Abstract
OBJECTIVE
We examined whether ingestion of medium-chain triglycerides could improve cognition during hypoglycemia in subjects with intensively treated type 1 diabetes and assessed potential underlying mechanisms by testing the effect of β-hydroxybutyrate and octanoate on rat hippocampal synaptic transmission during exposure to low glucose.
RESEARCH DESIGN AND METHODS
A total of 11 intensively treated type 1 diabetic subjects participated in stepped hyperinsulinemic- (2 mU · kg−1 · min−1) euglycemic- (glucose ∼5.5 mmol/l) hypoglycemic (glucose ∼2.8 mmol/l) clamp studies. During two separate sessions, they randomly received either medium-chain triglycerides or placebo drinks and performed a battery of cognitive tests. In vitro rat hippocampal slice preparations were used to assess the ability of β-hydroxybutyrate and octanoate to support neuronal activity when glucose levels are reduced.
RESULTS
Hypoglycemia impaired cognitive performance in tests of verbal memory, digit symbol coding, digit span backwards, and map searching. Ingestion of medium-chain triglycerides reversed these effects. Medium-chain triglycerides also produced higher free fatty acids and β-hydroxybutyrate levels compared with placebo. However, the increase in catecholamines and symptoms during hypoglycemia was not altered. In hippocampal slices β-hydroxybutyrate supported synaptic transmission under low-glucose conditions, whereas octanoate could not. Nevertheless, octanoate improved the rate of recovery of synaptic function upon restoration of control glucose concentrations.
CONCLUSIONS
Medium-chain triglyceride ingestion improves cognition without adversely affecting adrenergic or symptomatic responses to hypoglycemia in intensively treated type 1 diabetic subjects. Medium-chain triglycerides offer the therapeutic advantage of preserving brain function under hypoglycemic conditions without causing deleterious hyperglycemia.
Maintaining plasma glucose (PG) at near-normal levels in individuals with type 1 diabetes reduces the risk for developing long-term microvascular complications (1). However, intensive insulin therapy increases the risk of severe hypoglycemia, which can cause rapid deterioration of cognitive function and often occurs without warning symptoms (1,2). As a result, hypoglycemia limits the ability of patients to achieve target glycemic goals because the immediate fear of hypoglycemia exceeds the fear of long-term complications. Therefore, new strategies to protect the brain from hypoglycemia-induced injury are essential for optimizing the benefits of insulin therapy.
Although the brain relies primarily on glucose, it can use alternative fuels such as monocarboxylic acids, lactate, and ketones to maintain energy homeostasis (3–7). Exposure to prolonged fasting or hypoglycemia causes adaptive changes in the brain, including an enhanced ability to utilize alternative fuels (3,8,9). Thus, patients with intensively managed type 1 diabetes, by virtue of their increased exposure to hypoglycemia, may develop an enhanced ability to use alternate fuels, which, in turn, might provide neuroprotection during hypoglycemia.
Medium-chain triglycerides, constituents of coconut and palm kernel oils, are medium-chain fatty acid esters of glycerol. Medium-chain triglycerides have a favorable safety profile and are used to treat a variety of disorders (10–12). They offer a readily available noncarbohydrate fuel source because they are rapidly absorbed and quickly metabolized into medium-chain fatty acids (10). Medium-chain fatty acids do not require chylomicrons for transport or carnitine for entry into mitochondria (10). As a result, metabolism of medium-chain fatty acids promotes the generation of ketones (10). Furthermore, animal data suggest that medium-chain fatty acids can readily cross the blood-brain barrier (BBB) and be oxidized by the brain (13). Thus, medium-chain fatty acids may provide both a direct and an indirect brain fuel source via the generation of ketones, offering type 1 diabetic patients a prophylactic treatment strategy to preserve brain function during hypoglycemic episodes without raising blood glucose levels.
To explore this possibility, we evaluated whether oral medium-chain triglycerides could improve cognitive performance during acute insulin-induced hypoglycemia in intensively treated type 1 diabetic subjects. In addition, an in vitro hippocampal slice preparation from nondiabetic rats was used to assess the ability of β-hydroxybutyrate and octanoate to support neuronal activity when the glucose supply is deficient.
RESEARCH DESIGN AND METHODS
A total of 11 individuals (5 men, 6 women, aged [mean ± SD] 34.8 ± 8.9 years, BMI 24.2 ± 3.4 kg/m2) with type 1 diabetes for 15.9 ± 9.5 years participated in the study. Subjects had no medical problems other than type 1 diabetes and had a normal physical exam and electrocardiogram. Blood tests confirmed absent C-peptide levels and normal renal and liver function. Subjects were intensively controlled with insulin (10 with continuous subcutaneous insulin infusion [CSII] and 1 with multiple daily injections), as reflected by a mean A1C of 6.9 ± 0.6 and a history of frequent hypoglycemic episodes, defined as self-reported fingerstick blood glucose <60 mg/dl. The number of hypoglycemic episodes per month was between 1 and 5 in two subjects, between 6 and 10 in two subjects, between 11 and 30 in six subjects, and >30 in one subject. Subjects gave their written informed consent to participate in this study, which was approved by the Yale University human investigation committee.
Experimental protocol.
Nine subjects underwent two stepwise hyperinsulinemic-euglycemic-hypoglycemic clamp studies with ingestion of either the medium-chain triglycerides or placebo drink in random order in a crossover design, as described below. Of the 11 subjects, 2 participated in one study session (1 with placebo and 1 with medium-chain triglycerides). Cognitive data from all 11 subjects were included in the analysis as permitted by the mixed model. Paired Student's t tests were used to compare substrate and hormone levels between medium-chain triglycerides and control sessions for the nine subjects who completed both sessions.
Subjects were admitted to the Hospital Research Unit (HRU) of the Yale Center for Clinical Investigation on the evening before the study. Dinner was served at 6:00 p.m., and they were fasted overnight until the end of the study the following day. At approximately 9:00 p.m., an intravenous catheter was inserted into an antecubital vein for infusion of insulin (regular human insulin; Novo Nordisk, Bagsvaerd, Denmark) and dextrose to maintain euglycemia overnight. Subjects who used CSII had the option of being admitted to the HRU on the morning of each session, at which time their CSII infusion was suspended, and an intravenous catheter was inserted in an antecubital vein for insulin and glucose administration. Of the 10 CSII-treated patients, 3 chose this option and were admitted on the morning of each session. These subjects reduced their basal insulin dose by 20% and checked blood glucose at home before bedtime and on awakening. The study was cancelled if blood glucose was <70 mg/dl based on home glucose measurements.
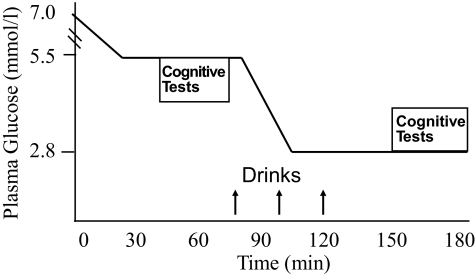
At approximately 7:30 a.m., a second catheter was placed in a retrograde fashion into a dorsal vein of the nondominant hand for blood sampling. The hand was placed in a heated box (∼50–55°C) to arterialize venous blood. At time = 0, PG was indistinguishable on the placebo (6.8 ± 0.4 mmol/l) and medium-chain triglyceride days (7.0 ± 0.7 mmol/l). A primed continuous infusion of insulin was then initiated and maintained at a constant rate of 2.0 mU · kg−1 · min−1, and a variable rate of 20% dextrose was infused concomitantly (Fig. 1). At 75 min, subjects ingested over a 5-min period the first of a series of three drinks, each in 50-ml volumes, containing either medium-chain triglycerides or sucralose, a sugar substitute. During the medium-chain triglycerides session, a total of 40 g of medium-chain triglycerides (derived from coconut oil containing 67% octanoate, 27% decanaote, and 6% other fatty acids; Novartis) was ingested at 25-min intervals with front loading of 20 g then 10 g twice. During the control session, cherry-flavored water sweetened with sucralose was ingested at identical time intervals. Drinks were prepared by the HRU. At 5 min after the first drink, PG (mean ± SE) was lowered to 2.8 ± 0.16 mmol/l for the hypoglycemic phase of the clamp. PG was measured in duplicate every 5 min to ensure a stable glucose plateau. Blood samples were collected for glucose, lactate, β-hydroxybutyrate, glycerol, free fatty acids (FFAs), insulin, glucagon, norepinephrine, and epinephrine levels at baseline and at 20-min intervals.
FIG. 1.
Variable rate glucose infusion and primed continuous infusion of insulin (2 mU · kg−1 · min−1). Hyperinsulinemic clamps were used to maintain euglycemic conditions for 90 min followed by a 90-min hypoglycemic phase. Cognitive tests were administered during steady-state euglycemia and hypoglycemia. The study drink (medium-chain triglycerides or placebo) was given at time = 75, 100, and 125 min. Upward arrows indicate time of drink administration.
During the euglycemic phase (from 45 to 70 min) and again during the hypoglycemic phase (from 155 to 180 min), subjects completed a battery of cognitive tasks. Tests of nonmemory function included digit symbol substitution, Tests of Everyday Attention, telephone book searching, and map searching in 1 and 2 min. Tests of immediate and delayed verbal memory and verbal memory recognition were adapted from the Wechsler Memory Scale logical memory tests (14). Working memory was assessed by modified versions of the standard Wechsler Memory Scale Digit Span and Letter/Number Sequencing Tests (14). These cognitive tests have been validated in studies of the effect of hypoglycemia on cognition (15,16). Hypoglycemic symptoms were assessed by a self-rating questionnaire during both the euglycemic and hypoglycemic phases. Symptoms of hypoglycemia were classified as autonomic (racing heart, sweating, warmness, trembling, hunger, anxiety) or neuroglycopenic (weakness, tiredness, double vision, difficulty speaking, difficulty concentrating, drowsiness, confusion, blurry vision); the total symptom score was equal to the autonomic plus neuroglycopenic symptom scores.
Measurement of hormones and metabolites.
PG and lactate were measured enzymatically using glucose and lactate oxidase, respectively (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin and glucagon were measured using a double-antibody radioimmunoassay (Millipore, St. Charles, MO), epinephrine and norepinephrine by high-performance liquid chromatography (ESA, Chelmsford, MA), and FFAs using NEFA-HR (Wako Diagnostics, Richmond, VA). Plasma glycerol was measured by an enzymatic end point reaction with a CMA 600 analyzer (CMA Microdialysis, Chelmsford, MA) and β-hydroxybutyrate using an ACE chemical analyzer (Wako Diagnostics, Richmond, VA).
Animal protocol.
Standard methods were used for hippocampal slice preparation (17) using adult Sprague-Dawley rats (29 male, 13 female). The standard artificial cerebrospinal fluid (aCSF) contained (in mmol/l): 124 NaCl, 3 KCl, 2 MgSO4, 1.2 NaH2PO4, 26 NaHCO3, 2.0 CaCl2, and 10 glucose, pH 7.4. The slices (400 μm) were placed on the stage of an interface recording chamber (Fine Science Tools, Foster City, CA), where they were superfused with aCSF and maintained at 32°C ± 0.5.
Local field potentials were recorded in the cell body layer of CA1 using a low-resistance (3 mol/lΩ) patch pipette filled with aCSF; a twisted bipolar electrode placed in the Schaffer collaterals was used to evoke synaptic responses. The baseline response used for the experiment was 50% of the maximal response recorded in aCSF. The stimulus intensity was not altered for the balance of the experiment. Synaptic responses were studied both at low frequencies (0.1 Hz) and after stimulus trains of 10 Hz for 10 s. At rest, brain slices have lower rates of oxidative phosphorylation than the intact brain. Therefore, to more accurately simulate the increased neural activity and metabolic demand seen during hippocampal memory processing (18) and to model cognitive activation, we used 10-Hz repetitive synaptic stimulation. The protocol will drive oxidative metabolism in slices (17) without causing significant synaptic plasticity (19).
Hypoglycemia was induced by a bath applying aCSF containing 2 mmol/l glucose with 8 mmol/l sucrose added to maintain osmolarity for 30 min. This concentration of bath glucose results in a tissue glucose of ∼0.5 mmol/l (20) compared with 5.0 mmol/l with a bath glucose of 10 mmol/l. The synaptic responses were delivered at 0.1 Hz during the wash-on period, and stimulus trains (10 Hz, 10 s) were delivered at 10-min intervals to investigate the relationship between the metabolic load and synaptic responses.
We examined three experimental conditions: 2 mmol/l glucose + 8 mmol/l β-hydroxybutyrate, 2 mmol/l glucose + 8 mmol/l octanoate, and 2 mmol/l glucose + 4 mmol/l β-hydroxybutyrate + 4 mmol/l octanoate. For each condition, the test compound(s) were bath applied for an additional 30 min with one stimulus train midway during the wash-on period. Control aCSF (10 mmol/l glucose) was then washed on to determine the ability of the tissue to recover from hypoglycemia.
Statistical analysis.
Data analysis was performed using SAS version 9.2 (Cary, NC). Clamp- and treatment-dependent changes were analyzed independently for each cognitive test using a mixed-model ANOVA. In the mixed-model ANOVA, fixed effects for the treatment order, treatment (medium-chain triglycerides vs. placebo), glucose (euglycemia vs. hypoglycemia), and their interactions were included, and correlation between repeated assessments was modeled using an unstructured covariance pattern (21). Linear contrasts were estimated to test differences in euglycemic to hypoglycemic cognitive changes between medium-chain triglycerides and placebo. The level of significance at individual time points was determined by paired Student's t tests with a Bonferroni correction for multiple testing. Paired Student's t tests were used to compare substrate and hormone levels between medium-chain triglycerides and control sessions during steady-state euglycemia and hypoglycemia. A P value <0.05 was considered significant. Except where noted, all data are reported as the means ± SE.
Physiology statistics.
The amplitude of the population spike was the primary measure. Paired Student's t tests, corrected for multiple comparisons, were used to test for significance at the different points in the experiment.
RESULTS
PG, insulin, and metabolite concentrations.
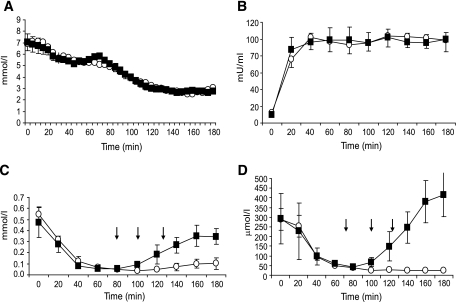
PG profiles were identical throughout medium-chain triglyceride and control sessions (Fig. 2). During steady-state euglycemia (from 30 to 75 min), PG was 5.5 ± 0.07 mmol/l in the medium-chain triglycerides and 5.4 ± 0.1 mmol/l in the control sessions (P = 0.4). Similarly, steady-state glucose levels during the final 40 min of the hypoglycemic phase were equivalent during the medium-chain triglyceride (2.74 ± 0.05 mmol/l) and control sessions (2.73 ± 0.06 mmol/l; P = 0.8). Plasma insulin also increased comparably in both sessions 99 ± 12 (medium-chain triglycerides) versus 98 ± 11 μU/ml (control; P = 0.4).
FIG. 2.
A–D: PG (A), plasma insulin (B), plasma FFA (C), and plasma β-hydroxybutyrate (D) profiles during the euglycemic-hypoglycemic clamp studies with medium-chain triglycerides or placebo ingestion. ■, medium-chain triglycerides; ○, placebo. Down arrows indicate drink administration.
During the euglycemic phase of both sessions, insulin suppressed plasma FFAs and β-hydroxybutyrate. During hypoglycemia, both metabolites remained suppressed in the control study but rose after administration of medium-chain triglycerides. During the final 40 min of hypoglycemia, plasma FFAs (0.323 ± 0.07 vs. 0.083 ± 0.04 mmol/l, P = 0.01) and β-hydroxybutyrate (356 ± 81 vs. 25 ± 1.4 μmol/l, P < 0.01) were significantly higher after medium-chain triglycerides compared with placebo. There were no differences between groups in plasma glycerol (22 ± 7 vs. 31 ± 8 μmol/l, P = 0.30) or lactate (0.94 ± 0.16 vs. 1.12 ± 0.17 mmol/l, P = 0.20) during hypoglycemia.
Cognitive tests.
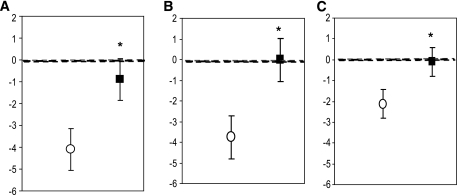
Acute hypoglycemia impaired cognitive performance in tests of immediate verbal memory (P < 0.001), delayed verbal memory (P = 0.005), verbal memory recognition (P < 0.001), digit symbol coding (P = 0.03), digit span backwards (P = 0.008), and map searching in 1 min (P = 0.04) as assessed by the change in performance from euglycemia to hypoglycemia after placebo ingestion (Fig. 3 and Table 1). When compared with ingestion of the placebo drink, medium-chain triglycerides prevented the decline in cognitive performance during hypoglycemia in tests of immediate verbal memory (P = 0.009), delayed verbal memory (P < 0.001), and verbal memory recognition (P = 0.0008). Medium-chain triglycerides also improved performance during hypoglycemia in digit symbol coding (P = 0.002) and total map searching (P = 0.04).
FIG. 3.
Medium-chain triglyceride ingestion preserved cognitive performance under hypoglycemic conditions in tests of verbal memory. A: Immediate verbal memory. B: Delayed verbal memory. C: Verbal memory recognition. Figures show change in test scores (euglycemia-hypoglycemia) after medium-chain triglycerides (■) or placebo (○). *P < 0.01 medium-chain triglycerides vs. placebo.
TABLE 1.
Cognitive test scores during euglycemia and hypoglycemia with medium-chain triglycerides or placebo ingestion
| Cognitive test | Medium-chain triglycerides euglycemia (∼5.5 mmol · l−1 · l−1) | Medium-chain triglycerides hypoglycemia (∼2.8 mmol · l−1 · l−1) | Placebo euglycemia (∼5.5 mmol · l−1 · l−1) | Placebo hypoglycemia (∼2.8 mmol · l−1 · l−1) |
|---|---|---|---|---|
| Immediate verbal memory | 15.85 ± 0.66 | 14.97 ± 1.13* | 17.36 ± 1.03 | 13.28 ± 1.04† |
| Delayed verbal memory | 14.82 ± 1.25 | 14.80 ± 1.31* | 15.33 ± 1.40 | 11.58 ± 0.71† |
| Verbal memory recognition | 13.19 ± 0.53 | 13.29 ± 0.50* | 14.27 ± 0.23 | 12.14 ± 0.19† |
| Digit span backwards | 0.60 ± 0.05 | 0.58 ± 0.05 | 0.64 ± 0.05 | 0.54 ± 0.05† |
| Letter/number sequencing | 12.04 ± 0.81 | 10.97 ± 0.76 | 11.07 ± 0.85 | 9.92 ± 0.71 |
| Digit symbol coding | 72.50 ± 5.27 | 74.99 ± 4.56* | 74.04 ± 4.59 | 68.56 ± 3.54† |
| Map search (1 min) | 53.11 ± 4.16 | 48.42 ± 3.19 | 50.35 ± 3.27 | 42.94 ± 2.31† |
| Map search (2 min) | 73.30 ± 1.70 | 75.11 ± 1.51* | 75.04 ± 1.92 | 74.67 ± 1.51 |
| Telephone search | 2.86 ± 0.17 | 3.06 ± 0.20 | 3.14 ± 0.26 | 3.46 ± 0.34 |
Data are least square means ± SE.
*P < 0.05 change from euglycemia to hypoglycemia after medium-chain triglycerides vs. placebo;
†P < 0.05 between euglycemia and hypoglycemia.
Counterregulatory hormones.
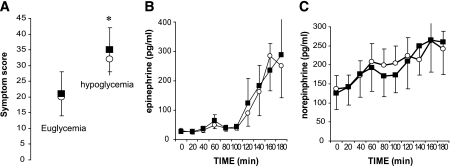
Hypoglycemia increased plasma epinephrine and norepinephrine levels in both treatment groups (Fig. 4). They were, however, not significantly different during the final 40 min of hypoglycemia (epinephrine 233 ± 102 in control subjects vs. 236 ± 90 pg/ml with medium-chain triglycerides, P = 0.8; norepinephrine 239 ± 45 in control subjects vs. 272 ± 69 pg/ml with medium-chain triglycerides, P = 0.2). As expected (22), there was no significant glucagon response to hypoglycemia during the control and medium-chain triglycerides sessions (46 ± 7.4 in control subjects vs. 47 ± 8.0 pg/ml with medium-chain triglycerides, P = 0.75).
FIG. 4.
A: Symptoms of hypoglycemia were significantly greater during hypoglycemia compared with euglycemia. *P < 0.05. There was no difference in symptoms of hypoglycemia after medium-chain triglyceride ingestion when compared with placebo ingestion. B and C: Plasma epinephrine (B) and plasma norepinephrine (C) profiles during euglycemic-hypoglycemic clamp studies with medium-chain triglycerides or placebo ingestion. ■, medium-chain triglycerides; ○, placebo.
Symptomatic responses.
Total hypoglycemic symptom scores were significantly elevated during hypoglycemia compared with euglycemia (control 30.80 ± 3.9 vs. 19.22 ± 1.40, medium-chain triglycerides 35.25 ± 5.8 vs. 21.35 ± 2.3, respectively; P = 0.002 for comparison of hypoglycemia to euglycemia). There was no difference in hypoglycemic symptoms after medium-chain triglycerides compared with placebo ingestion (Fig. 4).
Rat hippocampal slice studies.
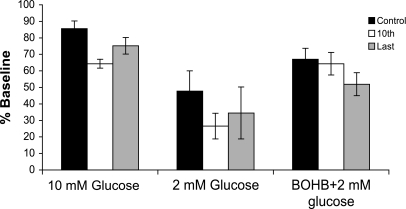
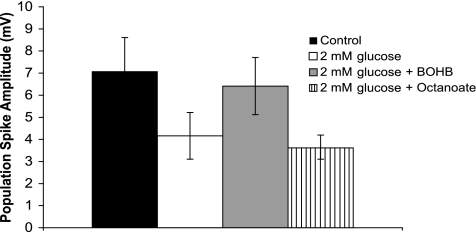
β-Hydroxybutyrate can partially substitute for glucose in vitro. When a 2 mmol/l glucose bath was applied for 30 min with intervening stimulus trains, the field potential amplitude decreased from 7.1 ± 1.2 to 4.1 ± 0.93 mV and then reached steady state. Across the population, this represented a 47.7 ± 12.3% decrease (P < 0.05, n = 21). When β-hydroxybutyrate was added iso-osmotically, there was a partial recovery to 5.74 ± 1.03 mV (83.8 ± 7.8% of control, n = 10). This was significantly (P < 0.05) different from the 2 mmol/l glucose values alone, but not different from values in control aCSF. We also assessed whether β-hydroxybutyrate could fully substitute for glucose using a bath applying 8 mmol/l β-hydroxybutyrate in 0 mmol/l added glucose aCSF. In all three slices studied, the synaptic response was lost after the first stimulus train and was only partially recoverable upon washing to 10 mmol/l glucose, indicating the need for a minimal level of glucose to maintain synaptic transmission under a metabolic load.
β-Hydroxybutyrate in 2 mmol/l glucose was also able to maintain synaptic function during both low- and moderate-frequency stimulation. In control aCSF, there was a modest decrease in the mean population spike amplitude 9.9 ± 2.5% (first vs. last response) during a 10-Hz train. In contrast, as shown in Fig. 5, the synaptic response was reduced by 80.0 ± 6.4% of control in 2 mmol/l glucose (P < 0.05). When β-hydroxybutyrate was added to the bath, the ability of the tissue to respond to synaptic stimulation was restored to 82.0 ± 22.4% (18% depression) of control (n = 10). It was also notable that β-hydroxybutyrate was able to prevent the initial depression seen in control studies (Fig. 5).
FIG. 5.
β-Hydroxybutyrate (BOHB) supports synaptic activity during a stimulus train. Data were taken from the 1st, 10th, and final stimulus during the last of a series of three 10-Hz, 10-s trains delivered under three conditions: control (10 mmol/l glucose), 2 mmol/l glucose, and 2 mmol/l glucose with 8 mmol/l β-hydroxybutyrate. Note that there was a profound decrease in the percent change in the amplitude of the evoked response in 2 mmol/l glucose that was reversed in the presence of 2 mmol/l glucose + 8 mmol/l β-hydroxybutyrate. Also note that β-hydroxybutyrate was able to sustain synaptic activity during the train to a greater degree than 10 mmol/l glucose, as shown by the effect on the 10th stimulus. Data are from a total of 21 slices: β-hydroxybutyrate was applied to 10 of these.
Octanoate does not substitute for glucose in hippocampal slice preparations.
In contrast, substitution of octanoate for glucose using the same experimental paradigm produced no recovery of synaptic function in any of the slices tested (n = 6). The response only recovered to 49.6 ± 12.8% of control after washing with 10 mmol/l glucose containing aCSF in three of six cases. Moreover, unlike β-hydroxybutyrate, octanoate did not preserve population spike amplitude during the stimulus train (Fig. 6).
FIG. 6.
Octanoate does not support synaptic transmission under hypoglycemic conditions. Graph shows the effect of bath application of 2 mmol/l glucose with or without equimolar substitution of either β-hydroxybutyrate or octanoate. Note that β-hydroxybutyrate was able to substitute for glucose under basal conditions, whereas octanoate had no effect. Data are shown 10 min after the last of three stimulus trains, n = 10 β-hydroxybutyrate, n = 6 octanoate.
Octanoate improves recovery after hypoglycemia.
To determine whether there was a synergistic effect of β-hydroxybutyrate and octanoate, they were bath-applied together (4 mmol/l each) using the same protocol as above. There was a partial recovery to 75.9 ± 12.6% of control, an effect not significantly different from that seen with 8 mmol/l β-hydroxybutyrate. However, there was an increase in the speed with which the synaptic response recovered to a stable baseline when washed with control aCSF compared with β-hydroxybutyrate alone (2 mmol/l glucose + 8 mmol/l β-hydroxybutyrate, 31.6 ± 8.7 min, n = 7; 2 mmol/l glucose + 4 mmol/l β-hydroxybutyrate + 4 mmol/l octanoate, 21.9 ± 8.2 min, n = 5).
DISCUSSION
This study tested the hypothesis that oral medium-chain triglycerides could provide an alternative fuel source to prevent the deterioration of higher brain function caused by acute hypoglycemia in intensively treated type 1 diabetic subjects. We used a battery of tasks to assess a range of cognitive domains. As expected, hypoglycemia impaired performance in tests of attention, short-term and delayed verbal memory, and working memory. Medium-chain triglyceride ingestion prevented this decline in performance in tests of short-term and delayed verbal memory and tests pertaining to attention. Medium-chain triglycerides' beneficial effect was most notable on tests of verbal memory, which to a large extent involves the hippocampus, a brain region particularly vulnerable to hypoglycemia (18,22–24). From the therapeutic perspective, it is reassuring that the cognitive benefit of medium-chain triglycerides was not associated with an adverse effect on hypoglycemia-induced adrenergic responses or symptoms.
Medium-chain triglycerides, a source of medium-chain fatty acids, have been widely used for nutritional support and in patients with malabsorption (10,25). Medium-chain fatty acids are rapidly absorbed and oxidized in the liver. This results in an excess of acetyl-CoA, and in turn the rapid production of ketones (10), an energy source for the brain (3,5,7). Furthermore, medium-chain fatty acids readily cross the BBB and are metabolized by the brain (13). Therefore, medium-chain fatty acids could directly and/or indirectly, via the generation of ketones, act to preserve brain function during hypoglycemia by provision of alternative fuels without raising blood glucose levels in patients with type 1 diabetes.
Medium-chain triglyceride ingestion raised plasma β-hydroxybutyrate and FFA levels during insulin-induced hypoglycemia, and thus both fuels might contribute to the observed effects on cognitive performance. The hippocampal slice data, however, suggest that the predominant impact of medium-chain fatty acids is mediated via the generation of ketones. β-Hydroxybutyrate supported synaptic transmission both at rest and during stimulus trains when glucose supply was deficient, whereas octanoate alone was ineffective. The failure to see an effect of octanoate in the hippocampal slice preparation reflects a time-dependent effect, and longer prior exposure to medium-chain fatty acids might have improved neuronal function. Alternatively, these findings may be explained by differences in brain metabolism of ketones and medium-chain fatty acids. Evidence suggests that octanoate is exclusively metabolized by astrocytes (13,26,27), whereas ketones are oxidized by both neurons and astrocytes (28,29). The finding that octanoate was able to improve the rate of recovery of synaptic function upon restoration of control glucose concentrations, but not the response to hypoglycemia itself, is consistent with the hypothesis that astrocytes may be critical for the restoration of synaptic function after a metabolic challenge such as hypoglycemia.
There is significant literature on the effects of alternative metabolic substrates on synaptic function in brain slice preparations (30–32). However, our studies on β-hydroxybutyrate differ from previous work (30,31) in two important aspects. First, we examined β-hydroxybutyrate in lowered (2 mmol/l) glucose compared with aglycemia, and second, we examined the ability of either β-hydroxybutyrate or octanoate to support synaptic transmission under a metabolic load. In glucose-free medium, β-hydroxybutyrate is able to maintain ATP but neither phosphocreatine levels nor synaptic function in slices prepared from adult rats (30,31). In contrast, our data indicate that β-hydroxybutyrate is able to sustain synaptic activity in adult rats only with some glucose present. It is also important to note that β-hydroxybutyrate is comparable to glucose in its ability to support synaptic activity under a metabolic load (Fig. 5). Taken together, these data suggest that there is an absolute requirement for a low concentration of glycolytic substrate to sustain robust synaptic transmission. This possibility is consistent with the data of Kanatani et al. (32); however, other investigators (33) have suggested that lactate can substitute for glucose under most conditions.
Although medium-chain triglyceride ingestion sustained cognitive function during acute hypoglycemia, it did not affect the adrenergic hormonal or symptomatic response to hypoglycemia. This finding might reflect a specific effect of medium-chain fatty acids on brain regions involved in cognition without affecting subcortical regions, such as the ventromedial hypothalamus, that are involved in the detection of hypoglycemia and the initiation of counterregulatory responses. This is consistent with evidence suggesting that there are regional differences in the brain's capacity to use alternative fuels during hypoglycemia (34). Evans et al. (34) demonstrated that intralipid infusion impaired the counterregulatory response to hypoglycemia without affecting cognitive performance, whereas Rossetti et al. (35) recently reported that amino acid ingestion preserved cognitive performance without affecting counterregulatory or symptomatic responses to acute hypoglycemia, much as we observed here.
Some studies, however, suggest that lactate and β-hydroxybutyrate sustain cognitive function while blunting counterregulatory responses during hypoglycemia (4–6). Notably, the β-hydroxybutyrate concentrations in those studies using β-hydroxybutyrate infusions (5,6) were much higher than in this study. Therefore, differences in circulating levels of β-hydroxybutyrate could explain the differences observed. In addition, prior studies (5,6) examined nondiabetic subjects, whereas we focused on intensively treated type 1 diabetic subjects. Pan et al. (8) suggest that it takes up to 72 h for ketones to be metabolized in the brain of nondiabetic individuals, probably because of the time required to increase BBB monocarboxylic acid transporters. In keeping with this view, acute in vitro studies using nondiabetic animals indicate that ketones can be immediately metabolized in the absence of the BBB (30). Moreover, it has been reported that brain acetate transport is increased in type 1 diabetic subjects receiving intensive insulin therapy compared with nondiabetic subjects (9). Thus, adaptive increases in the transport of β-hydroxybutyrate into the brain of intensively managed type 1 diabetic subjects may account for the ability of medium-chain triglycerides to rapidly attenuate hypoglycemic effects on cognitive function, and such adaptations in β-hydroxybutyrate transport may be region specific (36).
A potential limitation of the study is that cognitive performance may decline over time, thereby contributing to the deterioration in performance we observed in the hypoglycemic phase of the study. However, we anticipate that the dominant effect on cognitive decline was hypoglycemia per se, given that both the medium-chain triglycerides and placebo sessions were performed over identical time intervals in random order, making it highly unlikely that the specific benefit of medium-chain triglycerides could be specifically attributed to a time-associated decline in cognitive performance. Of note, all of the type 1 diabetic patients selected for this study were receiving intensive insulin therapy regimens and had a documented history of hypoglycemia. As a result, they had absent glucagon and reduced epinephrine responses during the hypoglycemic clamp. The increase in epinephrine in these patients was less than half that seen in other studies reported by our group in nondiabetic individuals (37). Our aim was to see whether medium-chain triglycerides could maintain brain function in the face of hypoglycemia in such individuals. Whether the prophylactic benefits of medium-chain triglyceride ingestion might differ in patients with and without hypoglycemia unawareness remains to be determined.
It should be emphasized that long-term effects of medium-chain triglycerides on cardiovascular risk factors and glucose metabolism are unknown. Short-term studies of the effects of medium-chain triglyceride ingestion on serum lipoprotein profiles in nondiabetic subjects are conflicting. Some report that medium-chain triglyceride intake causes only minor changes (38–40) or decreases (41) in serum lipid profiles, whereas others suggest it increases serum lipoprotein levels (42). Medium-chain triglycerides are marketed as a weight loss supplement based on reports that they increase energy expenditure and fat oxidation (43–45) and reduce body weight in animals and humans (46,47). Short-term studies of medium-chain triglycerides have also suggested beneficial effects on glucose metabolism in patients with type 2 diabetes (48,49). Whether similar metabolic effects of medium-chain triglycerides are observed in type 1 diabetes will require further investigation.
We conclude that ingestion of medium-chain triglycerides improves cognitive function without affecting the adrenergic hormonal or symptomatic responses to acute hypoglycemia in intensively controlled type 1 diabetic patients. These findings suggest that medium-chain triglycerides could be used as prophylactic therapy for such patients with the goal of preserving brain function during hypoglycemic episodes, such as when driving or sleeping, without producing hyperglycemia.
ACKNOWLEDGMENTS
This study was supported in part by a grant from the Juvenile Diabetes Research Foundation Center for the Study of Hypoglycemia (4-2004-807), the Yale Center of Clinical Investigation supported by a Clinical and Translational Science Awards Grant (UL1 RR024139) from the National Center for Research Resources, and National Institutes of Health grants (R37 DK20495, RO1NA045792, and DK069831).
No potential conflicts of interest relevant to this article were reported.
This study was presented at the American Diabetes Association 68th Scientific Sessions, San Francisco, CA, 6–10 June 2008, abstract no. 15-OR.
We thank Ellen Hintz, Melinda Zgorski, Donna D'eugenio, Osama Abdelghany, Donna Caseria, Mikhail Smolgovsky, Ralph Jacob, Aida Groszmann, and Brenda Wu for their help in executing these studies.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997; 46: 271– 286 [PubMed] [Google Scholar]
- 3.Hasselbalch SG, Knudsen GM, Jakobsen J, Hageman LP, Holm S, Paulson OB: Brain metabolism during short-term starvation in humans. J Cereb Blood Flow Metab 1994; 14: 125– 131 [DOI] [PubMed] [Google Scholar]
- 4.Maran A, Cranston I, Lomas J, Macdonald I, Amiel SA: Protection by lactate of cerebral function during hypoglycaemia. Lancet 1994; 343: 16– 20 [DOI] [PubMed] [Google Scholar]
- 5.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J: Effect of hyperketonemia and hyperlacticacidemia on symptoms, cognitive dysfunction, and counterregulatory hormone responses during hypoglycemia in normal humans. Diabetes 1994; 43: 1311– 1317 [DOI] [PubMed] [Google Scholar]
- 6.Amiel SA, Archibald HR, Chusney G, Williams AJ, Gale EA: Ketone infusion lowers hormonal responses to hypoglycaemia: evidence for acute cerebral utilization of a non-glucose fuel. Clin Sci (Lond) 1991; 81: 189– 194 [DOI] [PubMed] [Google Scholar]
- 7.Hawkins RA, Williamson DH, Krebs HA: Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J 1971; 122: 13– 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan JW, Rothman TL, Behar KL, Stein DT, Hetherington HP: Human brain beta-hydroxybutyrate and lactate increase in fasting-induced ketosis. J Cereb Blood Flow Metab 2000; 20: 1502– 1507 [DOI] [PubMed] [Google Scholar]
- 9.Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI: Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 2006; 55: 929– 934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traul KA, Driedger A, Ingle DL, Nakhasi D: Review of the toxicologic properties of medium-chain triglycerides. Food Chem Toxicol 2000; 38: 79– 98 [DOI] [PubMed] [Google Scholar]
- 11.Craig GB, Darnell BE, Weinsier RL, Saag MS, Epps L, Mullins L, Lapidus WI, Ennis DM, Akrabawi SS, Cornwell PE, Sauberlich HE: Decreased fat and nitrogen losses in patients with AIDS receiving medium-chain-triglyceride-enriched formula vs those receiving long-chain-triglyceride-containing formula. J Am Diet Assoc 1997; 97: 605– 611 [DOI] [PubMed] [Google Scholar]
- 12.Gracey M, Burke V, Anderson CM: Medium chain triglycerides in paediatric practice. Arch Dis Child 1970; 45: 445– 452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebert D, Haller RG, Walton ME: Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 2003; 23: 5928– 5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wechsler D: Wechsler Memory Scale Revised Manual San Antonio, TX, The Psychological Corp., 1987 [Google Scholar]
- 15.McAulay V, Deary IJ, Sommerfield AJ, Frier BM: Attentional functioning is impaired during acute hypoglycaemia in people with type 1 diabetes. Diabet Med 2006; 23: 26– 31 [DOI] [PubMed] [Google Scholar]
- 16.Sommerfield AJ, Deary IJ, McAulay V, Frier BM: Short-term, delayed, and working memory are impaired during hypoglycemia in individuals with type 1 diabetes. Diabetes Care 2003; 26: 390– 396 [DOI] [PubMed] [Google Scholar]
- 17.Kann O, Kovacs R, Heinemann U: Metabotropic receptor-mediated Ca2+ signaling elevates mitochondrial Ca2+ and stimulates oxidative metabolism in hippocampal slice cultures. J Neurophysiol 2003; 90: 613– 621 [DOI] [PubMed] [Google Scholar]
- 18.McNay EC, Fries TM, Gold PE: Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A 2000; 97: 2881– 2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dericioglu N, Garganta CL, Petroff OA, Mendelsohn D, Williamson A: Blockade of GABA synthesis only affects neural excitability under activated conditions in rat hippocampal slices. Neurochem Int 2008; 53: 22– 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tekkok SB, Godfraind JM, Krnjevic K: Moderate hypoglycemia aggravates effects of hypoxia in hippocampal slices from diabetic rats. Neuroscience 2002; 113: 11– 21 [DOI] [PubMed] [Google Scholar]
- 21.Brown H, Prescott R: Applied Mixed Models in Medicine Chichester, U.K., John Wiley and Sons, 1999 [Google Scholar]
- 22.McNay EC, Sherwin RS: Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes 2004; 53: 418– 425 [DOI] [PubMed] [Google Scholar]
- 23.Auer RN, Wieloch T, Olsson Y, Siesjo BK: The distribution of hypoglycemic brain damage. Acta Neuropathol 1984; 64: 177– 191 [DOI] [PubMed] [Google Scholar]
- 24.Fujioka M, Okuchi K, Hiramatsu KI, Sakaki T, Sakaguchi S, Ishii Y: Specific changes in human brain after hypoglycemic injury. Stroke 1997; 28: 584– 587 [DOI] [PubMed] [Google Scholar]
- 25.Bach AC, Frey A, Lutz O: Clinical and experimental effects of medium-chain-triglyceride-based fat emulsions: a review. Clin Nutr 1989; 8: 223– 235 [DOI] [PubMed] [Google Scholar]
- 26.Kuge Y, Yajima K, Kawashima H, Yamazaki H, Hashimoto N, Miyake Y: Brain uptake and metabolism of [1-11C]octanoate in rats: pharmacokinetic basis for its application as a radiopharmaceutical for studying brain fatty acid metabolism. Ann Nucl Med 1995; 9: 137– 142 [DOI] [PubMed] [Google Scholar]
- 27.Auestad N, Korsak RA, Morrow JW, Edmond J: Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem 1991; 56: 1376– 1386 [DOI] [PubMed] [Google Scholar]
- 28.Pan JW, de Graaf RA, Petersen KF, Shulman GI, Hetherington HP, Rothman DL: [2,4-13 C2]-beta-Hydroxybutyrate metabolism in human brain. J Cereb Blood Flow Metab 2002; 22: 890– 898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J: Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 1987; 18: 551– 561 [DOI] [PubMed] [Google Scholar]
- 30.Arakawa T, Goto T, Okada Y: Effect of ketone body (d-3-hydroxybutyrate) on neural activity and energy metabolism in hippocampal slices of the adult guinea pig. Neurosci Lett 1991; 130: 53– 56 [DOI] [PubMed] [Google Scholar]
- 31.Izumi Y, Ishii K, Katsuki H, Benz AM, Zorumski CF: Beta-hydroxybutyrate fuels synaptic function during development: histological and physiological evidence in rat hippocampal slices. J Clin Invest 1998; 101: 1121– 1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanatani T, Mizuno K, Okada Y: Effects of glycolytic metabolites on preservation of high energy phosphate level and synaptic transmission in the granule cells of guinea pig hippocampal slices. Experientia 1995; 51: 213– 216 [DOI] [PubMed] [Google Scholar]
- 33.Schurr A: Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab 2006; 26: 142– 152 [DOI] [PubMed] [Google Scholar]
- 34.Evans ML, Matyka K, Lomas J, Pernet A, Cranston IC, Macdonald I, Amiel SA: Reduced counterregulation during hypoglycemia with raised circulating nonglucose lipid substrates: evidence for regional differences in metabolic capacity in the human brain? J Clin Endocrinol Metab 1998; 83: 2952– 2959 [DOI] [PubMed] [Google Scholar]
- 35.Rossetti P, Porcellati F, Busciantella Ricci N, Candeloro P, Cioli P, Nair KS, Santeusanio F, Bolli GB, Fanelli CG: Effect of oral amino acids on counterregulatory responses and cognitive function during insulin-induced hypoglycemia in nondiabetic and type 1 diabetic people. Diabetes 2008; 57: 1905– 1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins RA, Biebuyck JF: Ketone bodies are selectively used by individual brain regions. Science 1979; 205: 325– 327 [DOI] [PubMed] [Google Scholar]
- 37.Goldberg PA, Weiss R, McCrimmon RJ, Hintz EV, Dziura JD, Sherwin RS: Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia-associated autonomic failure. Diabetes 2006; 55: 1121– 1126 [DOI] [PubMed] [Google Scholar]
- 38.Nosaka N, Kasai M, Nakamura M, Takahashi I, Itakura M, Takeuchi H, Aoyama T, Tsuji H, Okazaki M, Kondo K: Effects of dietary medium-chain triacylglycerols on serum lipoproteins and biochemical parameters in healthy men. Biosci Biotechnol Biochem 2002; 66: 1713– 1718 [DOI] [PubMed] [Google Scholar]
- 39.Cater NB, Heller HJ, Denke MA: Comparison of the effects of medium-chain triacylglycerols, palm oil, and high oleic acid sunflower oil on plasma triacylglycerol fatty acids and lipid and lipoprotein concentrations in humans. Am J Clin Nutr 1997; 65: 41– 45 [DOI] [PubMed] [Google Scholar]
- 40.Hashim SA, Arteaga A, Van Itallie TB: Effect of a saturated medium-chain triglyceride on serum-lipids in man. Lancet 1960; 1: 1105– 1108 [DOI] [PubMed] [Google Scholar]
- 41.Kasai M, Maki H, Nosaka N, Aoyama T, Ooyama K, Uto H, Okazaki M, Igarashi O, Kondo K: Effect of medium-chain triglycerides on the postprandial triglyceride concentration in healthy men. Biosci Biotechnol Biochem 2003; 67: 46– 53 [DOI] [PubMed] [Google Scholar]
- 42.Tholstrup T, Ehnholm C, Jauhiainen M, Petersen M, Hoy CE, Lund P, Sandstrom B: Effects of medium-chain fatty acids and oleic acid on blood lipids, lipoproteins, glucose, insulin, and lipid transfer protein activities. Am J Clin Nutr 2004; 79: 564– 569 [DOI] [PubMed] [Google Scholar]
- 43.Hill JO, Peters JC, Yang D, Sharp T, Kaler M, Abumrad NN, Greene HL: Thermogenesis in humans during overfeeding with medium-chain triglycerides. Metabolism 1989; 38: 641– 648 [DOI] [PubMed] [Google Scholar]
- 44.St-Onge MP, Bourque C, Jones PJ, Ross R, Parsons WE: Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord 2003; 27: 95– 102 [DOI] [PubMed] [Google Scholar]
- 45.St-Onge MP, Jones PJ: Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr 2002; 132: 329– 332 [DOI] [PubMed] [Google Scholar]
- 46.Simon E, Fernandez-Quintela A, Del Puy Portillo M, Del Barrio AS: Effects of medium-chain fatty acids on body composition and protein metabolism in overweight rats. J Physiol Biochem 2000; 56: 337– 346 [DOI] [PubMed] [Google Scholar]
- 47.Geliebter A, Torbay N, Bracco EF, Hashim SA, Van Itallie TB: Overfeeding with medium-chain triglyceride diet results in diminished deposition of fat. Am J Clin Nutr 1983; 37: 1– 4 [DOI] [PubMed] [Google Scholar]
- 48.Han JR, Deng B, Sun J, Chen CG, Corkey BE, Kirkland JL, Ma J, Guo W: Effects of dietary medium-chain triglyceride on weight loss and insulin sensitivity in a group of moderately overweight free-living type 2 diabetic Chinese subjects. Metabolism 2007; 56: 985– 991 [DOI] [PubMed] [Google Scholar]
- 49.Eckel RH, Hanson AS, Chen AY, Berman JN, Yost TJ, Brass EP: Dietary substitution of medium-chain triglycerides improves insulin-mediated glucose metabolism in NIDDM subjects. Diabetes 1992; 41: 641– 647 [PubMed] [Google Scholar]