Abstract
OBJECTIVE
To test the hypotheses that decreased insulin-mediated glucose disposal in muscle is associated with a reduced muscle microvascular exchange capacity (Kf) and that 6 months of high-dose statin therapy would improve microvascular function in people with central obesity.
RESEARCH DESIGN AND METHODS
We assessed skeletal muscle microvascular function, visceral fat mass, physical activity levels, fitness, and insulin sensitivity in skeletal muscle in 22 female and 17 male volunteers with central obesity whose age (mean ± SD) was 51 ± 9 years. We tested the effect of atorvastatin (40 mg daily) on muscle microvascular function in a randomized, double-blind, placebo-controlled trial lasting 6 months.
RESULTS
Kf was negatively associated with a measure of glycemia (A1C; r = −0.44, P = 0.006) and positively associated with insulin sensitivity (the ratio of insulin-stimulated glucose effectiveness, or M value, to the mean insulin concentration, or I value; r = 0.39, P = 0.02). In regression modeling, A1C, visceral fat mass, and M:I explained 38% of the variance in Kf (in a linear regression model with Kf as the outcome [R2 = 0.38, P = 0.005]). M:I was associated with Kf independently of visceral fat mass (B coefficient 3.13 [95% CI 0.22–6.02], P = 0.036). Although 6 months' treatment with atorvastatin decreased LDL cholesterol by 51% (P < 0.001) and plasma high-sensitivity C-reactive protein by 75% (P = 0.02), microvascular function was unchanged.
CONCLUSIONS
Decreased insulin-mediated glucose uptake in skeletal muscle is associated with impaired muscle microvascular exchange capacity (Kf), independently of visceral fat mass. Muscle microvascular function is not improved by 6 months of high-dose statin treatment, despite marked statin-mediated improvements in lipid metabolism and decreased inflammation.
Microvascular dysfunction is a cardinal long-term complication of type 2 diabetes. Reported microvascular defects in type 2 diabetes include impaired endothelium-dependent vasodilatation, reduced substrate delivery, and lower capillary density in insulin-sensitive tissues (1). Increased glycation of erythrocyte membrane proteins causing rigidity may result in an increased resistance to travel through the microcirculation (2), while concomitant alterations of the endothelial cell surface glycocalyx may modulate vascular permeability and exchange surface area (3). Obesity is the most important modifiable risk factor for the development of type 2 diabetes. Microvascular dysfunction has also been reported in obese subjects in the absence of diabetes, but it remains unclear which component(s) of obesity-linked pathophysiology contributes to microvascular dysfunction (1,4–6). Increased body fat mass is associated with molecular changes that contribute to altered vasodilatory responses, oxidative stress, abnormalities of vasoconstriction, and altered platelet adhesion; all of these defects could potentially influence solute delivery via the microvasculature (5). Insulin increases blood flow and microvascular perfusion in skin (7,8) and skeletal muscle (9,10), and impairment of insulin-induced microvascular dilator responses in skeletal muscle in animal models of insulin resistance, even at basal insulin concentrations, is believed to be a key factor in reduced glucose uptake (4,11). Thus, microvascular dysfunction might contribute to obesity-associated insulin resistance. Studies in insulin-resistant states in humans, such as obesity with or without the presence of type 2 diabetes (12), have shown impaired microvascular function where both insulin-mediated muscle microvascular perfusion and glucose uptake are reduced (5,13). The attenuation of insulin-stimulated muscle microvascular perfusion recruitment in obese humans is reminiscent of that reported in obese Zucker rats (6), being suggestive of common mechanistic pathways, including increased production of reactive oxygen species and reduced nitric oxide (NO) availability.
Based on results in animal models, it has been proposed that insulin acts to dilate the arterioles governing flow through capillary beds (14), thereby increasing substrate delivery (15). This occurs independently of, and appears to precede, increases in total blood flow and glucose disposal (14) resulting from dilatation of upstream arteriolar vessels (16). Entangled with this hypothesis is the concept of insulin-mediated redistribution of blood flow through the preferential perfusion of so-called nutritive vessels at the expense of nonnutritive routes (17). In healthy humans, many, but not all, experimental studies have shown insulin to have dose- and time-dependent effects, increasing blood flow in a manner that parallels glucose disposal (18). However, whether the capacity of insulin to increase the number of patent capillaries (capillary recruitment) is impaired in insulin-resistant states, such as obesity, remains uncertain (19–21).
It is well accepted that treatment with statins decreases risk of macrovascular disease and that statins have pleiotropic actions. Statins are also effective treatments for targeting vascular risk in people with features of the metabolic syndrome (22). However, the extent to which statin therapy has beneficial effects on the microvasculature has been little studied and remains unclear. Within the macrovasculature, statins have been shown to improve endothelial function and attenuate endothelial dysfunction in the presence of atherosclerotic risk factors through upregulation of endothelial NO synthase and the increased production of NO, and such an effect has been shown to occur after 6 months of therapy (rev. in (23). However, the potential for statins to modulate endothelial function in smaller vessels has yet to be elucidated.
Although it is known that microvascular dysfunction occurs in obesity, the nature of the relationship between microvascular function in an insulin-sensitive tissue such as skeletal muscle and insulin-mediated glucose disposal in skeletal muscle is uncertain. The role of potential confounders, such as fitness and physical activity levels, in the relationship between skeletal muscle microvascular function and insulin-mediated glucose disposal in skeletal muscle have not been fully clarified. Moreover, it is not certain whether statin therapy confers any benefit on muscle microvascular function via hypothesized pleiotropic actions independently of an effect on circulating LDL cholesterol concentrations.
The aims of our study were to assess the relationship between insulin-mediated glucose disposal and measures of skeletal muscle microvascular function, including microvascular filtration capacity (Kf), a measure of microvascular integrity (isovolumetric pressure [Pvi]), and resting limb blood flow (Qa), and to test the effect of statins on these factors in individuals with central obesity. We hypothesized 1) that decreased insulin-mediated glucose disposal in muscle is associated with a reduced muscle Kf and 2) that 6 months' intensive high-dose statin treatment would reverse this microvascular dysfunction (potentially via the pleiotrophic actions of statins on NO production). We took care to assess potential confounders, such as physical inactivity and cardiorespiratory fitness, that are known to influence Kf (24).
RESEARCH DESIGN AND METHODS
The study was approved by the Southampton General Hospital research ethics committee (LREC05/Q1704/38) and conducted in accordance with the Declaration of Helsinki. All participants were unpaid volunteers and gave informed written consent. White European subjects aged 18–75 years were invited to participate in the study. Volunteers were eligible for the main study if they had central obesity and at least one other feature of the metabolic syndrome as assessed by International Diabetes Federation criteria (25). For ethical reasons, subjects were only included in the study if estimated cardiovascular risk was <20% over 10 years based on the equation derived from the Framingham Heart Study because national guidelines indicate that people at higher cardiovascular risk should receive statin treatment for primary prevention of cardiovascular disease. Exclusion criteria were known diabetes; renal, liver, or uncontrolled thyroid disease; uncontrolled hypertension (blood pressure >160/100 mmHg); treatment with lipid-modifying drugs; antihypertensive medication; corticosteroid therapy; or hormone replacement therapy. For more information on the subjects and methods, please refer to the supplemental appendix, available online at http://dx.doi.org/10.2337/db08-1688.
After completing the baseline tests, subjects were randomized in a double-blind placebo-controlled trial study design by an independent pharmacist to either 40 mg atorvastatin daily or to matched placebo for 26 weeks. The primary end point of the trial was a change in microvascular function. Previously, Charles et al. (26) studied 12 individuals in a 14-week training program during which lower limbs were trained for endurance exercise, and these authors showed a 79% improvement in Kf (from 2.4 ± 0.8 to 4.3 ± 0.9, P < 0.05). Brown et al. (24), using electrical stimulation for 4 weeks in five sedentary individuals (8 Hz, 3 × 20 min/day for 5 days per week), showed that Kf increased ∼200%, from 3.38 ± 0.38 to 6.68 ± 0.62 (P < 0.05). We estimated that a sample size of n = 40 subjects would give us 99% power at the 5% significance level to detect a 1-SD increase in Kf and that, based on the changes shown with exercise and electrical stimulation studies, such a functional change would be physiologically relevant. Data are presented on 39 subjects because one person was unable to complete the study after suffering side effects of the prescribed trial medication.
Body composition, fat mass, and lean body mass were measured using a dual X-ray absorptiometry Delfia W 4500 instrument (coefficient of variation = 0.68%; Hologic, Bedford, MA) using a standard visual method to divide images into trunk, limb, and head. An abdominal magnetic resonance imaging scan was undertaken to assess visceral fat (27–29). An oral glucose tolerance test was performed with a 75-g glucose load with samples collected after 2 h.
The following tests were undertaken at baseline and at 26 weeks: microvascular function was assessed using a Filtrass venous occlusion plethysmographic system using a passive inductive transducer with an accuracy of ±5 μm (Compumedics DWL, Singen, Germany). Filtration (Jv in ml · min−1 · 100 ml−1) was measured from the slope of volume change in response to each pressure step over the last 2 min of its application, to allow for completion of vascular filling, and plotted against cuff pressure (Pcuff). The slope of this relationship, at pressures above those giving rise to net filtration, is a measure of microvascular filtration capacity (Kf) (30). Extrapolation of the relationship to its intercept on the Pcuff axis gives the isovolumetric venous pressure (Pvi) at which there is neither net filtration nor absorption (30,31). Muscle strength was assessed by measurement of handgrip strength using a Jamar dynamometer (Promedics, Blackburn, U.K.).
Hyperinsulinemic-euglycemic clamp.
A hyperinsulinemic-euglycemic clamp was undertaken to assess whole-body glucose uptake (insulin-stimulated glucose effectiveness, or M value) during the steady state of the clamp (final 30 min of the clamp), both at baseline and after intervention while subjects were taking their trial medication (32).
Measurement of insulin sensitivity.
Whole-body insulin sensitivity was measured as glucose uptake during the steady state of the clamp with an insulin infusion rate of 1.5 mU · kg−1 · min−1. All individuals achieved euglycemia during the clamp with glucose concentrations clamped at 5.0 mmol/l. Whole-body glucose uptake (M value) was defined as the glucose infusion rate during the final 30 min of the test (in mg · kg−1 · min−1) when steady-state insulin concentrations had been achieved. The ratio of M to the mean insulin concentration (I value) was used as an index of insulin sensitivity. M:I values were estimated by dividing the M value by the I value during the last 30 min of the clamp.
Cardiorespiratory fitness and physical activity energy expenditure.
Cardiorespiratory fitness measured in terms of maximal oxygen uptake (Vo2max) was determined using a treadmill test and Cortex Metalyzer, and physical activity (physical activity energy expenditure and metabolic equivalents) was assessed using an activity monitor (Armband Sensewear Pro2) (33).
Statistical analyses.
All statistical analyses were performed using SPSS for Windows version 16.0 (SPSS, Chicago, IL). Student's t test comparisons were undertaken to compare mean values of normally distributed data. Pearson correlation coefficients are presented for univariate analyses of normally distributed data. Where variables were not normally distributed, log transformation was undertaken to normalize the distribution. Multivariate linear regression models were used to describe factors that were independently associated with Kf as the dependent (outcome) variable. A P value of <0.05 was considered to be statistically significant for all analyses. Data are expressed as the mean ± SD and range unless otherwise stated. To test the effect of statin on measures of microvascular function, we analyzed microvascular function at the end of the trial, adjusting for randomization and baseline microvascular measures by factorial ANOVA.
RESULTS
Table 1 shows the baseline characteristics of subjects recruited to the study. The 39 healthy volunteers included 17 men and were aged 51 ± 9 years. Of the study subjects, 11 had two features, 18 had three features, 9 had four features, and 1 had all five features of the metabolic syndrome. Subjects were excluded if they had known diabetes at recruitment. On baseline testing one subject was found to have a fasting glucose of 7.4 mmol/l and therefore analyses were undertaken both including and excluding this person. Inclusion of data from this individual (who received no glucose-lowering medication during the study) did not change or affect the results, and the data are therefore presented for all 39 subjects who completed the 6-month trial. For more information on the results of this study, please refer to the supplementary material in the online appendix.
TABLE 1.
Baseline characteristics of participants
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 51.4 ± 9.0 | 29.0–69.6 |
| BMI (kg/m2) | 32.1 ± 4.6 | 26.0–47.9 |
| Waist (cm) | 105.3 ± 12.9 | 86.5–151.0 |
| Total fat (%) | 35.6 ± 7.4 | 21–48 |
| Truncal fat (% of total) | 52.2 ± 5.6 | 43–64 |
| Systolic blood pressure (mmHg) | 133 ± 14 | 93–155 |
| Diastolic blood pressure (mmHg) | 85 ± 9 | 64–104 |
| Cardiovascular disease risk (% per 10 years)* | 7.3 ± 5.1 | 0–17.3 |
| Total cholesterol (mmol/l) | 5.7 ± 1.1 | 3.2–9.3 |
| LDL cholesterol (mmol/l) | 3.7 ± 0.9 | 1.7–7.0 |
| HDL cholesterol (mmol/l) | 1.45 ± 0.36 | 0.92–2.45 |
| Triglyceride (mmol/l) | 1.4 ± 0.6 | 0.4–2.7 |
| Glucose (mmol/l) | 5.2 ± 0.7 | 4.0–7.4 |
| A1C (%) | 5.5 ± 0.3 | 4.9–6.3 |
% fat estimated by DEXA.
*Estimated using Framingham risk score.
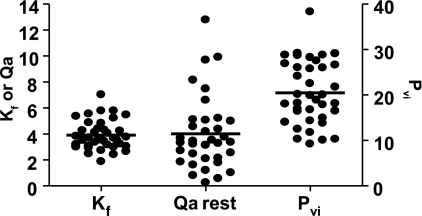
Figure 1 shows baseline Kf, Qa, and Pvi measurements. Mean values for Kf, Qa, and Pvi were 3.91 ± 0.18 × 10−3 ml · min−1 · 100 ml−1 · mmHg−1, 4.01 ± 0.48 ml · min−1 · 100 ml−1, and 20.5 ± 1.1 mmHg, respectively. There was considerable variability in all measures within the cohort with an approximately threefold difference in Kf levels between subjects.
FIG. 1.
Baseline measurements of filtration capacity (Kf), resting limb blood flow (Qa), and endothelial integrity (Pvi) from 39 individuals. All values were derived using venous congestion plethysmography from the raw data (see supplemental information in the online appendix). Qa = resting (ml · 100 ml−1 · min−1); Kf = × 10−3 ml · min−1 · 100 ml−1 · mmHg−1; Pvi = mmHg.
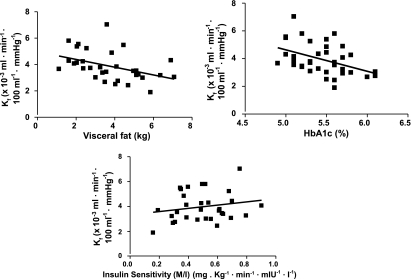
We investigated the relationships between measures of microvascular function (Kf, Qa, and Pvi), measures of obesity and features of the metabolic syndrome, together with measures of physical activity and fitness. Table 2 shows correlation coefficients describing the relationships between Kf, age, features of the metabolic syndrome, Vo2max, physical activity energy expenditure, and metabolic equivalents. Of the readily measurable features of the metabolic syndrome (waist circumference, blood pressure, glucose, HDL cholesterol, and triglyceride concentrations), Kf was statistically significantly associated with waist circumference (r = −0.36, P = 0.025). There was a borderline significant association with triglycerides (r = −0.30, P = 0.07), and there were no significant associations between Kf and age, blood pressure, glucose, or HDL cholesterol concentrations. Figure 2 shows the scatter plots for the relationships between Kf and visceral fat, A1C, and M:I. Kf was negatively associated with visceral fat (r = −0.43, P = 0.015) and A1C (r = −0.44, P = 0.006). Kf was also negatively associated with plasma high-sensitivity C-reactive protein (hsCRP) and soluble intracellular adhesion molecule (ICAM)-1 (both P < 0.05). Kf was positively associated with M:I (r = 0.39, P = 0.02). There were no significant associations of note with Pvi. Resting Qa was associated with respiratory exchange ratio (r = 0.52, P = 0.002). Neither Pvi nor Qa were associated with age (r = −0.20, P = 0.24, and r = −0.12, P = 0.94, respectively).
TABLE 2.
Correlations between filtration capacity (Kf) and features of the metabolic syndrome, abdominal obesity, fitness, and physical activity
| r | P | |
|---|---|---|
| Age (years) | 0.02 | 0.89 |
| Waist (cm) | −0.36 | 0.025 |
| Visceral fat (kg) | −0.43 | 0.015 |
| Subcutaneous fat (kg) | −0.28 | 0.12 |
| Systolic blood pressure (mmHg) | −0.15 | 0.36 |
| Diastolic blood pressure (mmHg) | 0.08 | 0.63 |
| Cardiovascular disease risk (% per 10 years) | −0.15 | 0.38 |
| HDL cholesterol (mmol/l) | 0.13 | 0.43 |
| Triglyceride (mmol/l) | −0.30 | 0.07 |
| Glucose (mmol/l) | −0.05 | 0.77 |
| A1C (%) | −0.44 | 0.006 |
| Steps (n) | 0.35 | 0.04 |
| Physical activity energy expenditure (metabolic equivalents) | 0.20 | 0.24 |
| Vo2max (ml · min−1 · kg−1) | 0.21 | 0.20 |
| Glucose disposal (mg · kg−1 · min−1 · mIU−1 · l−1) | 0.39 | 0.021 |
FIG. 2.
Scatter plots for the relationships between Kf and visceral fat, A1C, and M:I from 39 individuals at baseline. Kf was negatively associated with visceral fat (r = −0.43, P = 0.015) and A1C (r = −0.44, P = 0.006) and positively associated with M:I (r = 0.39, P = 0.02).
Multiple regression modeling was undertaken to further explore factors that were associated with Kf. In a regression model that included Kf as the outcome, 38% of the variance in Kf was explained by A1C, M:I, and visceral fat mass as the explanatory variables in the model (R2 = 0.38, P = 0.005). To determine whether the association between M:I and Kf (observed in univariate analysis) (Table 2) was independent of visceral fat, we undertook regression modeling with Kf as the outcome variable and included M:I and visceral fat as explanatory variables. In this model, M:I and visceral fat explained 30% of the variance in Kf (R2 = 0.30, P = 0.008). M:I was associated with Kf independently of visceral fat (B coefficient = 3.13, 95% CI 0.22–6.02, P = 0.036), whereas visceral fat was not associated with Kf independently of M:I (B coefficient = −0.09, 95% CI −0.40 to 0.22, P = 0.55). There was no effect of sex in our model.
Having observed associations with measures of insulin sensitivity and microvascular function in muscle, we investigated whether a functional measure of muscle performance (grip strength) was associated with measures of microvascular function. Only Pvi was associated with grip strength {mean handgrip strength [(right + left)/2]} and Pvi (r = −0.55, P < 0.001). We observed similar results for the relationship between Pvi and left handgrip strength (r = −59, P < 0.001) and right handgrip strength (r = −0.51, P = 0.001).
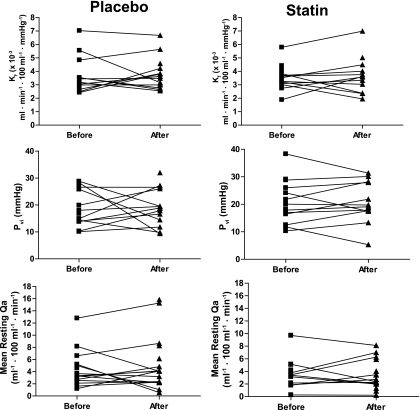
We investigated the effect of 6 months of 40 mg/day of atorvastatin treatment in the randomized placebo controlled trial. In subjects in the placebo arm of the study (n = 20), mean baseline LDL cholesterol was 3.78 ± 1.05 mmol/l, and it was 3.70 ± 0.90 mmol/l at follow-up (P = 0.51). In contrast, in the treatment arm of the study (n = 19), LDL cholesterol fell from 3.53 ± 0.81 mmol/l at baseline to 1.73 ± 0.71 mmol/l at follow-up (P < 0.001). Subjects in the placebo arm of the study had a mean baseline triglyceride of 1.31 ± 0.68 mmol/l, and triglyceride was 1.19 ± 0.61 mmol/l at follow-up (P = 0.34). Mean baseline fasting triglyceride concentration in the treatment arm of the study was 1.41 ± 0.62 mmol/l, compared with 1.00 ± 0.58 mmol/l at follow-up (P = 0.001). Median baseline hsCRP in subjects in the placebo arm of the study was 2.0 mg/l (95% CI 1.09–10.47) compared with 3.0 mg/l (1.62–6.35) at follow-up (P = 0.73). Statins reduced hsCRP from a baseline value of 2.0 mg/l (95% CI 1.31–5.59) to 0.5 mg/l (0.35–4.65) at follow-up (P = 0.02). The change in LDL cholesterol concentration was positively correlated with the change in hsCRP (r = 0.27, P < 0.002). In both the placebo and treatment arms of the study, there was no change in body fat, M:I, A1C, or ICAM-1 measurements after 6 months of treatment (data not shown). Figure 3 shows Kf, Pvi, and Qa measurements before and after 6 months' treatment with atorvastatin. There was no change in Kf, Pvi, and Qa measurements with statin treatment, adjusting for baseline measures, age, and sex (Kf: P = 0.99; Pvi: P = 0.28; Qa: P = 0.29).
FIG. 3.
Kf, isovolumetric pressure (Pvi), and resting limb blood flow (Qa) measured before and after 26 weeks' treatment with atorvastatin (40 mg once daily) or matched placebo.
DISCUSSION
Our results show that in adults with central obesity, decreased insulin-mediated glucose uptake in skeletal muscle is associated with impaired muscle Kf independently of visceral fat mass. Our data demonstrate that the association between Kf and insulin-mediated glucose disposal is independent of visceral fat mass, with no confounding by physical inactivity or low fitness levels. Despite a wealth of evidence showing that statins confer a benefit in the macrovasculature, and despite a marked statin effect on LDL cholesterol levels (lowered by ∼50%) and hsCRP (decreased by 75%), our results clearly show no effect of statin on measures of muscle microvascular function.
In our study, insulin-mediated glucose disposal (M:I) was positively associated with exchange capacity (Kf), suggesting that the more insulin-sensitive individuals have a greater exchange capacity, thereby facilitating muscle nutrient delivery. Although the range of values of Kf measured in our study group was considerable, they were similar to those reported previously for similarly aged individuals (26,34). The values of Kf are also within the range reported for individuals with pre-diabetes or diabetes without microvascular complications (35–38). We failed to show any association between age and any of the measures of microvascular function in our study. However, the mean age of our volunteers was 51.4 years with an SD of only 9 years. Thus, it is plausible that in our relatively small sample size of predominantly middle-aged subjects, we have failed to detect true associations between aging and measures of microvascular function. It is also possible that because we have not studied very aged individuals, we have not detected any obesity-independent, age-related change in microvascular function.
Having shown associations between insulin-mediated glucose uptake in skeletal muscle and Kf, we also explored whether a functional measure of skeletal muscle performance (grip strength) was associated with measures of muscle microvascular function, since we have recently shown in a large cohort study that decreased grip strength was associated with metabolic syndrome (39) and type 2 diabetes (40). The mechanism underlying decreased handgrip strength in type 2 diabetes and metabolic syndrome is uncertain, but, interestingly, our results show that one measure of microvascular integrity (Pvi) was associated with decreased grip strength. In other studies, increases in Pvi have been associated with inflammatory disease (41), but whether vascular inflammation within skeletal muscle may contribute to loss of muscle strength in people with type 2 diabetes has yet to be explored.
Our results showed a marked effect of atorvastatin to decrease LDL cholesterol and CRP, but, in keeping with previous work (42) testing the effects of 12 weeks' treatment with atorvastatin, we showed no effect of atorvastatin on levels of inflammatory markers (e.g., ICAM-1, tumor necrosis factor-α, interleukin-6, endothelin 1, retinol binding protein 4, leptin, adiponectin, and resistin) (data not shown). Whereas statins have been shown to have a beneficial effect on endothelial function and blood flow within the macrovasculature (e.g., flow-mediated dilation), our data with high-dose atorvastatin for 6 months showed no effect of statin on any of the measured aspects of muscle microvascular function. Fegan et al. (43) showed, in individuals with type 2 diabetes, no improvement in cutaneous vascular response after 3 months' treatment with single or combined lipid-lowering therapy. Although no effect of 4 weeks' treatment with 20 mg/day atorvastatin was observed on vasomotor function by high-resolution ultrasound examination of the brachial artery (flow-mediated dilation and sublingual nitrate) (44), a beneficial effect of statins on aspects of endothelial function has been noted over 6 months (45). It is plausible that turnover of endothelial cells or neovascularization is needed to improve aspects of microvascular function as measured in our study. Six months' treatment with statins may be insufficient time for this to occur. It is possible that our failure to detect a difference in microvascular function with statin treatment represents a type 2 statistical error. However, our randomized placebo-controlled trial sample size gave us 97% power to detect a 1.0-SD change in Kf at the 5% significance level, and a 1.0-SD change in Kf represents a ∼1.1-unit change in Kf, or an increase in mean Kf from 3.9 to 5.0 × 10−3 ml · min−1 · 100 ml−1 · mmHg−1. Our study was therefore powered to detect relatively modest changes in Kf, and more marked changes in Kf have been observed with electrical stimulation and with exercise. Electrical stimulation for 4 weeks increased Kf from 3.38 ± 0.38 to 6.68 ± 0.62 (P < 0.05) (24), and a 14-week training program during which lower limbs were trained for endurance caused a 79% improvement in Kf (from 2.4 ± 0.8 to 4.3 ± 0.9, P < 0.05) (26,35–38). Thus, these data suggest that our study was powered to detect physiologically relevant changes in Kf.
The methods available to investigate muscle microvasculature are limited, and those used to measure the whole tissue or insulin-mediated capillary perfusion and flow are very invasive, for example, radiolabeled imaging techniques (46), contrast enhanced ultrasound using albumin microbubbles (9), needle-inserted laser Doppler probes (8), or measurements of the distribution of blood flow by [15O]H2O as an index of flow heterogeneity (47). One way of assessing blood/muscle exchange, and hence to assess impaired muscle microvascular function, is to quantify the capacity of the microvascular bed to filter fluid. Plethysmography is a well-validated, noninvasive technique that uses small-step increases in venous occlusion pressure and measurement of the resultant changes in limb volume to provide a measure of microvascular filtration capacity (Kf) (48). Kf, which is measured predominantly in the muscle of the lower limb, has been shown to be differentially sensitive to increases in capillary surface area, as found in training schedules (26), as well as sensitive enough to detect increases in capillary perfusion, as in studies involving chronic electrical stimulation (24). Kf thus appears to be an important and sensitive measure to detect impairment of microvascular function, and the methodology used (plethysmography) does not affect the function of the vasculature under study (17). In the current study, a noninvasive measure was used that was acceptable to subjects returning for testing at the end of the study. We reasoned that the greater technical ease of the Filtrass system and its good reproducibility, together with its noninvasive nature, would result in better compliance in our nonpaid volunteers who were required to return for follow-up measurements at the end of the intensive 6-month clinical trial. Many of the techniques mentioned above used to assess changes in blood flow in muscle rely on visualization of erythrocyte movement or their particulate surrogates. Moreover, the movement of plasma, which determines bulk transfer and microvascular surface interchange of small solutes to sustain the optimal diffusion gradient, cannot be readily visualized. Plethysmographic assessment of Kf goes some way toward addressing this matter by measuring the rate of fluid exchange across the whole muscle microvascular bed. This enables evaluation of microvascular filtration parameters, by which significant differences due to pathophysiology and/or therapeutic interventions can be studied. Other more invasive techniques provide evidence of an insulin-mediated microvascular “recruitment” in human muscle through a selective action on precapillary arterioles (9–11,13,49) to redirect blood to “nutritive” vascular beds. The measurement of Kf or Qa by plethysmography does not allow us to distinguish between variations in muscle blood flow due to shifts, or redistribution, within microvascular networks and those in total blood flow, as determined by the resistance vessels supplying the muscle. It is possible that variation in muscle blood flow due to shifts, or redistribution, within microvascular networks could explain some of the wide variance in microvascular measurements (Fig. 1) that we observed across our cohort.
In summary, we have shown a strong association between skeletal muscle Kf and decreased insulin sensitivity in skeletal muscle in men and women with central obesity. Despite marked decreases in LDL cholesterol and hsCRP concentrations caused by 6 months' high-dose statin treatment, there was no improvement in any measure of skeletal muscle microvascular function, suggesting that these factors do not make an important contribution to control of microvascular function. Our data emphasize that further studies are now required to investigate the effects of insulin-sensitizing agents on microvascular function both in individuals at risk of type 2 diabetes and in those who have type 2 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by an Independent Research Grant from Pfizer U.K. to C.D.B. and A.J.K. No other potential conflicts of interest relevant to this article were reported.
The work was presented in abstract form at the European Association for the Study of Diabetes, Amsterdam, 17–21 September 2007 annual scientific meeting.
The authors thank Debbie Smith and Gina Schreiber, School of Medicine, University of Southampton, for their technical help in data collection and analysis; the nurses of the Wellcome Trust Clinical Research facility, especially Clare Grocott, for their help with the physiological measurements; and Lucinda England for help with recruitment. Dr. Margaret Brown, University of Birmingham, is acknowledged for her help in protocol development.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Clinical trial reg. no. EUDRACT 2005000512-28.
REFERENCES
- 1.Wiernsperger N, Nivoit P, De Aguiar LG, Bouskela E: Microcirculation and the metabolic syndrome. Microcirculation 2007; 14: 403– 438 [DOI] [PubMed] [Google Scholar]
- 2.Shin S, Ku YH, Suh JS, Singh M: Rheological characteristics of erythrocytes incubated in glucose media. Clin Hemorheol Microcirc 2008; 38: 153– 161 [PubMed] [Google Scholar]
- 3.Goldin A, Beckman JA, Schmidt AM, Creager MA: Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006; 114: 597– 605 [DOI] [PubMed] [Google Scholar]
- 4.Stepp DW: Impact of obesity and insulin resistance on vasomotor tone: nitric oxide and beyond. Clin Exp Pharmacol Physiol 2006; 33: 407– 414 [DOI] [PubMed] [Google Scholar]
- 5.Stapleton PA, James ME, Goodwill AG, Frisbee JC: Obesity and vascular dysfunction. Pathophysiology 2008; 15: 79– 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisbee JC: Obesity, insulin resistance, and microvessel density. Microcirculation 2007; 14: 289– 298 [DOI] [PubMed] [Google Scholar]
- 7.Serne EH, IJzerman RG, Gans RO, Nijveldt R, de Vries G, Evertz R, Donker AJ, Stehouwer CD: Direct evidence for insulin-induced capillary recruitment in skin of healthy subjects during physiological hyperinsulinemia. Diabetes 2002; 51: 1515– 1522 [DOI] [PubMed] [Google Scholar]
- 8.de Jongh RT, Clark AD, IJzerman RG, Serne EH, de Vries G, Stehouwer CD: Physiological hyperinsulinaemia increases intramuscular microvascular reactive hyperaemia and vasomotion in healthy volunteers. Diabetologia 2004; 47: 978– 986 [DOI] [PubMed] [Google Scholar]
- 9.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E: Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 2001; 50: 2682– 2690 [DOI] [PubMed] [Google Scholar]
- 10.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ: Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 2006; 290: E1191– E1197 [DOI] [PubMed] [Google Scholar]
- 11.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S: Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 2003; 284: E241– E258 [DOI] [PubMed] [Google Scholar]
- 12.de Jongh RT, Serne EH, IJzerman RG, de Vries G, Stehouwer CD: Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes 2004; 53: 2873– 2882 [DOI] [PubMed] [Google Scholar]
- 13.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ: Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006; 55: 1436– 1442 [DOI] [PubMed] [Google Scholar]
- 14.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ: Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004; 53: 1418– 1423 [DOI] [PubMed] [Google Scholar]
- 15.Renkin EM: Microcirculation. In Handbook of Physiology Sect. 2, vol. IV, pt. 2 Renkin EM, Michel CC. Eds. Bethesda, MD, American Physiological Society, 1984, p. 627– 686 [Google Scholar]
- 16.Segal KR, Edano A, Abalos A, Albu J, Blando L, Tomas MB, Pi-Sunyer FX: Effect of exercise training on insulin sensitivity and glucose metabolism in lean, obese, and diabetic men. J Appl Physiol 1991; 71: 2402– 2411 [DOI] [PubMed] [Google Scholar]
- 17.Clark MG: Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 2008; 295: E732– E750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mather K, Laakso M, Edelman S, Hook G, Baron A: Evidence for physiological coupling of insulin-mediated glucose metabolism and limb blood flow. Am J Physiol Endocrinol Metab 2000; 279: E1264– E1270 [DOI] [PubMed] [Google Scholar]
- 19.Poole DC, Brown MD, Hudlicka O: Counterpoint: there is not capillary recruitment in active skeletal muscle during exercise. J Appl Physiol 2008; 104: 891– 893 [DOI] [PubMed] [Google Scholar]
- 20.Poole D, Brown M, Hudlicka O: Last word on Point:Counterpoint: there is/is not capillary recruitment in active skeletal muscle during exercise. J Appl Physiol 2008; 104: 901. [DOI] [PubMed] [Google Scholar]
- 21.Clark MG, Rattigan S, Barrett EJ, Vincent MA: Point: there is capillary recruitment in active skeletal muscle during exercise. J Appl Physiol 2008; 104: 889– 891 [DOI] [PubMed] [Google Scholar]
- 22.Athyros VG, Mikhailidis DP, Papageorgiou AA, Didangelos TP, Peletidou A, Kleta D, Karagiannis A, Kakafika AI, Tziomalos K, Elisaf M: Targeting vascular risk in patients with metabolic syndrome but without diabetes. Metabolism 2005; 54: 1065– 1074 [DOI] [PubMed] [Google Scholar]
- 23.Korkmaz H, Onalan O: Evaluation of endothelial dysfunction: flow-mediated dilation. Endothelium 2008; 15: 157– 163 [DOI] [PubMed] [Google Scholar]
- 24.Brown MD, Jeal S, Bryant J, Gamble J: Modifications of microvascular filtration capacity in human limbs by training and electrical stimulation. Acta Physiol Scand 2001; 173: 359– 368 [DOI] [PubMed] [Google Scholar]
- 25.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J: The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb 2005; 12: 295– 300 [DOI] [PubMed] [Google Scholar]
- 26.Charles M, Charifi N, Verney J, Pichot V, Feasson L, Costes F, Denis C: Effect of endurance training on muscle microvascular filtration capacity and vascular bed morphometry in the elderly. Acta Physiol (Oxf) 2006; 187: 399– 406 [DOI] [PubMed] [Google Scholar]
- 27.Holt HB, Wild SH, Wood PJ, Zhang J, Darekar AA, Dewbury K, Poole RB, Holt RI, Phillips DI, Byrne CD: Non-esterified fatty acid concentrations are independently associated with hepatic steatosis in obese subjects. Diabetologia 2006; 49: 141– 148 [DOI] [PubMed] [Google Scholar]
- 28.Holt HB, Wild SH, Wareham N, Ekelund U, Umpleby M, Shojaee-Moradie F, Holt RI, Phillips DI, Byrne CD: Differential effects of fatness, fitness and physical activity energy expenditure on whole-body, liver and fat insulin sensitivity. Diabetologia 2007; 50: 1698– 1706 [DOI] [PubMed] [Google Scholar]
- 29.Holt HB, Wild SH, Postle AD, Zhang J, Koster G, Umpleby M, Shojaee-Moradie F, Dewbury K, Wood PJ, Phillips DI, Byrne CD: Cortisol clearance and associations with insulin sensitivity, body fat and fatty liver in middle-aged men. Diabetologia 2007; 50: 1024– 1032 [DOI] [PubMed] [Google Scholar]
- 30.Gamble J, Gartside IB, Christ F: A reassessment of mercury in silastic strain gauge plethysmography for microvascular permeability assessment in man. J Physiol 1993; 464: 407– 422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pappenheimer JR, Soto-Rivera A: Effective osmotic pressure of the plasma proteins and other quantities associated with the capillary circulation in the hindlimbs of cats and dogs. Am J Physiol 1947; 152: 471– 491 [DOI] [PubMed] [Google Scholar]
- 32.Brackenridge A, Pearson ER, Shojaee-Moradie F, Hattersley AT, Russell-Jones D, Umpleby AM: Contrasting insulin sensitivity of endogenous glucose production rate in subjects with hepatocyte nuclear factor-1beta and -1alpha mutations. Diabetes 2006; 55: 405– 411 [DOI] [PubMed] [Google Scholar]
- 33.Mignault D, St-Onge M, Karelis AD, Allison DB, Rabasa-Lhoret R: Evaluation of the Portable HealthWear Armband: a device to measure total daily energy expenditure in free-living type 2 diabetic individuals. Diabetes Care 2005; 28: 225– 227 [DOI] [PubMed] [Google Scholar]
- 34.Gamble J, Bethell D, Day NP, Loc PP, Phu NH, Gartside IB, Farrar JF, White NJ: Age-related changes in microvascular permeability: a significant factor in the susceptibility of children to shock? Clin Sci (Lond) 2000; 98: 211– 216 [PubMed] [Google Scholar]
- 35.Alpert JS, Coffman JD, Balodimos MC, Koncz L, Soeldner JS: Capillary permeability and blood flow in skeletal muscle of patients with diabetes mellitus and genetic prediabetes. N Engl J Med 1972; 286: 454– 460 [DOI] [PubMed] [Google Scholar]
- 36.Poulsen HL, Nielsen SL: Water filtration of the forearm in short- and long-term diabetes mellitus. Diabetologia 1976; 12: 437– 440 [DOI] [PubMed] [Google Scholar]
- 37.Jaap AJ, Shore AC, Gamble J, Gartside IB, Tooke JE: Capillary filtration coefficient in type II (non-insulin-dependent) diabetes. J Diabetes Complications 1994; 8: 111– 116 [DOI] [PubMed] [Google Scholar]
- 38.Jaap AJ, Shore AC, Tooke JE: Differences in microvascular fluid permeability between long-duration type I (insulin-dependent) diabetic patients with and without significant microangiopathy. Clin Sci (Lond) 1996; 90: 113– 117 [DOI] [PubMed] [Google Scholar]
- 39.Sayer AA, Syddall HE, Dennison EM, Martin HJ, Phillips DI, Cooper C, Byrne CD: Grip strength and the metabolic syndrome: findings from the Hertfordshire Cohort Study. QJM 2007; 100: 707– 713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C: Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care 2005; 28: 2541– 2542 [DOI] [PubMed] [Google Scholar]
- 41.Anim-Nyame N, Gamble J, Sooranna SR, Johnson MR, Sullivan MH, Steer PJ: Evidence of impaired microvascular function in pre-eclampsia: a non-invasive study. Clin Sci (Lond) 2003; 104: 405– 412 [DOI] [PubMed] [Google Scholar]
- 42.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A: Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care 2004; 27: 2450– 2457 [DOI] [PubMed] [Google Scholar]
- 43.Fegan PG, Shore AC, Mawson D, Tooke JE, MacLeod KM: Microvascular endothelial function in subjects with type 2 diabetes and the effect of lipid-lowering therapy. Diabet Med 2005; 22: 1670– 1676 [DOI] [PubMed] [Google Scholar]
- 44.Crisostomo LM, Souza CA, Mendes CM, Coimbra SR, Favarato D, Luz PL: Vascular and metabolic response to statin in the mildly hypertensive hypercholesterolemic elderly. Clinics 2008; 63: 589– 594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao JK, Laufs U: Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005; 45: 89– 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raitakari M, Nuutila P, Ruotsalainen U, Laine H, Teras M, Iida H, Makimattila S, Utriainen T, Oikonen V, Sipila H, Haaparanta M, Solin O, Wegelius U, Knuuti J, Yki-Jarvinen H: Evidence for dissociation of insulin stimulation of blood flow and glucose uptake in human skeletal muscle: studies using [15O]H2O, [18F]fluoro-2-deoxy-D-glucose, and positron emission tomography. Diabetes 1996; 45: 1471– 1477 [DOI] [PubMed] [Google Scholar]
- 47.Utriainen T, Nuutila P, Takala T, Vicini P, Ruotsalainen U, Ronnemaa T, Tolvanen T, Raitakari M, Haaparanta M, Kirvela O, Cobelli C, Yki-Jarvinen H: Intact insulin stimulation of skeletal muscle blood flow, its heterogeneity and redistribution, but not of glucose uptake in non-insulin-dependent diabetes mellitus. J Clin Invest 1997; 100: 777– 785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gamble J, Christ F, Gartside IB: Human calf precapillary resistance decreases in response to small cumulative increases in venous congestion pressure. J Physiol 1998; 507: 611– 617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ: Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 2004; 53: 447– 453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.