Abstract
OBJECTIVE
Intestinal L-cells secrete the incretin glucagon-like peptide-1 (GLP-1) in response to ingestion of nutrients, especially long-chain fatty acids. The Gαs-coupled receptor GPR119 binds the long-chain fatty acid derivate oleoylethanolamide (OEA), and GPR119 agonists enhance GLP-1 secretion. We therefore hypothesized that OEA stimulates GLP-1 release through a GPR119-dependent mechanism.
RESEARCH DESIGN AND METHODS
Murine (m) GLUTag, human (h) NCI-H716, and primary fetal rat intestinal L-cell models were used for RT-PCR and for cAMP and GLP-1 radioimmunoassay. Anesthetized rats received intravenous or intraileal OEA, and plasma bioactive GLP-1, insulin, and glucose levels were determined by enzyme-linked immunosorbent assay or glucose analyzer.
RESULTS
GPR119 messenger RNA was detected in all L-cell models. OEA treatment (10 μmol/l) of mGLUTag cells increased cAMP levels (P < 0.05) and GLP-1 secretion (P < 0.001) in all models, with desensitization of the secretory response at higher concentrations. GLP-1 secretion was further enhanced by prevention of OEA degradation using the fatty acid amide hydrolase inhibitor, URB597 (P < 0.05–0.001 vs. OEA alone), and was abolished by H89-induced inhibition of protein kinase A. OEA-induced cAMP levels and GLP-1 secretion were significantly reduced in mGLUTag cells transfected with GPR119-specific small interfering RNA (P < 0.05). Application of OEA (10 μmol/l) directly into the rat ileum, but not intravenously, increased plasma bioactive GLP-1 levels in euglycemic animals by 1.5-fold (P < 0.05) and insulin levels by 3.9-fold (P < 0.01) but only in the presence of hyperglycemia.
CONCLUSIONS
The results of these studies demonstrate, for the first time, that OEA increases GLP-1 secretion from intestinal L-cells through activation of the novel GPR119 fatty acid derivate receptor in vitro and in vivo.
Glucagon-like peptide-1 (GLP-1) is an intestinal hormone with potent insulinotropic effects that are essential to the maintenance of normal glucose homeostasis (1). In addition to glucose-dependent stimulation of insulin secretion, GLP-1 shows other favorable effects, increasing β-cell proliferation in rodents, as well as enhancing β-cell survival in both rodent and in human islets (2,3). Additionally, GLP-1 has been shown to protect cardiomyocytes from ischemia, and GLP-1 infusion improves cardiac function in patients with heart failure (4,5). Finally, the central nervous system effects of GLP-1 include inhibition of gastric emptying, reduction of appetite, and promotion of satiety in humans (6,7) and rodents (8,9). As a result of its potent antidiabetes and anorexic effects, GLP-1 analogs and GLP-1 degradation inhibitors have been successfully introduced to the clinic for pharmacologic treatment of patients with type 2 diabetes (10).
While the biological effects of GLP-1 have been well established, the mechanisms underlying GLP-1 secretion are less well understood. GLP-1 is secreted from intestinal endocrine L-cells, localized predominantly in the distal ileum and colon (11). Rapid GLP-1 release after food intake (12,13) may be regulated by afferent innervation by the vagus nerve (14,15) as well as, in rodents, by proximal gut hormones, such as glucose-dependent insulinotropic peptide (GIP) from the duodenal K-cells (16). However, L-cells also release GLP-1 in response to direct stimulation by nutrients (16), such as carbohydrates and, most notably, fat (17,18). Monounsaturated long-chain fatty acids, such as oleic acid, are strong stimulators of GLP-1 secretion from the L-cells, both in vitro and in vivo, through a signaling pathway that requires protein kinase C (PKC) ζ (17,18). Additional studies have indicated roles for the orphan G-protein–coupled receptors, GPR40 and GPR120, in the response of the L-cell to saturated fat and α-linolenic acid, respectively (19,20). Very recently, the fatty acid derivate receptor, GPR119, was also found to be expressed in a highly tissue-specific fashion by the intestinal L-cell and the pancreatic β-cell (21,22). Furthermore, a GPR119-specific pharmacological agonist was demonstrated to increase the plasma levels of both GLP-1 and insulin in mice. However, the relevance of physiological ligands of GPR119 to GLP-1 secretion by the L-cell currently remains unknown.
Oleoylethanolamide (OEA) and lysophosphatidylcholine (LPC) are endogenously occurring fatty acid derivates that are specific ligands of GPR119 (23,24). While LPC is often associated with pathophysiological processes such as atherosclerosis (25), OEA is found in a variety of tissues, including the intestinal epithelium, under physiological conditions (26). OEA is synthesized in vivo from membrane phospholipids through an N-acylphosphatidylethanolamine (NAPE)-phospholipase D (PLD)-dependent pathway (27); OEA can also be degraded into oleic acid and ethanolamide by the naturally occurring enzyme fatty acid amide hydrolase (FAAH), which is also expressed by the intestinal epithelium (28,29). Intestinal OEA levels are known to decrease during fasting and increase upon refeeding, and OEA administration to rats reduces food intake, suggesting a role for OEA in the regulation of satiety (26,30–32). As OEA was first identified as a ligand for the intranuclear peroxisome proliferator–activated receptor (PPAR) α, it has been generally assumed that the appetite reduction is dependent on PPARα activation (33). However, as GLP-1 is known to induce satiety, we hypothesized that OEA may also stimulate GLP-1 secretion from the intestinal L-cells in a GPR119-dependent fashion.
RESEARCH DESIGN AND METHODS
Cell models.
The murine (m) GLUTag L-cell line was cultured in Dulbecco's modified Eagle's medium (DMEM) containing 25 mmol/l glucose and supplemented with 10% FBS; the medium was changed every 2–3 days and cells were passaged by trypsinization and reseeding at a 1:3 dilution (17,34,35). The human (h) NCI-H716 L-cell line was obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in suspension in RPMI-1640 supplemented with 10% FBS (35,36). Fetal rat intestinal cultures (FRICs) were prepared by enzymatic dispersal of term fetal intestines from 19 to 20 days' pregnant Wistar rats, and cells were maintained overnight in DMEM containing 25 mmol/l glucose, 10% FBS, and penicillin-streptomycin, as previously reported (15,17,35,37). All three models of the intestinal L-cell have been validated with respect to the regulation of GLP-1 secretion, such that GLP-1 is secreted in response to known secretagogues, including muscarinic/cholinergic agonists, leptin and long-chain fatty acids in all models, as well as glucose-dependent insulinotropic peptide (GIP) in the rodent cells (17,34–38).
In vitro experiments.
mGLUTag cells were plated in poly-d-lysine–coated 24-well culture plates and grown to 80% confluence. For experiments with hNCI-H716 cells, adhesion of the cells was initiated by plating on Matrigel matrix (Becton Dickinson, Bedford, MA) in DMEM medium, supplemented with 10% FBS, 2 days before the experiment, as described (35,38). FRIC cells were investigated the day after cell dispersal and plating. Adenosine 3′,5′-cyclic monophosphate (cAMP) responses to OEA were determined by washing mGLUTag cells with Hanks' balanced salt solution followed by treatment for 30 min with FBS-free medium containing 1% DMSO alone (negative control), 10 μmol/l forskolin (positive control), or 10–15 μmol/l OEA, as previously described (39). To prevent cAMP degradation, all media contained 10 μmol/l 3-isobutyl-1-methylxanthine (Sigma-Aldrich, Oakville, ON, Canada). Cells were then extracted in ethanol for radioimmunoassay (RIA) of cAMP content. Secretion experiments were performed as described (17,34–36). In brief, mGLUTag, hNCI-H716, and FRIC cells were washed and then incubated for 2 h with FBS-free DMEM containing 1% DMSO alone (negative control), 10 μmol/l forskolin (Sigma-Aldrich), or 1 μmol/l GIP (positive controls) (Bachem, Torrance, CA) or with different concentrations of OEA, palmitoylethanolamide (PEA), or LPC (all from Sigma-Aldrich). Some cells were preincubated for 30 min with 10 μmol/l or 30 μmol/l H89 (Sigma-Aldrich) to inhibit protein kinase A (PKA) or with 1 μmol/l URB597 (Calbiochem, Mississauga, ON, Canada), an FAAH inhibitor (29). DMSO was used as a solvent to prepare stock solutions of fatty acid derivates and inhibitors. For secretion experiments, medium and cells were collected separately and peptides were extracted by reversed-phase adsorption using C18 silica cartridges (Sep-Pak; Waters Scientific, Mississauga, ON, Canada), as previously described (17,34–36), for RIA of GLP-1 content. GLP-1 secretion was calculated as total GLP-1 content of medium normalized for the total amount of GLP-1 in the medium plus cells. Average basal secretion of mGLUTa, hNCI-H716, and FRIC cells was 3.4 ± 0.3% (n = 31), 2.7 ± 0.4% (n = 28), and 2.86 ± 0.6% (n = 4), respectively, of total GLP-1. No changes in total GLP-1 content were found under any of the experimental conditions (data not shown).
Small interfering RNA transfection.
mGLUTag cells were plated in a 24-well plate, as described above. Scrambled small interfering RNA (siRNA) (control) and two siRNAs targeting murine GPR119 coding sequences were purchased from Ambion (Austin, TX). Transfection was performed by 5-h incubation in Opti-Mem medium using 20 pmol/l siRNA and 1 μl Lipofectamine 2000 (Invitrogen, Burlington, ON, Canada) as instructed by the manufacturer. Cells were allowed to recover for 2 days before cAMP and/or secretion experiments. Transfection efficiency was quantified by real-time RT-PCR. In brief, total RNA was extracted from mGLUTag cells using an RNeasy kit as instructed by the manufacturer (Qiagen, Mississauga, ON, Canada) and subjected to reverse transcription using SuperScript II and random hexamers (Invitrogen), followed by real-time PCR using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) for GPR119 (Mm00731497_s1) and 18S (Hs99999901_s1; endogenous control). Relative quantification of GPR119 mRNA expression was calculated using the ΔΔ cycle threshold method (40).
In vitro assays.
Viability of the mGLUTag cells after treatment was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich) assay, as reported (17). Cells were plated in poly-d-lysine–coated 96-well plates and treated for 2 h with medium containing 1% DMSO alone (control), 5 mmol/l H2O2 (positive control), or fatty acid derivates or inhibitors at concentrations used for the secretion experiment, after which the MTT reaction was carried out. The resulting absorbance was measured at 570 nm; higher absorbance correlates with cell viability and lower absorbance with cell death. cAMP was measured by RIA of ethanol extracts as described (39) (Biomedical Technologies, Stoughton, MA). RIA for COOH-terminal GLP-1 immunoreactivity was conducted using an established lab assay (17,34–36).
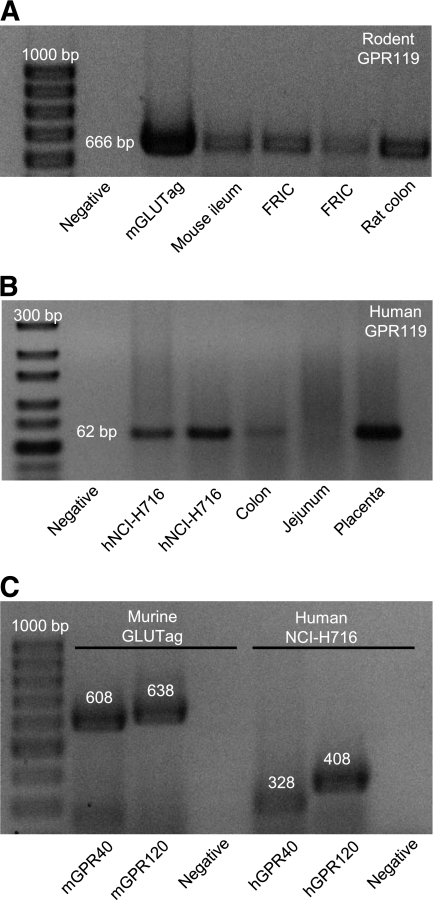
As there is currently no good GPR119 antiserum commercially available, GPR119 expression was determined by RT-PCR. Human jejunum and colon total RNA was purchased from Ambion, and human placental RNA was a kind gift from Dr. J.R. Challis (University of Toronto, Toronto, ON, Canada). Total RNA from mGLUTag, hNCI-H716, and FRIC cells, as well as from rodent tissues, was extracted as described above. The primer pairs used for amplification of human GPR119 were described by Soga et al. (23) and for mouse GPR40 and GPR120 by Iakoubov et al. (17); all other primer pairs were designed using PrimerQuest (IDT, Coralville, IA) (sequences are shown in Table 1). PCR were carried out at 57°C for 35 cycles, and PCR without RNA template was used as the negative control. Products were analyzed by agarose gel electrophoresis and visualized with ethidium bromide.
TABLE 1.
PCR primers
| Gene (GenBank accession no.) | Primers (5′–3′) | Position | Amplicon (bp) |
|---|---|---|---|
| m/rGPR119 (NM_181751) | TGATGGTGTTGGCCTTTGCTTCAC | 328–351 | 666 |
| TGGTAAAGGCAGCATTTGTGGCAG | 993–970 | ||
| hGPR119 (NM_178471) | TCTCGGCCCACACAGAAGA | 208–226 | 62 |
| GCTGCGGAGGAAGTGACAAA | 269–250 | ||
| mGPR40 (NM_194057) | TCTGATCTCCTACTGGCCATCACT | 151–175 | 608 |
| AGGTCCGGGTTTATGAAACTAGCC | 734–758 | ||
| hGPR40 (NM_005303) | TTCAGCCTCTCTCTCCTGCTCTTT | 550–573 | 328 |
| TTCTTGCCGCACACACTGTCTTCA | 877–854 | ||
| mGPR120 (NM_181748) | TTTCTTCTCGGATGTCAAGGGCGA | 126–149 | 638 |
| TCCGCGATGCTTTCGTGATCTGTA | 763–740 | ||
| hGPR120 (NM_181745) | TCGATTTGCACACTGATTTGGCCC | 630–653 | 408 |
| AAATGTGAAGGCCACCACCCAGAA | 1,037–1,014 |
In vivo experiments.
In vivo animal protocols were approved by the University of Toronto Animal Care Committee. Male Wistar rats (200–300 g) were obtained from Charles River Laboratories (St. Constant, QC, Canada) and maintained on a standard laboratory diet with free access to water under a 12-h light-dark schedule. Following an overnight fast, rats were anesthetized with isoflurane (Baxter, Mississauga, ON, Canada). One hour before blood sampling, some rats were intraperitoneally injected with 3 mg/kg URB597 to inhibit FAAH (29,41). URB597 alone did not affect the levels of glucose, insulin, or bioactive GLP-1 in these studies; therefore, data from rats with and without URB597 injection were combined. The carotid artery was cannulated for blood sampling and the jugular vein for injections. To prevent bioactive GLP-1 degradation by dipeptidylpeptidase (DPP) IV, rats received 5 mg/kg Sitagliptin (Januvia; MSD Sharp & Dohme, Haar, Germany), a selective inhibitor of DPP-IV (10,42), intravenously 30 min before blood collection. Some rats also received a femoral vein cannula for continuous infusion of 37.5% glucose to maintain glycemia at 13 mmol/l for at least 30 min. The glucose infusion rate was adjusted based on frequent (every 5–10 min) blood glucose measurements, as described in Goh et al. (43). Rats undergoing a hyperglycemic clamp were not pretreated with Sitagliptin in order to reduce the number of variables that might affect glycemia. The abdominal cavity of all rats was then opened and a 10-cm section of the distal ileum was cleansed by perfusion with saline and tied off to create a luminally distinct compartment that retained vascular perfusion. Subsequent to collection of the basal blood sample at t = 0 min, either the luminal compartment was filled with 2 ml of 10 μmol/l OEA or vehicle (0.9% saline/10% Tween 80; Sigma-Aldrich), or 5 mg/kg OEA or vehicle (0.9% saline/10% Tween 80) was administered intravenously, and additional blood samples were collected at 5, 15, 30, and 60 min. All samples (1 ml each) were collected into 10% (vol/vol) Trasylol (5,000 Kalikrein inactivating units/ml; Bayer, Toronto, ON, Canada), EDTA (12 mg/ml), and diprotin A (a DPP-IV inhibitor, 68 mg/ml; Sigma-Aldrich), and plasma was stored at −80°C until analysis. Plasma glucose levels were analyzed on a Beckman Analyzer II (Beckman, Fullerton, CA), and plasma insulin levels were measured by enzyme-linked immunosorbent assay (Crystal Chem, Downers Grove, IL) in normoglycemic animals and by RIA (Millipore, Billerica, MA) in hyperglycemic animals due to the wider insulin detection range. Plasma levels of bioactive GLP-1 were determined by electrochemiluminescence-based detection assay (Meso Scale Discovery, Gaithersburg, MD).
Statistical analyses.
All results are expressed as means ± SE. Area under the curve (AUC) was determined using the trapezoidal rule, and the data are expressed per min. Statistical analysis was performed using SAS software (SAS Institute, Cary, NC). One- and two-way ANOVA was followed by Student's t test or n-1 custom hypotheses post hoc tests, as appropriate. To reduce interassay variations, some data were normalized to basal levels. Significance was assumed at P < 0.05.
RESULTS
Expression of GPR40, GPR119, and GPR120.
RT-PCR for rodent GPR119 mRNA transcripts was performed on total RNA extracted from mGLUTag and FRIC cells, as well as from mouse ileum and rat colon tissue. As shown in Fig. 1A, GPR119 mRNA was detected in both rodent L-cell models, as well as in the intestinal samples. Additionally, GPR119 mRNA was detected in the hNCI-H716 cells and in human colon and placental tissue (Fig. 1B). In contrast to previous reports (22), we did not detect GPR119 mRNA in human jejunum, possibly due to a low number of L-cells in this tissue (11). Consistent with our previous findings (17), mRNA transcripts for both GPR40 and GPR120 were detected in the mGLUTag cells, and both receptor mRNAs were also found to be expressed in the hNCI-H716 cells (Fig. 1C).
FIG. 1.
Expression of GPR119 mRNA in L-cell models. A: Total RNA from mGLUTag cells, FRICs, and murine intestinal tissues was analyzed for expression of GPR119 mRNA by RT-PCR. B: Total RNA from hNCI-H716 cells, human intestinal tissues, and human placenta was analyzed for expression of GPR119 mRNA by RT-PCR. C: Total RNA from mGLUTag cells and hNCI-H716 cells was analyzed for expression of GPR40 and GPR120 mRNA by RT-PCR. All products were separated on agarose gels and visualized with ethidium bromide, with the molecular-size ladder on the left. Negative controls did not include RNA template. The anticipated band sizes of products are indicated in base pairs (bp).
OEA induces GLP-1 secretion in vitro.
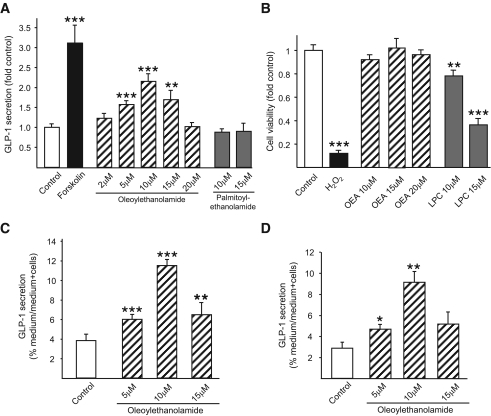
Possible effects of GPR119 receptor activation on GLP-1 secretion were first investigated in mGLUTag cells. Release of GLP-1 was increased to 3.1 ± 0.4–fold of basal values by treatment with forskolin, a direct activator of adenylyl cyclase and strong L-cell secretagogue (34). OEA (5–20 μmol/l), a known ligand of GPR119 (24), significantly increased GLP-1 secretion to a maximum of 2.1 ± 0.2–fold of basal levels at 10 μmol/l (P < 0.001) (Fig. 2A). The same concentrations of PEA, a saturated fatty acid ethanolamide (16:0) that is a very weak agonist of GPR119 (23,24), did not increase GLP-1 secretion from mGLUTag cells. Importantly, OEA treatment did not affect the viability of the GLUTag cells (Fig. 2B). In contrast, although LPC treatment of mGLUTag cells at concentrations reported to activate GPR119 (10–15 μmol/l) also enhanced GLP-1 release (to a maximum of 10.4 ± 0.5–fold of control values; data not shown), LPC was found to markedly reduce cell viability by up to 60.0 ± 6.8% compared with control cells (P < 0.05 and P < 0.001) (Fig. 2B); LPC was, therefore, not used in further experiments. The effects of OEA on GLP-1 secretion were also confirmed in hNCI-H716 and FRIC cells (Fig. 2C and D), wherein OEA treatment significantly increased GLP-1 secretion to 2.6 ± 0.2– and 5.8 ± 2.5–fold of basal levels at 10 μmol/l (P < 0.001 and P < 0.01), respectively. Interestingly, higher concentrations of OEA were associated with diminished GLP-1 release in all cell models, suggestive of desensitization.
FIG. 2.
Effects of OEA on GLP-1 secretion. mGLUTag cells (n = 9–12) (A), hNCI-H716 cells (n = 8) (C), and FRIC cells (n = 4) (D) were incubated with medium alone (1% DMSO, negative control), forskolin (10 μmol/l, positive control), OEA (2–20 μmol/l), or PEA (10–15 μmol/l, negative control) for 2 h. GLP-1 content of media and cells was determined by RIA. B: To determine potential effects on cell viability, mGLUTag cells were incubated with medium alone (1% DMSO, negative control), H2O2 (5 mmol/l, positive control), OEA (10–20 μmol/l), or LPC (10–15 μmol/l) for 2 h, followed by MTT assay (n = 8–16). *P < 0.05; **P < 0.01; ***P < 0.001 vs. control.
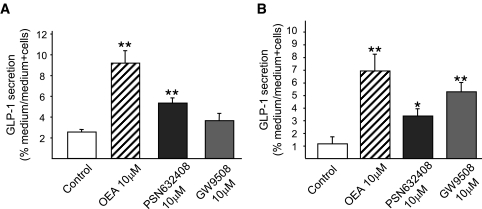
To verify the activity of GPR119 in the L-cell, mGLUTag and hNCI-H716 cells were treated with a specific GPR119 agonist, PSN632408 (10 μmol/l) (24). PSN632408 increased GLP-1 secretion by both cell lines to 2.1 ± 0.2– and 2.9 ± 0.5–fold of basal values (P < 0.01 for mGLUTag and P < 0.05 for hNCI-H716 cells) (Fig. 3), demonstrating that GPR119 is functional and can initiate GLP-1 secretion in these cells. To determine possible function of the other fatty acid receptors expressed in the L-cell lines GPR40 and GPR120 (Fig. 1C), both mGLUTag and hNCI-H716 cells were also treated with the combined GPR40/GPR120 agonist GW9508 (10 μmol/l) (24). Consistent with previous findings from our group (17), GPR40 and GPR120 were not found to be involved in GLP-1 secretion from mGLUTag cells, as treatment with GW9508 did not increase GLP-1 secretion (Fig. 3). In contrast, GW9508 increased GLP-1 secretion by hNCI-H716 cells to 4.5 ± 0.6–fold of basal values (P < 0.01), providing indication for a role of GPR40 and/or GPR120 in human L-cells.
FIG. 3.
Effects of GPR119 and GPR40/120 agonists on GLP-1 secretion. mGLUTag (n = 4) (A) and hNCI-H716 (n = 4) (B) cells were incubated with medium alone (1% DMSO, negative control), OEA (10 μmol/l), the GPR119 agonist PSN632408 (10 μmol/l), or the combined GPR40/GPR120 agonist GW9508 (10 μmol/l) for 2 h. GLP-1 content of media and cells was determined by RIA. *P < 0.05; **P < 0.01 vs. control.
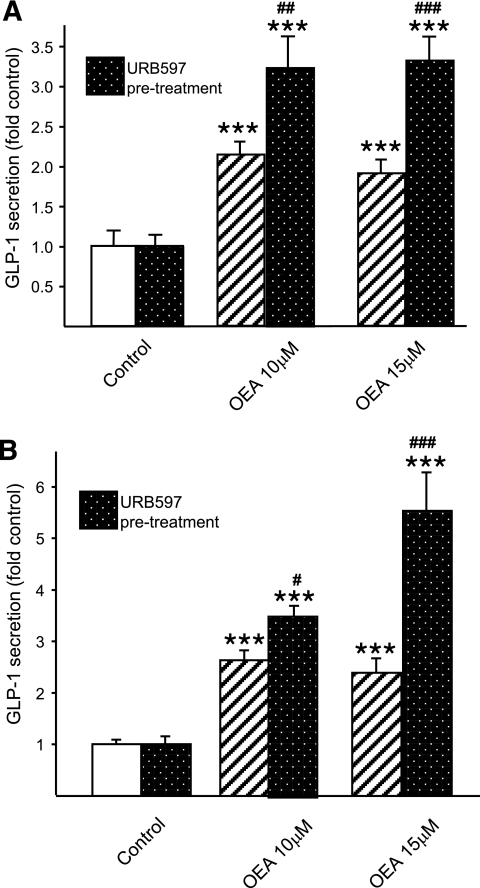
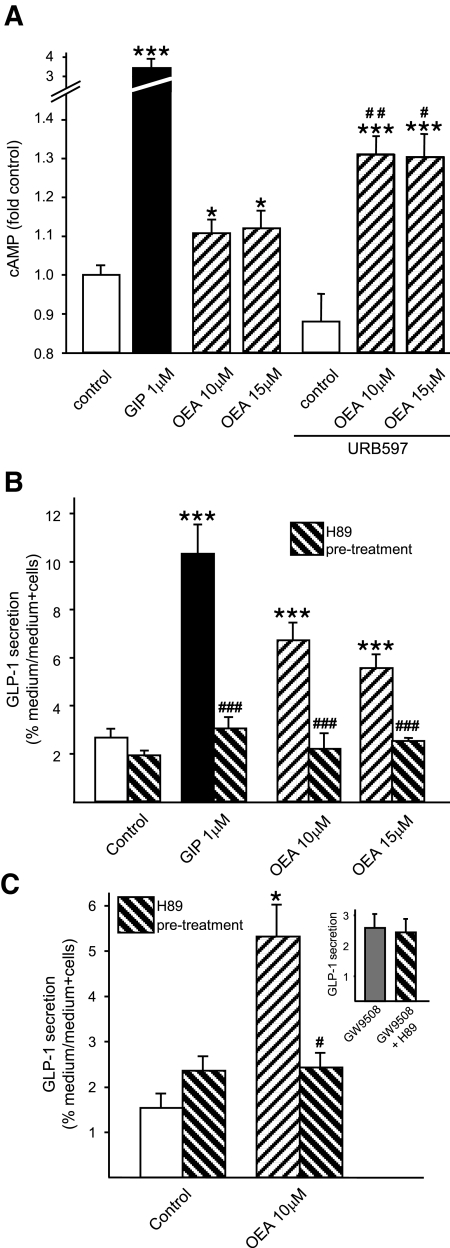
Prevention of OEA degradation with URB597 significantly increased OEA-induced GLP-1 secretion to 3.2 ± 0.4– and 3.3 ± 0.3–fold of control values at 10 and 15 μmol/l OEA in mGLUTag cells (P < 0.001 compared with URB597 alone and P < 0.01–0.001 compared with OEA treatment without URB597) (Fig. 4A), as well as in hNCI-H716 cells (to 3.5 ± 0.2– and 5.5 ± 0.7–fold of control values at 10 and 15 μmol/l OEA (P < 0.001 compared with URB597 alone and P < 0.05–0.001 compared with OEA treatment without URB597) (Fig. 4B). These findings indicate that degradation by FAAH limits the effects of OEA on GLP-1 secretion in both of the intestinal L-cell lines utilized.
FIG. 4.
Effect of inhibition of OEA degradation on OEA-induced GLP-1 secretion. mGLUTag (n = 6–18) (A) and hNCI-H716 (n = 12) (B) cells were pretreated for 30 min with URB597 (1 μmol/l) to inhibit FAAH and prevent OEA degradation before incubation with medium alone (1%DMSO, negative control) or OEA (10–15 μmol/l) for 2 h. GLP-1 content of media and cells was determined by RIA. ***P < 0.001 vs. control; #P < 0.05; ##P < 0.01; ###P < 0.001 vs. OEA treatment alone.
OEA signals through a PKA- and GPR119-dependent mechanism.
As ligand binding to GPR119 leads to activation of adenylyl cyclase, increased production of cAMP, and enhanced PKA activity (24), mGLUTag cells were first examined for cAMP responses to OEA (10–15 μmol/l) and GIP, which is known to signal through cAMP- and PKA-dependent pathways (positive control) (44). OEA treatment alone caused a small but significant increase in intracellular cAMP to 1.11 ± 0.03 – and 1.12 ± 0.04–fold of control values at 10 and 15 μmol/l, respectively (P < 0.05) (Fig. 5A). This effect was enhanced when OEA degradation was prevented with URB597, such that cAMP levels increased by an additional 1.3 ± 0.04 – and 1.3 ± 0.03–fold, respectively (P < 0.001 compared with URBB597 alone and P < 0.05–0.01 compared with OEA treatment without URB597). Furthermore, pretreatment of the mGLUTag cells with the PKA inhibitor H89 (10 μmol/l) completely abolished OEA (10–15 μmol/l)-induced GLP-1 secretion (Fig. 5B). Similar results were found in hNCI-H716 cells, although an increased concentration of H89 (30 μmol/l) was required to abrogate OEA-induced GLP-1 release (Fig. 5C). H89 treatment did not affect GPR40/120-induced GLP-1 secretion (Fig. 5C, inset), demonstrating the specificity of this inhibitor for PKA-mediated signaling.
FIG. 5.
Effect of PKA inhibition on OEA-induced GLP-1 secretion. mGLUTag (n = 6–9) (A and B) and hNCI-H716 (n = 4–6) (C) cells were pretreated for 30 min with medium alone (1% DMSO, negative control), H89 (10 μmol/l for mGLUTag and 30 μmol/l for hNCI-H716), or URB597 (1 μmol/l) to inhibit PKA or FAAH, respectively, followed by incubation with medium alone (1% DMSO, negative control), GIP (1 μmol/l, positive control), or OEA (10–15 μmol/l) for 2 h. C, inset: hNCI-H716 cells were pretreated for 30 min with media alone (1% DMSO) or with H89 (30 μmol/l), followed by incubation with the GPR40/120 agonist GW9508 (10 μmol/l). cAMP content of cells and GLP-1 content of media and cells were determined by RIA. *P < 0.05; ***P < 0.001 vs. appropriate control; #P < 0.05; ##P < 0.01; ###P < 0.001 vs. paired treatment alone.
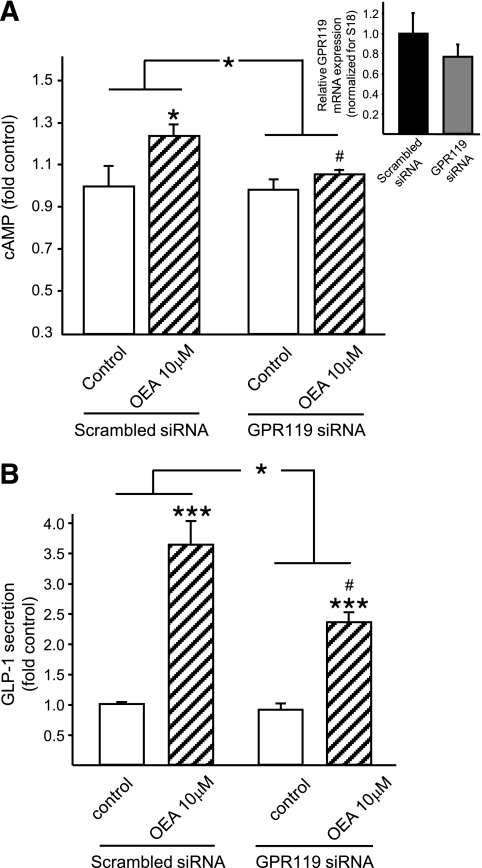
To determine whether the effects of OEA on cAMP production and GLP-1 secretion are dependent upon GPR119, mGLUTag cells were transfected with specific GPR119 siRNA or scrambled siRNA (control), resulting in 23% knockdown of GPR119 mRNA, as determined by real-time RT-PCR (Fig. 6A, inset). Despite the relatively low level of GPR119 knockdown, OEA (10 μmol/l) failed to enhance cAMP levels in cells treated with GPR119 siRNA, whereas the cAMP response to OEA treatment was preserved in the control cells (P < 0.05) (Fig. 6A). Furthermore, GPR119 knockdown led to a 45% reduction in the GLP-1 secretory response to OEA (P < 0.05) (Fig. 5B). These data therefore provide support for a role of GPR119 and the PKA signaling pathway in OEA-induced GLP-1 secretion.
FIG. 6.
Effect of GPR119 knockdown on OEA-induced GLP-1 secretion. mGLUTag cells were transfected with scrambled siRNA (20 pmol/l, control) or GPR119 siRNA (20 pmol/l) 2 days before the experiment. Cells were then incubated with medium alone (1% DMSO, negative control) or OEA (10–15 μmol/l) for 2 h. cAMP content of cells (n = 6) (A) and GLP-1 content of media and cells (n = 9) (B) were determined by RIA. A, inset: GPR119 mRNA transcript levels were determined by quantitative RT-PCR relative to 18S transcript levels. *P < 0.05; ***P < 0.001 vs. control or vs. the Δ change in control cells, as indicated by the lines. #P < 0.05 vs. OEA treatment with scrambled siRNA.
OEA enhances GLP-1 secretion in vivo.
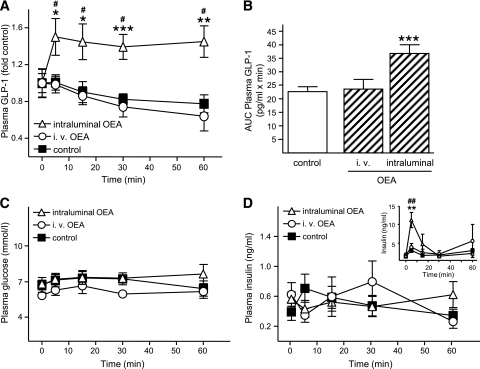
To establish the effects of OEA on the L-cell in vivo, rats were treated with OEA either intraluminally or intravenously. Intraluminal application of OEA (10 μmol/l; e.g., 20 nmol/rat) to euglycemic rats significantly increased plasma bioactive GLP-1 concentrations to 1.5 ± 0.2–fold of basal values (P < 0.05) within 5 min of administration, and this stimulation was maintained throughout the entire 60-min time course of the experiment (Fig. 7A). Thus, the AUC for the bioactive GLP-1 response was significantly increased to 1.6 ± 0.1–fold of vehicle-infused rats (P < 0.001) (Fig. 7B). In contrast, intravenous administration of OEA at a 200-fold–higher dose (e.g., 4 μmol/250 g rat) than that used intraluminally demonstrated no effect on bioactive GLP-1 concentrations compared with rats treated with vehicle alone. Throughout the 60-min experiment, the plasma levels of glucose (Fig. 7C) and insulin (Fig. 7D) remained stable at basal levels and did not differ between treatment and control groups.
FIG. 7.
In vivo effect of OEA on GLP-1 secretion. Anesthetized rats received intraluminal or intravenous injections of vehicle (saline/10% Tween 80; combined controls), intraluminal OEA (2 ml of 10 μmol/l), or intravenous OEA (5 mg/kg), and blood samples were collected over a 1-h period. Plasma concentrations of bioactive GLP-1 (A), glucose (C), and insulin (D) were determined by ELISA and glucose analyzer, as appropriate (n = 5–11). B: AUC for the absolute plasma bioactive GLP-1 concentrations was determined using the trapezoidal rule and is expressed per min. D, inset: Rats (n = 4–5) were maintained at 13 mmol/l plasma glucose (hyperglycemic clamp) for a minimum of 30 min, and this was maintained throughout the OEA treatment procedure. Plasma insulin levels were determined by RIA. A: To reduce interassay variations due to use of separate kits, bioactive GLP-1 concentrations were calculated as fold increase over basal GLP-1 levels (control: 29.4 ± 8.6 pg/ml; intraluminal OEA: 18.9 ± 4.9 pg/ml; and intravenous OEA: 32.7 ± 3.4 pg/ml; P = NS between the basal values). *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.001 vs. control; #P < 0.05 and ##P < 0.01 vs. basal values.
As GLP-1 is known to lose its insulinotropic effects under normoglycemic conditions, changes in insulin levels upon OEA treatment were also measured under hyperglycemic conditions. Glycemia was maintained at 13 mmol/l, the upper physiological level in rats, for at least 30 min before the start of OEA application and throughout the remainder of the procedure. The basal concentration of insulin before OEA application was 1.4 ± 0.4 pg/ml and did not differ between the groups. Intraluminal application of OEA caused a 3.9 ± 0.7–fold increase in insulin plasma levels within 5 min of application (11.2 ± 2.1 vs. 2.8 ± 2.0 pg/ml in the control group; P < 0.01) (Fig. 7D, inset). In contrast, intravenous infusion of OEA did not affect insulin levels during the entire treatment period in the hyperglycemic rats.
DISCUSSION
Previous studies have indicated that the fatty acid derivate receptor GPR119 is present on pancreatic β-cells and intestinal L-cells, and its stimulation by a GPR119 agonist increases insulin and GLP-1 secretion, respectively (21,22,24). However, the role of physiologically occurring ligands of GPR119, such as OEA, and the intracellular mechanisms underlying GPR119-dependent GLP-1 secretion from the intestinal L-cell have remained undefined. The results of the present study demonstrate, for the first time, that OEA stimulates GLP-1 secretion from both mouse and human intestinal L-cell lines, as well as from primary rat L-cells in vitro. Additionally, application of OEA directly into the intestinal lumen in rats induced a significant and persistent increase in bioactive GLP-1 levels over 1 h, supporting the in vitro findings and demonstrating a role for OEA as a GLP-1 secretagogue in vivo.
To further establish the role of OEA in GLP-1 secretion, the intracellular signaling mechanisms underlying its effects on the L-cell were investigated. OEA induced a small, but significant, increase in intracellular cAMP concentration in both the mGLUTag and hNCI-H716 cells, comparable with findings in OEA-treated RINm5 and MIN6 pancreatic β-cell lines (45). Furthermore, GLP-1 secretion in OEA-treated cells was strictly dependent on PKA in both the human and mouse L-cells, as indicated by complete abrogation of the response in H89-treated cells. These findings are consistent with studies by our lab and others showing that increased cAMP levels, in response to cAMP analogs as well as to secretagogues such as GIP, stimulate GLP-1 release by the intestinal L-cell (16,34,46). Furthermore, while initial studies on OEA identified the intranuclear receptor PPARα and transient receptor potential vanilloid type 1 (TRPV1) as targets for OEA (27,33,47), neither of these receptors has been reported to stimulate the cAMP/PKA signaling pathway. In contrast, the deorphanization of GPR119 as a cAMP-linked OEA receptor (24) implicated GPR119 as more likely target for OEA in the intestinal L-cell. Consistent with this hypothesis, treatment of human and mouse intestinal L-cells with a GPR119-specific agonist PSN632408 significantly increased GLP-1 secretion in both cell lines. Furthermore, transfection of mGLUTag cells with GPR119 siRNA significantly diminished both the cAMP and GLP-1 responses to OEA. When taken together, these findings provide support for both the presence of functional GPR119 in the intestinal L-cell and the requirement for this novel G-protein–coupled receptor in OEA-induced GLP-1 secretion.
We have previously reported that oleic acid is a strong L-cell secretagogue, increasing GLP-1 secretion both in vivo and in vitro (17,18,48). It is therefore interesting that OEA is rapidly degraded to oleic acid and ethanolamide via FAAH-dependent hydrolysis (29), thereby providing a possible GPR119-independent mechanism underlying OEA-induced GLP-1 secretion. Therefore, to exclude the possible effects of oleic acid on the actions of OEA, L-cells were pretreated with the FAAH inhibitor URB597 to reduce OEA hydrolysis and resultant oleic acid accumulation. The increase in OEA-induced GLP-1 secretion found with URB597 treatment provides support for a direct role of OEA, and not of oleic acid, in OEA-induced GLP-1 secretion.
While intraluminal application of OEA in vivo significantly increased GLP-1 secretion in normal rats, intravenous injection of OEA at a 200-fold–higher dose failed to increase circulating levels of bioactive GLP-1. Additionally, while intraluminal OEA application clearly increased insulin levels in hyperglycemic rats, intravenous injection of OEA did not affect insulin levels in these animals under either euglycemic or hyperglycemic conditions. Taken together, this data suggests that circulating OEA does not cause significant increases in hormone release from either of its known target tissues (the intestinal L-cell and the pancreatic β-cell). There are several possible explanations for these findings. First, the volume of distribution for OEA as a lipophilic substance should be high, therefore increasing the concentration of OEA required for administration into the jugular vein to reach distant target tissues at stimulatory concentrations. However, higher levels of OEA cannot be achieved in the circulation of the rat due to the limited solubility of OEA in solvents. Furthermore, whether OEA is cleared through the liver and/or lungs is unknown and may be significant.
The actions of both OEA and a GRP119 agonist on insulin secretion have previously been reported to be glucose dependent (21,45), as further confirmed in the present study. Previous studies have also demonstrated that ∼45% of the glucose-lowering effect of an oral GPR119 agonist given concurrently with oral glucose is mediated through enhancement of GLP-1 release (22), suggesting that the insulinotropic effect of GPR119 activation is mediated both directly, through GPR119 expressed on the β-cell, and indirectly, through enhanced release of GLP-1. Nonetheless, although both the L-cell and the β-cell are responsive to OEA, the glucose sensitivity of the β-cell response, compared with the glucose insensitivity of the L-cell response that we have observed, suggests that oral administration of this fatty acid derivate alone to enhance GLP-1 secretion, without influencing insulin release, will permit the insulin-independent biological actions of GLP-1. In contrast, administration of OEA in the setting of a glucose-containing meal would facilitate release of both GLP-1 and insulin, thereby also modulating glycemic responses (22,49).
The observation of apparent desensitization in all of the dose-response curves was unexpected, as this has not previously been reported for GPR119. However, Gαs-coupled receptors are well established to undergo homologous desensitization (50); indeed, preliminary studies in which hNCI-716 cells were pretreated with the GPR119 agonist PSN632408 (10 μmol/l for 6 h) demonstrated a 70.9 ± 4.6% and 50.7 ± 11.7% decrease in subsequent OEA- and PSN632408-induced GLP-1 secretion, respectively (data not shown). Collectively, these findings indicate that GPR119 may undergo homologous desensitization, a phenomenon that clearly warrants further investigation.
In summary, the results of this study establish, for the first time, the role of GPR119 in OEA-induced GLP-1 secretion and add GPR119 to the growing list of fatty acid–responsive pathways that function to modulate release of GLP-1, including GPR40, GPR120, and PKCζ. When combined with the reported insulinotropic effects of GPR119 agonists, these findings implicate GPR119 as a potential pharmacological target, as well as OEA as a nutriceutical approach to enhance GLP-1 in patients with type 2 diabetes.
ACKNOWLEDGMENTS
L.M.L. was supported by postdoctoral fellowships from the European Foundation for the Study of Diabetes (EFSD; Albert Renold and EFSD/Lilly Research Fellowships). R.I. was supported by postdoctoral fellowships from the Banting and Best Diabetes Centre, University of Toronto (Toronto, ON, Canada), and from Deutsche Forschungsgemeinschaft (DFG; the German Research Foundation). P.L.B. was supported by the Canada Research Chairs Program. This work was supported by an operating grant from the Canadian Diabetes Association (no. 2374). No other potential conflicts of interest relevant to this article were reported.
The authors are grateful to J.R. Challis (University of Toronto, Toronto, ON, Canada) for the gift of placental RNA.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Drucker DJ: The biology of incretin hormones. Cell Metab 2006; 3: 153– 165 [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Brubaker PL: Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 2002; 45: 1263– 1273 [DOI] [PubMed] [Google Scholar]
- 3.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R: Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003; 144: 5149– 5158 [DOI] [PubMed] [Google Scholar]
- 4.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP: Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006; 12: 694– 699 [DOI] [PubMed] [Google Scholar]
- 5.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M: Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008; 117: 2340– 2350 [DOI] [PubMed] [Google Scholar]
- 6.Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A: The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 2001; 25: 781– 792 [DOI] [PubMed] [Google Scholar]
- 7.Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA: Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003; 88: 2719– 2725 [DOI] [PubMed] [Google Scholar]
- 8.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP: Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol 1996; 271: R848– R856 [DOI] [PubMed] [Google Scholar]
- 9.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL: Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 2005; 146: 3748– 3756 [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ, Nauck MA: The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696– 1705 [DOI] [PubMed] [Google Scholar]
- 11.Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Goke B: Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 1992; 22: 283– 291 [DOI] [PubMed] [Google Scholar]
- 12.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V: Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 1993; 138: 159– 166 [DOI] [PubMed] [Google Scholar]
- 13.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B: Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 1996; 97: 92– 103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca AS, Brubaker PL: Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 1999; 140: 1687– 1694 [DOI] [PubMed] [Google Scholar]
- 15.Anini Y, Hansotia T, Brubaker PL: Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology 2002; 143: 2420– 2426 [DOI] [PubMed] [Google Scholar]
- 16.Roberge JN, Brubaker PL: Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 1993; 133: 233– 240 [DOI] [PubMed] [Google Scholar]
- 17.Iakoubov R, Izzo A, Yeung A, Whiteside CI, Brubaker PL: Protein kinase Czeta is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 2007; 148: 1089– 1098 [DOI] [PubMed] [Google Scholar]
- 18.Rocca AS, LaGreca J, Kalitsky J, Brubaker PL: Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology 2001; 142: 1148– 1155 [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G: Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005; 11: 90– 94 [DOI] [PubMed] [Google Scholar]
- 20.Edfalk S, Steneberg P, Edlund H: Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008; 57: 2280– 2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J: A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 2007; 148: 2601– 2609 [DOI] [PubMed] [Google Scholar]
- 22.Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, Chen R, Jones RM, Behan DP, Leonard J: A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology 2008; 149: 2038– 2047 [DOI] [PubMed] [Google Scholar]
- 23.Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, Matsumoto S, Kamohara M, Hiyama H, Yoshida S, Momose K, Ueda Y, Matsushime H, Kobori M, Furuichi K: Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun 2005; 326: 744– 751 [DOI] [PubMed] [Google Scholar]
- 24.Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C: Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 2006; 3: 167– 175 [DOI] [PubMed] [Google Scholar]
- 25.Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C: Lysophosphatidylcholine and secretory phospholipase A2 in vascular disease: mediators of endothelial dysfunction and atherosclerosis. Med Sci Monit 2006; 12: RA5– 16 [PubMed] [Google Scholar]
- 26.Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, Piomelli D: Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem 2007; 282: 1518– 1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D: Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci 2005; 62: 708– 716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB: Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996; 384: 83– 87 [DOI] [PubMed] [Google Scholar]
- 29.Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D: Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 2005; 313: 352– 358 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D: An anorexic lipid mediator regulated by feeding. Nature 2001; 414: 209– 212 [DOI] [PubMed] [Google Scholar]
- 31.Oveisi F, Gaetani S, Eng KT, Piomelli D: Oleoylethanolamide inhibits food intake in free-feeding rats after oral administration. Pharmacol Res 2004; 49: 461– 466 [DOI] [PubMed] [Google Scholar]
- 32.Astarita G, Di Giacomo B, Gaetani S, Oveisi F, Compton TR, Rivara S, Tarzia G, Mor M, Piomelli D: Pharmacological characterization of hydrolysis-resistant analogs of oleoylethanolamide with potent anorexiant properties. J Pharmacol Exp Ther 2006; 318: 563– 570 [DOI] [PubMed] [Google Scholar]
- 33.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D: Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 2003; 425: 90– 93 [DOI] [PubMed] [Google Scholar]
- 34.Brubaker PL, Schloos J, Drucker DJ: Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology 1998; 139: 4108– 4114 [DOI] [PubMed] [Google Scholar]
- 35.Anini Y, Brubaker PL: Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 2003; 52: 252– 259 [DOI] [PubMed] [Google Scholar]
- 36.Anini Y, Brubaker PL: Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L cells. Endocrinology 2003; 144: 3244– 3250 [DOI] [PubMed] [Google Scholar]
- 37.Brubaker PL: Control of glucagon-like immunoreactive peptide secretion from fetal rat intestinal cultures. Endocrinology 1988; 123: 220– 226 [DOI] [PubMed] [Google Scholar]
- 38.Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K: A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology 2001; 142: 4522– 4528 [DOI] [PubMed] [Google Scholar]
- 39.Shin ED, Estall JL, Izzo A, Drucker DJ, Brubaker PL: Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology 2005; 128: 1340– 1353 [DOI] [PubMed] [Google Scholar]
- 40.Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D: Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 2003; 9: 76– 81 [DOI] [PubMed] [Google Scholar]
- 42.Beconi MG, Reed JR, Teffera Y, Xia YQ, Kochansky CJ, Liu DQ, Xu S, Elmore CS, Ciccotto S, Hora DF, Stearns RA, Vincent SH: Disposition of the dipeptidyl peptidase 4 inhibitor sitagliptin in rats and dogs. Drug Metab Dispos 2007; 35: 525– 532 [DOI] [PubMed] [Google Scholar]
- 43.Goh TT, Mason TM, Gupta N, So A, Lam TK, Lam L, Lewis GF, Mari A, Giacca A: Lipid-induced beta-cell dysfunction in vivo in models of progressive beta-cell failure. Am J Physiol Endocrinol Metab 2007; 292: E549– E560 [DOI] [PubMed] [Google Scholar]
- 44.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI: Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993; 133: 2861– 2870 [DOI] [PubMed] [Google Scholar]
- 45.Ning Y, O'Neill K, Lan H, Pang L, Shan LX, Hawes BE, Hedrick JA: Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol 2008; 155: 1056– 1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson AK, Ward PS, Wong KY, Collord GJ, Habib AM, Reimann F, Gribble FM: Cyclic AMP triggers glucagon-like peptide-1 secretion from the GLUTag enteroendocrine cell line. Diabetologia 2007; 50: 2181– 2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahern GP: Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem 2003; 278: 30429– 30434 [DOI] [PubMed] [Google Scholar]
- 48.Rocca AS, Brubaker PL: Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology 1995; 136: 5593– 5599 [DOI] [PubMed] [Google Scholar]
- 49.Lauffer L, Iakoubov R, Brubaker PL: GPR119: “double-dipping” for better glycemic control. Endocrinology 2008; 149: 2035– 2037 [DOI] [PubMed] [Google Scholar]
- 50.Kelly E, Bailey CP, Henderson G: Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol 2008; 153 ( Suppl) 1: S379– S388 [DOI] [PMC free article] [PubMed] [Google Scholar]