of AMPK are not fully clear but may be related to the relative expression of the AMPK isoforms in these different cells or organs. AMPK α1 was markedly more abundantly expressed in HAECs, whereas the AMPK α2 isoform was expressed in very low concentration in HAECs relative to skeletal muscle (Fig. 2B).
FIG. 2.
IL-6 decreased phosphorylation of AMPK in HAECs. A: Phosphorylation (Thr172)/total AMPK in HAECs treated with or without (PBS control) IL-6 (10 ng/ml) for 2 h, followed by treatment with or without (PBS control) insulin (100 nmol/l) for 15 min. B: Representative blots and quantification of AMPK α1 and α1 isoforms/α-tubulin in HAECs and skeletal muscle (SkM). *Difference (P < 0.05) IL-6 versus control (A) and HAEC versus skeletal muscle (B); **difference (P < 0.05) IL-6 + insulin versus control (A) and HAECs versus skeletal muscle (B) (data are means ± SE; n = 3–6 replicates from 2–3 different experiments).
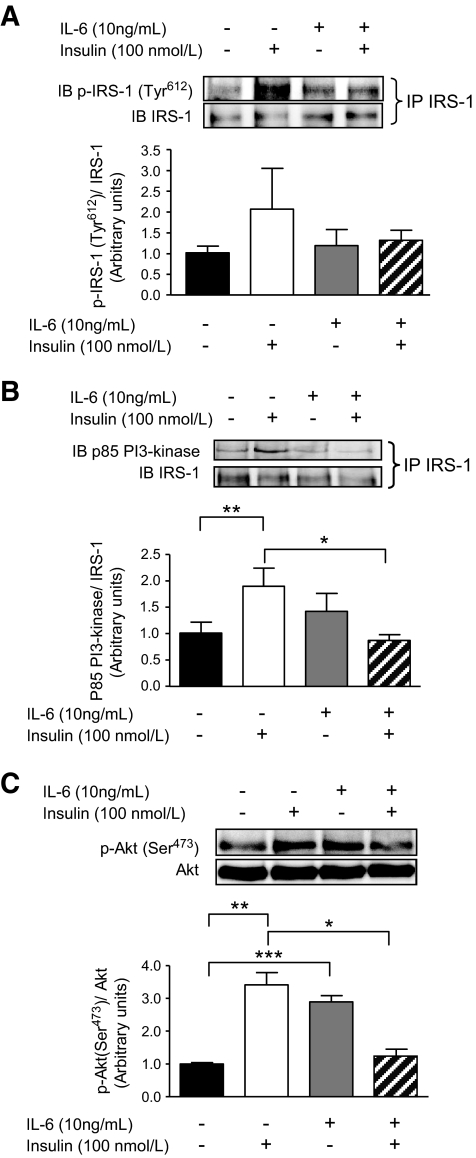
IL-6 decreases insulin signaling in HAECs.
It has been widely reported that IL-6 activates insulin signal transduction in skeletal muscle, adipose tissue, and liver (rev. in ref. 33). As activation of Akt is known to release NO from endothelial cells by phosphorylating eNOS (Ser1177) (34), we examined whether or not IL-6 would affect insulin signaling in HAECs. In the absence of insulin, IL-6 tended to increase the association of the p85 subunit of PI3-kinase with insulin receptor substrate (IRS)-1 (NS) (Fig. 3B). IL-6 alone increased the phosphorylation of its downstream target Akt (Ser473) (Fig. 3C). Pretreatment of HAECs with IL-6 suppressed the tendency (NS) for increased insulin-mediated phosphorylation of IRS-1 (Tyr612) (Fig. 3A), completely blunting the association of the p85 subunit of PI3-kinase with IRS-1 (Fig. 3B) and the phosphorylation of Akt (Ser473) (Fig. 3C).
FIG. 3.
IL-6 decreases insulin signaling in HAECs. Representative blots and quantification of phosphorylation (Tyr612) of IRS-1/total IRS-1 pulled down by immunoprecipation (IP) (A), expression of the p85 subunit of PI3-kinase associated with immunoprecipitated IRS-1 (B), and phosphorylation (Ser473)/total Akt (C) in HAECs treated with or without (PBS control) IL-6 (10 ng/ml) for 2 h, followed by treatment with or without (PBS control) insulin (100 nmol/l) for 15 min. *Difference (P < 0.05) IL-6 + insulin versus insulin; **difference (P < 0.05) insulin versus control; ***Difference (P < 0.05) IL-6 versus control (data are means ± SE; n = 3–5 replicates from three different experiments). IB, immunoblot.
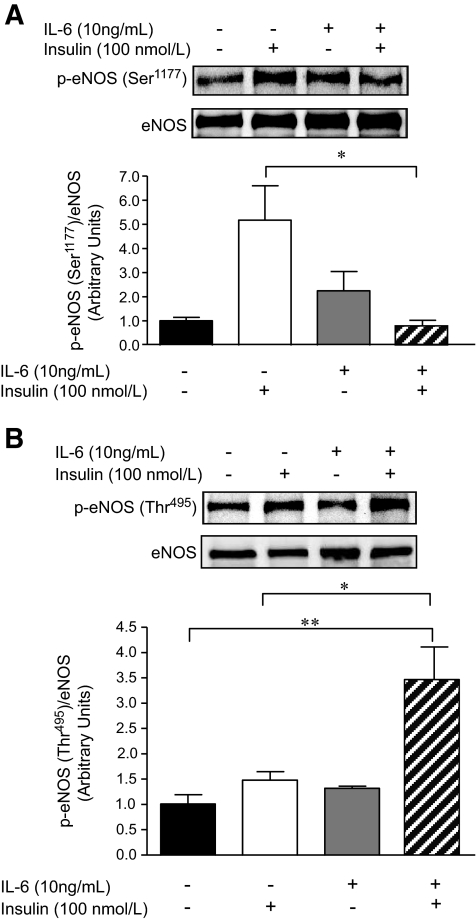
IL-6 blunts the insulin-mediated activation of eNOS in, and NO production from, HAECs.
We tested the effect of IL-6 on phosphorylation of eNOS at sites that activate (Ser1177) or inhibit (Thr495) the enzyme. Treatment with IL-6 alone did not significantly affect the basal phosphorylation of eNOS at either residue, although consistent with the effect of IL-6 alone on Akt phosphorylation (Fig. 3C), this treatment tended (NS) to increase eNOS phosphorylation at Ser1177 (Fig. 4A). Although treatment of HAECs with insulin markedly phosphorylated eNOS at Ser1177, pretreatment with IL-6 completely prevented this effect (Fig. 4A). In contrast, although neither insulin nor IL-6 treatment in isolation affected phosphorylation of eNOS at Thr495, the regulatory site that reduces synthase activity, pretreatment with IL-6 markedly increased insulin-mediated eNOS phosphorylation at this site (Fig. 4B). To determine whether the previously described signaling events resulted in parallel physiological changes, we examined the production of NO from endothelial cells in vitro. Consistent with the eNOS phosphorylation results, insulin treatment resulted in an approximately ninefold increase in NO production, but pretreatment with IL-6 abolished this effect (Fig. 5A and B).
FIG. 4.
IL-6 blunts the insulin-mediated activation of eNOS in HAECs. Representative blots and quantification of phosphorylation (Ser1177 [A] or Thr495 [B])/total eNOS in HAECs treated with or without (PBS control) IL-6 (10 ng/ml) for 2 h, followed by treatment with or without (PBS control) insulin (100 nmol/l) for 15 min. *Difference (P < 0.05) IL-6 + insulin versus insulin; **difference (P < 0.05) IL-6 + insulin versus control (data are means ± SE; n = 3–4 replicates from 3–4 different experiments).
FIG. 5.
IL-6 blunts the insulin-mediated production of NO from HAECs and decreases insulin-stimulated capillary recruitment in vivo. Representative fluorescence (A) and quantification (B) of NO production in HAECs treated with or without (PBS control) IL-6 (10 ng/ml) for 2 h, followed by treatment with or without (PBS control) insulin (100 nmol/l) for 15 min. Cells were viewed for 10 min, and fluorescence intensity was recorded at 15-s intervals. Calcium ionophore and S-nitroso-N-acetyl-penicilamine (SNAP) were used as positive controls for NO synthesis. C: 1-MX disappearance in hindlimb muscles from rats that underwent control (vehicle) and euglycemic-hyperinsulinemic clamps (insulin; 3 mU · min–1 · kg–1 ) for 120 min with and without the infusion of IL-6 (5.0 μg · h−1 · kg−1 ). *Difference (P < 0.05) IL-6 + insulin versus insulin (A) and insulin versus all other conditions (C); **difference (P < 0.05) insulin versus control (data are means ± SE; n = 3–5 replicates from three different experiments for A and B, n = 6 for C). (A high-quality digital representation of this figure is available in the online issue.)
IL-6 decreases insulin-stimulated capillary recruitment in vivo.
Our in vitro data clearly showed that IL-6 inhibited insulin-mediated endothelial cell signaling and NO production. Whether or not this translated into impaired vascular function in vivo was the next question that we sought to answer. Consistent with our in vitro data, whereas IL-6 had no effect per se on 1-MX disappearance, it completely prevented the insulin-mediated increase in capillary recruitment in the rat hindlimb (Fig. 5C).
IL-6 augments insulin action in muscle and fat but results in hepatic insulin resistance in vivo.
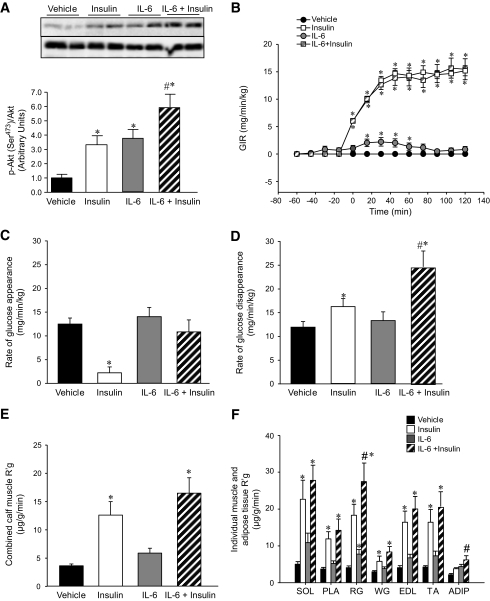
Having established that IL-6 has a negative effect on insulin-mediated endothelial cell function and capillary recruitment in vivo, we examined how the effect of IL-6 on capillary recruitment impacted insulin action and glucose uptake in rats under euglycemic-hyperinsulinemic clamp conditions. Unfortunately, muscle and fat tissue were only examined after 2-h clamp experiments, and, therefore, early signaling events were not captured. No differences were observed in phosphorylation of AMPK (Thr172), the downstream target acetyl-CoA carboxylase (Ser79), IRS-1 (Tyr612), or the association of the p85 subunit of PI3-kinase with IRS-1 in skeletal muscle with any treatment condition (data not shown). As activation of these signal transduction proteins by insulin and/or IL-6 is a rapid, transient event, it is likely that any effects were missed by sampling tissue after 2 h. Nonetheless, at this time point, both insulin and IL-6 independently increased Akt phosphorylation (Ser473). Consistent with previous studies (14,18), the effects of IL-6 and insulin on Akt phosphorylation were additive (Fig. 6A). We assessed insulin sensitivity via the glucose infusion rates (GIRs) during the clamp. Under non–insulin-stimulated conditions, IL-6 increased the GIR above that of the vehicle control. During insulin clamp conditions, we observed no effect of IL-6 infusion (Fig. 6B). These data contrast with those of previous studies from our group, in which IL-6 increased GIR during a euglycemic-hyperinsulinemic clamp in humans (14). In this previous study, IL-6 had no effect on the full suppression of hepatic glucose production by insulin in humans. In contrast, in the present study, whereas insulin markedly decreased the rate of glucose appearance (Ra), IL-6 restored this rate to levels seen in the absence of insulin (Fig. 6C). These data highlight the differences between rodents and humans in the capacity for IL-6 to affect liver glucose metabolism under insulin-stimulated conditions. Given that GIR was the same (Fig. 6B), but Ra was greater (Fig. 6C) with IL-6 under insulin-stimulated conditions, and given the Akt results, it was not surprising that IL-6 markedly enhanced the rate of whole body glucose disappearance during euglycemic-hyperinsulinemic clamp conditions (Fig. 6D). To investigate this effect further, we examined the rate of glucose uptake (R′g) into specific insulin-responsive depots. As expected, insulin increased R′g into the combined calf muscles, whereas there was a tendency (NS) for IL-6 to augment this effect (Fig. 6E). When we examined individual muscle and adipose tissue depots individually, we observed significant differences in R′g in red gastrocnemius muscles and adipose tissue when we compared insulin stimulation in the absence or presence of IL-6 (Fig. 6F). These data demonstrate that, despite compromising capillary blood flow, IL-6 can paradoxically enhance glucose uptake in skeletal muscle and fat under insulin-stimulated conditions.
FIG. 6.
IL-6 augments insulin action in muscle and fat but results in hepatic insulin resistance in vivo. Representative blots and quantification of phosphorylation (Ser473 )/total Akt in mixed hindlimb muscle (A), GIR (B), rate of glucose appearance (C) and disappearance (D), combined calf muscle glucose uptake (R′g) (E) and individual hindlimb muscle (SOL, soleus; PLA, plantaris; RG, red gastrocnemius; WG, white gastrocnemius, EDL, extensor digitorum longus; TA, tibialis anterior) and adipose tissue (ADIP) (F) from rats that underwent control (vehicle) and euglycemic-hyperinsulinemic clamps (insulin 3 mU · min–1 · kg–1 ) for 120 min with and without the infusion of IL-6 (5.0 μg · h–1 · kg–1 ). *Difference (P < 0.05) from vehicle; #difference IL-6 + insulin versus insulin (data are means ± SE; n = 6).
Negative effects of IL-6 on insulin signaling and NO production are not mediated by activation of c-Jun NH2-terminal kinase, extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase, or IκB kinase.
From these results, it was clear that IL-6 had different effects on insulin action in endothelial cells compared with those in skeletal muscle. We sought to elucidate a mechanism for such an effect. A recent article suggested that IL-6 impairs insulin-mediated NO production in human umbilical vein endothelial cells (HUVECs) through activation of the mitogen-activated protein kinases (MAPKs) c-Jun NH2-terminal kinase (JNK) and extracellular signal-regulated kinase 1/2 (ERK1/2) (35). In addition, the serine threonine kinase IκB kinase (IKK) has been shown to negatively affect insulin signaling in liver via an IL-6–dependent mechanism (36), whereas an alternative proinflammatory cytokine, TNF-α, is known to induce insulin resistance in endothelial cells via a p38 MAPK-dependent pathway (37). Accordingly, we examined whether these pathways were involved in the IL-6–dependent insulin resistance in our experimental model. We found no differences when examining the effects on these various signaling pathways when cells were incubated from 0 to 60 min (supplementary Fig. 2A). Unexpectedly, we found that incubation with IL-6 for 120 min markedly reduced the insulin-mediated increase in phosphorylation of JNK (Thr183)/Tyr185), ERK1/2 (p44/42 Thr202)/Tyr204), p38 MAPK (Thr180)/Tyr182), and IKKαβ (Ser180)/Ser181) (supplementary Fig. 2B–E). These pathways could not explain why IL-6 and insulin resulted in dysregulated endothelial cell signaling.
IL-6 combined with insulin increases membrane-bound TNF-α protein expression in HAECs but not in skeletal muscle.
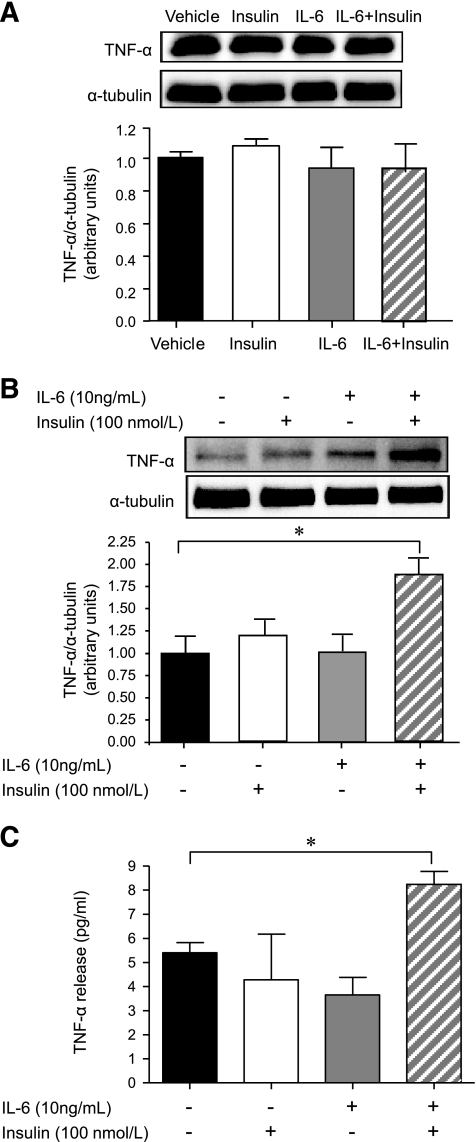
TNF-α is known to downregulate eNOS expression and activity in endothelial cells (37), white and brown adipose tissue (38), and skeletal muscle (38). In addition, as discussed, we have previously shown that the proinflammatory cytokine TNF-α not only reduces insulin signaling and AMPK activity in skeletal muscle (25) but also attenuates insulin-stimulated increases in capillary recruitment (26). Accordingly, we measured both TNF-α mRNA and membrane-bound TNF-α protein expression in HAECs and in mixed hindlimb skeletal muscles from the in vivo study. We observed no effect of insulin either with or without IL-6 on TNF-α mRNA in either HAECs (vehicle 1.0 ± 0.06; insulin [100 nmol/l] 1.1 ± 0.19; IL-6 [10 ng/ml] 0.98 ± 0.10; insulin + IL-6 1.31 ± 0.10, fold change from vehicle, NS) or mixed hindlimb skeletal muscle (vehicle 1.0 ± 0.41; insulin [3 mU · min−1 · kg−1] 2.0 ± 0.42; IL-6 [5.0 μg · h−1 · kg−1] 2.40 ± 0.80; insulin + IL-6 2.01 ± 0.83, fold change from vehicle, NS), and we did not find any effects of treatment on TNF-α protein expression in hindlimb mixed skeletal muscle (Fig. 7A). Although insulin and IL-6 alone had no effect on TNF-α protein expression in HAECs, an approximate twofold increase in membrane-bound TNF-α protein expression was observed in IL-6–pretreated, insulin-stimulated HAECs (Fig. 7B). When we measured TNF-α protein expression in the media from treated HAECs, we observed an increase in TNF-α release when comparing the IL-6 + insulin-treated cells with control cells (Fig. 7C).
FIG. 7.
The combined treatment of IL-6 and insulin increases TNF-α protein expression in and release from HAECs but not skeletal muscle. Representative blots and quantification of membrane-bound TNF-α in mixed hindlimb muscle that underwent control (vehicle) and euglycemic-hyperinsulinemic clamps (insulin 3 mU · min–1 · kg–1 ) for 120 min with and without the infusion of IL-6 (5.0 μg · h–1 · kg–1 ) (A). Representative blots and quantification of membrane-bound TNF-α (B) and TNF-α release into media (C) in HAECs treated with or without (PBS control) IL-6 (10 n/ml) for 2 h, followed by treatment with or without (PBS control) insulin (100 nmol/l) for 15 min *Difference (P < 0.05) from vehicle control (data are means ± SE; n = 3–5 replicates from 1–3 different experiments for in vitro experiments, n = 6 for in vivo experiments).
Negative effects of IL-6 on insulin signaling are mediated by TNF-α protein expression.
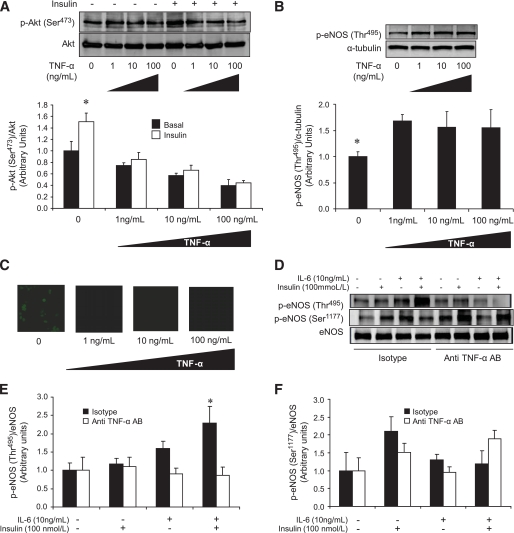
To determine whether the increase in TNF-α could have accounted for our observations in HAECs treated with IL-6 and insulin, we stimulated HAECs with TNF-α in a dose-dependent manner. TNF-α, even at the lowest dose of 1 ng/ml, completely blunted insulin-stimulated Akt phosphorylation (Ser473) (Fig. 8A), thus increasing eNOS phosphorylation at the inhibitory site (Thr495) (Fig. 8B). Such treatment completely blocked basal NO production (Fig. 8C). To determine whether the negative effects of IL-6 and insulin on eNOS signaling were mediated by the elevated TNF-α levels observed in this treatment condition, we performed TNF-α neutralization experiments. As expected, when pretreated with the isotype control, the combination of IL-6 and insulin increased phosphorylation of eNOS at the inhibitory site (Thr495). In contrast, this increase was completely prevented when cells were pretreated with an anti-TNF-α–neutralizing antibody (Fig. 8D and E). In addition, although not statistically significant, treatment with an anti-TNF-α–neutralizing antibody tended (P = 0.14) to rescue the blunted phosphorylation of eNOS at Ser1177 observed when cells were treated with IL-6 and insulin (Fig. 8D and F).
FIG. 8.
The negative effects of IL-6 on insulin signaling are mediated by TNF-α protein expression. Representative blots and quantification of phosphorylation (Ser473 )/total Akt (A), eNOS phosphorylation (Thr495 )/α-tubulin (B), and representative NO production images (C) in HAECs treated with or without (PBS control) TNF-α (1, 10, or 100 ng/ml) for 2 h followed by treatment with or without (PBS control) insulin (100 nmol/l). Representative blots (D) and quantification of phosphorylation of eNOS (Thr495 [E] ot Ser1177 [F]) total eNOS in HAECs pretreated for 1 h with 0.1 μg/ml of either mouse IgG1 isotype control antibody (Isotype) or mouse IgG1 anti-human TNF-α neutralization antibody (anti-TNF-α AB) then treated with or without (PBS control) IL-6 (10 ng/ml) for 2 h, followed by treatment with or without (PBS control) insulin (100 nmol/l) for 15 min. *Difference insulin versus basal in the absence of TNF-α (A), basal versus all doses of TNF-α (B), isotype-pretreated IL-6 + insulin versus isotype-pretreated vehicle control (E) (data are means ± SE; n = 3–5 replicates from 2–3 different experiments). (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
IL-6 is a biologically active cytokine involved in the acute inflammatory response but is also secreted by adipose tissue and skeletal muscle in the absence of inflammation to modify metabolism (39). Although recent studies have suggested that IL-6 may stimulate metabolic processes by enhancing insulin action (14) and preventing obesity (40), this cytokine has been linked to endothelial dysfunction (41) and increased risk of developing coronary heart disease (6). Here we provide evidence that IL-6 can enhance insulin action in peripheral tissues such as skeletal muscle. Paradoxically, IL-6 decreases insulin signaling and activation of AMPK in endothelial cells in the presence, but not the absence, of insulin. Furthermore, IL-6 attenuates insulin-mediated increases in capillary recruitment in hindlimb muscles.
Recent evidence has demonstrated that IL-6 is produced in (10) and released from (11) skeletal muscle. During vigorous, prolonged exercise, circulating IL-6 levels can increase up to 100-fold above basal levels (42). Our data demonstrating that IL-6 impairs endothelial cell signaling and capillary recruitment may appear surprising because exercise, which results in such a marked release of IL-6, also results in enhanced endothelial cell function (43) and capillary recruitment (44). Note that IL-6 treatment of cells in the absence of insulin resulted in an increase in Akt phosphorylation (Fig. 3C) and tended to elevate eNOS phosphorylation at serine residue 1177 (Fig. 4A) and NO production (Fig. 5A and B). It was only in the presence of insulin that IL-6 had such a marked inhibitory effect on both endothelial cell and vascular function. These data suggest that IL-6 plays an inhibitory role in vascular function in states of insulin resistance and may help to explain why IL-6 may be linked to vascular disease (6). Our data may explain why IL-6 is linked to the metabolic syndrome, in which patients present with a cluster of disorders, including atherosclerosis, hypertension, inflammation, and insulin resistance (45). It should be noted that the elevated IL-6 observed during exercise has a permissive effect on capillary recruitment because, in separate experiments, we demonstrated that hindlimb IL-6 infusion during muscle contraction does not affect contraction-induced increases in 1-MX disappearance (data not shown). Although previous studies have shown a tight correlation between insulin-mediated capillary recruitment and glucose uptake by muscle, this is the first study in which insulin-mediated capillary recruitment has been inhibited and insulin-mediated glucose uptake has been unaffected or augmented. This finding may mean that although microvascular perfusion of muscle is incomplete because of the global inhibition of capillary recruitment by the hindlimb as indicated by 1-MX metabolism, those regions of muscle receiving insulin, IL-6, and glucose are stimulated. Such a scenario is suggested by the further activation of muscle Akt by IL-6 and insulin that has already been activated by insulin alone (Fig. 7A).
It is important to note that our data showing that IL-6 and insulin increase phosphorylation of Akt and glucose uptake by skeletal muscle and adipose tissue support previous work from our group in skeletal muscle and adipocyte cell culture (14). However, in contrast with previous studies in humans (14), we showed that the GIR during a clamp was not augmented by IL-6. This was because, unlike our previous data from studies in humans, the increase in glucose disappearance (Rd) seen with IL-6 treatment (Fig. 6D) was completely offset by an increase in Ra. Thus, in contrast with human studies, in which IL-6 does not affect hepatic insulin sensitivity, in studies with rats it induces hepatic insulin resistance, augmenting insulin action in both skeletal muscle and adipose tissue.
These data demonstrate that IL-6 has marked opposing effects in endothelial cells compared with skeletal muscle. In separate experiments in which we have incubated muscle cells and adipocytes in a fashion similar to our treatment of HAECs, we have shown activation of AMPK and insulin signaling (14). In addition, consistent with our in vivo experiments reported here, we have previously demonstrated in human euglycemic-hyperinsulinemic clamps that IL-6 augments muscle glucose uptake (14). Our in vivo capillary recruitment measurements (Fig. 5C), which are indicative of vascular function, were entirely consistent with our cell culture data. We assessed many potential candidates to account for the differential effects of IL-6 on endothelial cells compared with muscle cells and adipocytes. In a previous study in HUVECs, it was shown that IL-6 induces insulin resistance via activation of JNK and ERK1/2 pathways (36). This was clearly not the case in our study because IL-6 markedly blocked insulin-induced increases in both JNK and ERK1/2 (see supplementary Fig. 2B and C). This is not surprising to us because the phenotype of endothelial cells is specific to the vascular bed from which they originate, and global gene expression profiling studies have demonstrated marked endothelial cell diversity (46). Indeed, in preliminary experiments in which we characterized our endothelial cell culture model, we observed a markedly different phenotype when comparing HUVECs with HAECs (data not shown). In addition, IKK is known to negatively affect insulin signaling via an IL-6–dependent mechanism (36), whereas TNF-α is known to induce insulin resistance in endothelial cells via a p38 MAPK–dependent pathway (37). In our model, activation of IKK or p38 MAPK could not account for our observations (see supplementary Fig. 2D and E). In contrast, we were able to show that in the presence, but not in the absence, of insulin, membrane-bound TNF-α protein expression in (Fig. 7B) and released from (Fig. 7C) HAECs increased approximately twofold, but no such effect was seen in skeletal muscle (Fig. 7A). It was indeed surprising that IL-6 alone did not increase TNF-α, but our data clearly pointed toward the endothelial cell–specific synergistic effect of IL-6 and insulin leading to a posttranscriptional increase in the membrane-bound form of TNF-α as the mechanism behind the differences we observed in endothelium versus skeletal muscle. Given the relatively short (15 min) exposure to insulin, it is likely that the combination of insulin and IL-6 led to an increase in the trafficking of TNF-α out of the cells rather than an increase in protein synthesis per se. Incubation of HAECs with various doses of TNF-α increased phosphorylation of eNOS at the negative regulatory site (Thr495) and completely suppressed both insulin-stimulated Akt phosphorylation and NO release (Fig. 8A–C). When we pretreated HAECs with a TNF-α–neutralizing antibody and then incubated them with IL-6 followed by insulin, the increase in eNOS phosphorylation (Thr495) was completely abrogated (Fig. 8D and E), whereas the blunted phosphorylation of eNOS at the active site (Ser1177) tended to be rescued (Fig. 8D and F).
In summary, we demonstrate that IL-6 decreases insulin-stimulated NO production from endothelial cells via a decreased activation of insulin signaling mediated by enhanced TNF-α production. Consistent with these cellular observations, we show that IL-6 decreases insulin-stimulated capillary recruitment. Paradoxically, however, IL-6 increases insulin-stimulated glucose uptake into skeletal muscle and adipose tissue via enhanced insulin signaling, at least in skeletal muscle, in which the combination of IL-6 and insulin does not lead to elevated TNF-α expression. Our results highlight the complex role of this cytokine in the etiology of whole-body metabolism.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by a grant from the National Health and Medical Research Council of Australia (NHMRC) (Project Grant 342115 awarded to M.A.F., M.G.C., and S.R.). V.B.M. is supported, in part, by a Baker Heart Research Institute Early Career Scientist Grant. B.G.D. is supported by a Dora Lush postgraduate scholarship from the NHMRC. S.R. is a Senior Research Fellow, and B.A.K. and M.A.F. are Principal Research Fellows of the NHMRC.
D.Y.C.Y. is supported by a postgraduate support grant from GlaxoSmithKline Australia. No other potential conflicts of interest relevant to this article were reported.
We thank Robert Southgate for his technical support.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Wellen KE, Hotamisligil GS: Inflammation, stress, and diabetes. J Clin Invest 2005; 115: 1111– 1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE: Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res 2001; 9: 414– 417 [DOI] [PubMed] [Google Scholar]
- 3.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B: Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 2000; 85: 3338– 3342 [DOI] [PubMed] [Google Scholar]
- 4.Carey AL, Bruce CR, Sacchetti M, Anderson MJ, Olson DB, Saltin B, Hawley JA, Febbraio MA: Interleukin-6 and tumor necrosis factor-α are not elevated in patients with type 2 diabetes: evidence that plasma IL-6 is related to fat mass and not insulin responsiveness. Diabetologia 2004; 47: 1029– 1037 [DOI] [PubMed] [Google Scholar]
- 5.Lazar M: How obesity causes diabetes: not a tall tale. Science 2005; 307: 373– 375 [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V: Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med 2008; 5: e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey AL, Febbraio MA: Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia 2004; 47: 1135– 1142 [DOI] [PubMed] [Google Scholar]
- 8.Kristiansen OP, Mandrup-Poulsen T: Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 2006; 54 ( Suppl. 2): S114– S124 [DOI] [PubMed] [Google Scholar]
- 9.Penkowa M, Keller C, Keller P, Jauffred S, Pedersen BK: Immunohistochemical detection of interleukin-6 in human skeletal muscle fibers following exercise. FASEB J 2003; 17: 2166– 2168 [DOI] [PubMed] [Google Scholar]
- 10.Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA: Skeletal myocytes are the source of Interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J 2004; 18: 992– 994 [DOI] [PubMed] [Google Scholar]
- 11.Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK: Low glycogen content increases interleukin-6 in contracting human skeletal muscle. J Physiol 2001; 537: 633– 639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King DS, Dalsky GP, Clutter WE, Young DA, Staten MA, Cryer PE, Holloszy JO: Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J Appl Physiol 1998; 64: 1942– 1946 [DOI] [PubMed] [Google Scholar]
- 13.Wojtaszewski JF, Hansen BF, Gade Kiens B, Markuns JF, Goodyear LJ, Richter EA: Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 2000; 49: 325– 331 [DOI] [PubMed] [Google Scholar]
- 14.Carey AL, Steinberg GR, Macaulay SL, Thomas WJ, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Petersen BK, Febbraio MA: IL-6 increases insulin stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMPK. Diabetes 2006; 55: 2688– 2697 [DOI] [PubMed] [Google Scholar]
- 15.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA: Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 2002; 282: E18– E23 [DOI] [PubMed] [Google Scholar]
- 16.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE: 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem 2001; 276: 46912– 46916 [DOI] [PubMed] [Google Scholar]
- 17.Weigert C, Hennige AM, Brodbeck K, Haring HU, Schleicher ED: Interleukin-6 (IL-6) acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser-473 of Akt. Am J Physiol Endocrinol Metab 2005; 289: E251– E257 [DOI] [PubMed] [Google Scholar]
- 18.Febbraio MA: gp130 receptor ligands: potential therapeutic targets in obesity. J Clin Invest 2007; 117: 841– 849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laakso M, Edelman SV, Brechtel G, Baron AD: Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man: a novel mechanism for insulin resistance. J Clin Invest 1990; 85: 1844– 1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S: Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 2003; 284: E241– E258 [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ: Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 2004; 53: 447– 453 [DOI] [PubMed] [Google Scholar]
- 22.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ: Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004; 53: 1418– 1423 [DOI] [PubMed] [Google Scholar]
- 23.Zeng G, Quon MJ: Insulin-stimulated production of nitric oxide is inhibited by wortmannin: direct measurement in vascular endothelial cells. J Clin Invest 1996; 98: 894– 898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE: AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 1999; 443: 285– 289 [DOI] [PubMed] [Google Scholar]
- 25.Steinberg GR, Michell BJ, van Denderen B, Watt MJ, Fam BC, Andrikopoulos S, Gorgun CZ, Proietto J, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE: Tumor necrosis factor-α induced skeletal muscle insulin resistance involves the suppression of AMP-kinase signaling. Cell Metab 2006; 4: 465– 474 [DOI] [PubMed] [Google Scholar]
- 26.Youd JM, Rattigan S, Clark MG: Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-α. Diabetes 2000; 49: 1904– 1909 [DOI] [PubMed] [Google Scholar]
- 27.Rattigan S, Clark MG, Barrett EJ: Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes 1997; 46: 1381– 1388 [DOI] [PubMed] [Google Scholar]
- 28.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR: Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 2002; 282: E714– E720 [DOI] [PubMed] [Google Scholar]
- 29.James DE, Jenkins AB, Kraegen EW: Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol 1985; 248: E567– 574 [DOI] [PubMed] [Google Scholar]
- 30.Kraegen EW, James DE, Jenkins AB, Chisholm DJ: Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol 1985; 248: E353– E362 [DOI] [PubMed] [Google Scholar]
- 31.Starkie RL, Arkinstall R MJ, Koukoulas I, Hawley JA, Febbraio MA: Carbohydrate attenuates the increase in plasma IL-6, but not in skeletal muscle IL-6 mRNA, during exercise in humans. J Physiol 2001; 533: 585– 591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watt MJ, Dzamko N, Thomas W, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR: CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med 2006; 12: 541– 548 [DOI] [PubMed] [Google Scholar]
- 33.Hoene M, Weigert C: The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev 2008; 9: 20– 29 [DOI] [PubMed] [Google Scholar]
- 34.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM: Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999; 399: 601– 605 [DOI] [PubMed] [Google Scholar]
- 35.Andreozzi F, Laratta E, Procopio C, Hribal ML, Sciacqua A, Perticone M, Miele C, Perticone F, Sesti G: Interleukin-6 impairs the insulin signaling pathway, promoting production of nitric oxide in human umbilical vein endothelial cells. Mol Cell Biol 2007; 27: 2372– 2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE: Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 2005; 11: 183– 190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G, Barrett EJ, Barrett MO, Cao W, Liu Z: Tumor necrosis factor-α induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology 2007; 148: 3356– 3363 [DOI] [PubMed] [Google Scholar]
- 38.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E: TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 2006; 116: 2791– 2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Febbraio MA, Pedersen BK: Muscle derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 2002; 16: 1335– 1347 [DOI] [PubMed] [Google Scholar]
- 40.Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO: Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 2002; 8: 75– 79 [DOI] [PubMed] [Google Scholar]
- 41.Fernández-Real JM, Ricart W: Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev 2003; 24: 278– 301 [DOI] [PubMed] [Google Scholar]
- 42.Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA: Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-α levels after prolonged running. Am J Physiol Cell Physiol 2001; 280: C769– C774 [DOI] [PubMed] [Google Scholar]
- 43.Kingwell BA: Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB J 2000; 14: 1685– 1696 [DOI] [PubMed] [Google Scholar]
- 44.Rattigan S, Bradley EA, Richards SM, Clark MG: Muscle metabolism and control of capillary blood flow: insulin and exercise. Essays Biochem 2006; 42: 133– 144 [DOI] [PubMed] [Google Scholar]
- 45.Semenkovich CF: Insulin resistance and atherosclerosis. J Clin Invest 2006; 116: 1813– 1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO: Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 2003; 100: 10623– 10628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.