Abstract
OBJECTIVE
We evaluated insulin sensitivity and insulin secretion across the entire range of fasting (FPG) and 2-h plasma glucose (PG), and we investigated the differences in insulin sensitivity and insulin release in different glucose tolerance categories.
RESEARCH DESIGN AND METHODS
A total of 6,414 Finnish men (aged 57 ± 7 years, BMI 27.0 ± 3.9 kg/m2) from our ongoing population-based METSIM (Metabolic Syndrome in Men) study were included. Of these subjects, 2,168 had normal glucose tolerance, 2,859 isolated impaired fasting glucose (IFG), 217 isolated impaired glucose tolerance (IGT), 701 a combination of IFG and IGT, and 469 newly diagnosed type 2 diabetes.
RESULTS
The Matsuda index of insulin sensitivity decreased substantially within the normal range of FPG (−17%) and 2-h PG (−37%) and was approximately −65 and −53% in the diabetic range of FPG and 2-h PG, respectively, compared with the reference range (FPG and 2-h PG <5.0 mmol/l). Early-phase insulin release declined by only approximately −5% within the normal range of FPG and 2-h PG but decreased significantly in the diabetic range of FPG (by 32–70%) and 2-h PG (by 33–51%). Changes in insulin sensitivity and insulin secretion in relation to hyperglycemia were independent of obesity. The predominant feature of isolated IGT was impaired peripheral insulin sensitivity. Isolated IFG was characterized by impaired early and total insulin release.
CONCLUSIONS
Peripheral insulin sensitivity was already decreased substantially at low PG levels within the normoglycemic range, whereas impairment in insulin secretion was observed mainly in the diabetic range of FPG and 2-h PG. Obesity did not affect changes in insulin sensitivity or insulin secretion in relation to hyperglycemia.
Type 2 diabetes is preceded by a long pre-diabetic state, characterized by mild elevation of fasting and/or postprandial glucose levels. This asymptomatic phase may last for years, and about one-third of these individuals finally develop type 2 diabetes (1). The pre-diabetic state, defined by an oral glucose tolerance test (OGTT), includes impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or their combination (2). Epidemiological studies have shown that IFG and IGT represent two distinct subgroups of abnormal glucose tolerance (1,3–5) that differ in their age and sex distribution (6,7) and associated cardiovascular risk (8). Therefore, IFG and IGT are likely to have different pathophysiologies.
Impaired insulin secretion and impaired insulin action are the two main pathophysiological disturbances leading to abnormal glucose tolerance. Previous studies on the role of impaired insulin secretion and insulin resistance in the development of IFG and IGT have yielded contradictory results (4–23). Inconsistencies across the studies are explained by differences in study populations, study designs and methods to assess insulin resistance and insulin secretion, and most importantly by a small sample size. Categorization of glucose tolerance is based on arbitrary cutoff points of glucose levels, and therefore different subgroups cannot fully account for changes in β-cell function and insulin action with increasing glycemia. Only a few studies have examined insulin secretion and/or insulin sensitivity as a function of glucose concentrations (13,24–28). These studies have been, however, relatively small, and most of them were conducted in non-Caucasian populations.
The aim of this study was to evaluate insulin sensitivity and insulin secretion across the entire range of fasting and 2-h plasma glucose (PG) from normal glucose tolerance (NGT) to type 2 diabetes to understand better the pathophysiology of the pre-diabetic state. Furthermore, we investigated the differences in insulin sensitivity and insulin release in different glucose tolerance subgroups. To address these questions, we collected a large sample of carefully phenotyped middle-aged Finnish men.
RESEARCH DESIGN AND METHODS
A total of 6,414 men from the ongoing population-based cross-sectional Metabolic Syndrome in Men (METSIM) study were included in the study. Subjects, aged from 45 to 70 years, were randomly selected from the population register of the town of Kuopio in eastern Finland (population 95,000). Every participant had a 1-day outpatient visit to the Clinical Research Unit at the University of Kuopio, including an interview on the history of previous diseases and current drug treatment and an evaluation of glucose tolerance and cardiovascular risk factors. Fasting blood samples were drawn after 12 h of fasting followed by an OGTT. The study was approved by the ethics committee of the University of Kuopio and Kuopio University Hospital, and it was in accordance with the Helsinki Declaration.
Clinical measurements.
Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as weight (kg) divided by height (m) squared. Waist (at the midpoint between the lateral iliac crest and lowest rib) and hip circumference (at the level of the trochanter major) were measured to the nearest 0.5 cm. Body composition was determined by bioelectrical impedance (RJL Systems) in subjects in the supine position after a 12-h fast (29).
OGTT.
A 2-h OGTT (75 g of glucose) was performed, and samples for PG and insulin were drawn at 0, 30, and 120 min. Glucose tolerance was evaluated based on OGTT as follows: NGT (fasting PG [FPG] <5.6 mmol/l and 2-h PG <7.8 mmol/l), isolated IFG (FPG 5.6–6.9 mmol/l and 2-h PG <7.8 mmol/l), isolated IGT (FPG <5.6 mmol/l and 2-h PG between 7.8 and 11.0 mmol/l), IFG + IGT (FPG 5.6–6.9 mmol/l and 2-h PG 7.8–11.0 mmol/l), and newly diagnosed type 2 diabetic subjects (FPG ≥7.0 mmol/l and/or 2-h PG ≥11.1 mmol/l) (2).
Laboratory determinations.
Plasma glucose was measured by enzymatic hexokinase photometric assay (Konelab Systems reagents; Thermo Fischer Scientific, Vantaa, Finland). Insulin was determined by immunoassay (ADVIA Centaur Insulin IRI no. 02230141; Siemens Medical Solutions Diagnostics, Tarrytown, NY).
Calculations.
The trapezoidal method was used to calculate glucose area under the curve (AUC) and insulin AUC during the OGTT. Surrogate indexes of insulin sensitivity and insulin secretion were calculated according to published formulas (Tables 1 and 2) (25,30–33), using glucose and insulin concentrations at 0, 30, and 120 min.
TABLE 1.
Spearman correlation coefficients between surrogate indexes of β-cell function and insulin release during the first phase (0–10 min) and second phase (10–60 min) and total (0–60 min) during an IVGTT
| β-Cell function indexes | Insulin release during an IVGTT |
||
|---|---|---|---|
| First phase | Second phase | Total | |
| HOMA-β | 0.568 | 0.645 | 0.663 |
| Insulinogenic index | 0.579 | 0.469 | 0.541 |
| InsAUC30/GluAUC30 | 0.666 | 0.707 | 0.743 |
| ΔInsAUC120/ΔGluAUC120 | 0.375 | 0.409 | 0.405 |
| InsAUC120/GluAUC120 | 0.648 | 0.750 | 0.775 |
| First-phase Stumvoll | 0.651 | 0.663 | 0.702 |
| Second-phase Stumvoll | 0.662 | 0.692 | 0.730 |
| Fasting insulin | 0.548 | 0.747 | 0.735 |
| Insulin at 30 min of an OGTT | 0.615 | 0.691 | 0.715 |
| Insulin at 120 min of an OGTT | 0.429 | 0.673 | 0.645 |
| Insulin AUC during an OGTT | 0.558 | 0.770 | 0.776 |
P < 0.001 for all correlation coefficients. Indexes of β-cell function were calculated as described previously: HOMA-β(30), insulinogenic index (31), ΔInsAUC120/ΔGluAUC120 (25), InsAUC120/GluAUC120 (31), first phase Stumvoll (32), and second phase Stumvoll (32). InsAUC30/GluAUC30 = (insulin at 0 min + insulin at 30 min of an OGTT)/(glucose at 0 min + glucose at 30 min of an OGTT).
TABLE 2.
Spearman correlation coefficients between surrogate indexes of insulin sensitivity and insulin sensitivity assessed by a euglycemic-hyperinsulinemic clamp (M value)
| Insulin sensitivity during clamp |
||
|---|---|---|
| M value | MLBM/I | |
| Insulin sensitivity indexes | ||
| 1/fasting insulin | 0.625 | 0.716 |
| 1/HOMA-IR | 0.605 | 0.708 |
| QUICKI* | 0.605 | 0.708 |
| Matsuda ISI* | 0.699 | 0.776 |
| MCR Stumvoll | 0.701 | 0.683 |
| ISI Stumvoll* | 0.705 | 0.693 |
Indexes of insulin sensitivity were calculated as described previously: HOMA-IR (30), quantitative insulin sensitivity index (30), Matsuda ISI (33), MCR Stumvoll (31), and ISI Stumvoll (31). For all correlation coefficients, P < 0.001.
*Surrogate indexes were validated against MLBM/I. ISI, insulin sensitivity index; MCR, metabolic clearance rate; QUICKI, quantitative insulin sensitivity index.
Statistical analysis.
Data are presented as the means ± SD, median (25th, 75th percentile) for continuous variables, or as count (percentage) for categorical variables. Variables with nonnormal skewed distribution were logarithmically transformed before analysis. Continuous variables were compared across the categories of glucose tolerance by ANOVA or after adjustment for covariates using the general linear model. Pairwise comparisons between the groups were performed by Bonferroni post hoc tests (with P value adjustment for multiple testing for each variable). Categorical variables were examined by χ2 test. Spearman's rank correlation was used to compare the surrogate indexes with the reference measures. Analyses were conducted with SPSS version 14 (SPSS, Chicago, IL). P values <0.05 were considered statistically significant.
Validation study.
A separate sample of 287 nondiabetic Finnish offspring of type 2 diabetic patients from the region of Kuopio was used to validate the OGTT-derived indexes of insulin secretion and insulin sensitivity against parameters measured by the intravenous glucose tolerance test (IVGTT) and euglycemic-hyperinsulinemic clamp. The study protocol has been described previously (10). All subjects underwent a 2-h OGTT (75 g of glucose) and on a separate occasion a frequently sampled IVGTT, followed by a euglycemic-hyperinsulinemic clamp for 120 min, as described previously (10). The study was approved by the ethics committee of the University of Kuopio and was in accordance with the Helsinki Declaration.
Spearman's correlations were calculated between insulin AUCs during the first 10 min and during 10–60 and 0–60 min of the IVGTT, considered as the reference indexes of first-phase, second-phase, and total insulin secretion, respectively, and surrogate indexes of insulin secretion (Tables 1 and 2). The ratio of total insulin AUC and total glucose AUC during 0–30 min of the OGTT (InsAUC30/GluAUC30) displayed the highest correlation with the first-phase insulin release during the IVGTT (rho = 0.67, P < 0.001) (Table 1). Insulin AUC during 120 min of the OGTT (InsAUC120), alone or adjusted for the corresponding glucose AUC (InsAUC120/GluAUC120), showed the highest correlation with the second-phase and total insulin release (rho ranging from 0.75 to 0.78, P < 0.001) (Table 1). Spearman's correlation analysis was also performed between the ratio of whole-body glucose uptake to lean body mass and to mean insulin concentration during the last 60 min of the euglycemic clamp (MLBM/I), considered as a reference index of insulin sensitivity, and surrogate indexes of insulin sensitivity. Matsuda ISI (calculated from glucose and insulin levels at 0, 30, and 120 min of the OGTT) showed the highest correlation with the clamp-derived MLBM/I (rho = 0.78, P < 0.001) (Table 2).
Based on the above correlation analyses, we used Matsuda ISI as a surrogate index of insulin sensitivity, InsAUC30/GluAUC30 as a surrogate index of the early-phase insulin release, and InsAUC120/GluAUC120 as a surrogate index of total insulin release in the METSIM study. We also calculated two disposition indexes as the products of insulin sensitivity × insulin secretion (DI30 = Matsuda ISI × InsAUC30/GluAUC30, DI120 = Matsuda ISI × InsAUC120/GluAUC120) as a measure of the β-cell response to insulin sensitivity. In a previous study analyzing several measures of disposition indexes based on insulin secretion and insulin sensitivity from an OGTT, the product of InsAUC/GluAUC and Matsuda ISI was the only combination that followed the hyperbolic relationship that is the requirement for a disposition index (34). A recent study indicated that oral disposition index (insulinogenic index × 1/HOMA-IR, where HOMA-IR is the homeostasis model assessment for insulin resistance) is predictive of diabetes over 10 years (35). We used 1/HOMA-IR as an approximate measure of insulin sensitivity in the liver (33,36). The limitation of this index is that it also measures peripheral insulin sensitivity.
RESULTS
Baseline characteristics.
The age of 6,414 men in this study was 57 ± 7 years, and their BMI was 27.0 ± 3.9 kg/m2. Altogether, 2,168 subjects (34%) had NGT, 2,859 (45%) had isolated IFG, 217 (3%) had isolated IGT, 701 (11%) had a combination of IFG and IGT, and 469 (7%) had newly diagnosed type 2 diabetes. A total of 492 subjects with previously diagnosed diabetes were excluded from statistical analyses. As shown in Table 3, subjects with NGT and isolated IFG were significantly younger than subjects with isolated IGT, IFG + IGT, and newly diagnosed type 2 diabetes. Subjects with isolated IGT were significantly more obese than subjects with isolated IFG (according to waist-to-hip ratio and fat mass). Subjects with IFG + IGT and newly diagnosed type 2 diabetes were significantly more obese than subjects with isolated IFG or isolated IGT.
TABLE 3.
Clinical and anthropometric characteristics of study participants according to glucose tolerance status
| n | NGT | Isolated IFG | Isolated IGT | IFG + IGT | Newly diagnosed diabetes | P | Total | |
|---|---|---|---|---|---|---|---|---|
| n | — | 2,168 | 2,859 | 217 | 701 | 469 | — | 6,414 |
| Age (years) | 6,414 | 57.5 ± 6.7 | 56.9 ± 6.8 | 60.3 ± 6.9 | 59.2 ± 6.8* | 59.6 ± 6.4*† | 6 × 10−31 | 57.7 ± 6.8 |
| BMI (kg/m2) | 6,410 | 25.8 ± 3.4 | 27.0 ± 3.6 | 26.9 ± 3.7‡ | 28.9 ± 4.4 | 29.7 ± 4.9 | 8 × 10−131 | 27.0 ± 3.9 |
| Weight (kg) | 6,411 | 79.8 ± 11.7 | 83.9 ± 12.7 | 82.4 ± 13.4§‡ | 88.7 ± 14.8 | 91.0 ± 15.6 | 3 × 10−93 | 83.5 ± 13.3 |
| Waist circumference (cm) | 6,410 | 94.6 ± 9.6 | 97.9 ± 10.4 | 98.7 ± 10.1‡ | 103.5 ± 11.5 | 105.7 ± 12.0 | 2 × 10−140 | 98.0 ± 10.9 |
| Hip circumference (cm) | 6,409 | 99.4 ± 6.2 | 101.1 ± 6.4 | 100.6 ± 6.7§‡ | 103.4 ± 7.9 | 104.5 ± 8.1* | 3 × 10−73 | 101.1 ± 6.8 |
| Waist-to-hip ratio | 6,409 | 0.95 ± 0.06 | 0.97 ± 0.06 | 0.98 ± 0.06 | 1.00 ± 0.06 | 1.01 ± 0.07 | 1 × 10−119 | 0.97 ± 0.06 |
| Fat mass (%) | 6,401 | 22.5 ± 6.3 | 23.4 ± 6.0 | 26.2 ± 6.8 | 27.2 ± 6.7* | 27.7 ± 6.1† | 3 × 10−108 | 23.9 ± 6.5 |
| BMI ≥27 kg/m2 (median) | 6,410 | 669 (31) | 1,276 (45) | 110 (51) | 452 (65) | 324 (69) | 1 × 10−84 | 2,831 (44) |
| Positive family history of diabetes | 6,412 | 889 (41) | 1,340 (47) | 104 (48) | 348 (50) | 254 (54) | 5 × 10−8 | 2,935 (46) |
Data are means ± SD or n (%). P values for overall comparison between five categories of glucose tolerance are shown (ANOVA for continuous variables, χ2 test for categorical variables). Bonferroni post hoc tests (continuous variables): all pairwise comparisons between categories of glucose tolerance were significant at P < 0.05, except for those marked as follows:
*P > 0.05 vs. isolated IGT;
†P > 0.05 vs. IFG + IGT;
‡P > 0.05 vs. isolated IFG;
§P > 0.05 vs. NGT.
Insulin sensitivity according to fasting and 2-h PG concentration.
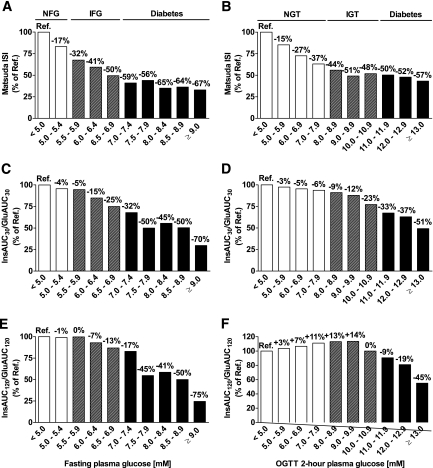
We generated different categories of FPG and 2-h PG to investigate the relationship between the peripheral insulin sensitivity (Matsuda ISI) or markers of early-phase and total glucose-stimulated insulin secretion and glycemia. Categories with FPG <5.0 mmol/l and 2-h PG <5.0 mmol/l were set as the reference categories. We observed a considerable decrease in age- and BMI-adjusted peripheral insulin sensitivity (−17%) within the normal range of FPG, compared with the reference category. Insulin sensitivity further decreased to −50% within the range of IFG and decreased to −67% in the diabetic range of FPG (Fig. 1A). A substantial decrease in insulin sensitivity (−37%) was also observed within the normal range of 2-h PG. Insulin sensitivity further decreased to −51% within the IGT range and to −57% within the diabetic range of 2-h PG (Fig. 1B). When changes in insulin sensitivity according to the levels of both FPG and 2-h PG were examined, the highest insulin sensitivity was observed in subjects with FPG <5.0 mmol/l and 2-h PG <5.0 mmol/l, and the lowest insulin sensitivity was seen in subjects in the diabetic range of FPG and 2-h PG (supplementary Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.ord/cgi/content/full/db08-1607/DCI).
FIG. 1.
Insulin sensitivity (Matsuda ISI) (A and B), early-phase insulin release (InsAUC30/GluAUC30) (C and D), and total insulin release during the OGTT (InsAUC120/GluAUC120) (E and F) across the categories of FPG and 2-h PG. Bars display the value of insulin sensitivity or insulin release relative to the reference category (FPG <5.0 mmol/l, 2-h PG <5.0 mmol/l). Calculations were based on geometric means, adjusted for age and BMI with the general linear model. Cutoff values for different categories of FPG were (in mg/dl): 90.1 (5.0 mmol/l), 99.1 (5.5 mmol/l), 108.1 (6.0 mmol/l), 117.1 (6.5 mmol/l), 126.1 (7.0 mmol/l), 135.1 (7.5 mmol/l), 144.1 (8.0 mmol/l), 153.2 (8.5 mmol/l), and 162.2 (9.0 mmol/l). Cutoff values for different categories of 2-h PG were (in mg/dl): 90.1 (5.0 mmol/l), 108.1 (6.0 mmol/l), 126.1 (7.0 mmol/l), 144.1 (8.0 mmol/l), 162.2 (9.0 mmol/l), 180.2 (10.0 mmol/l), 198.2 (11.0 mmol/l), 216.2 (12.0 mmol/l), and 234.2 (13.0 mmol/l).
Insulin release according to fasting and 2-h PG concentration.
Age- and BMI-adjusted early-phase insulin release (InsAUC30/GluAUC30) decreased only slightly (−4%) within the normal range of FPG. It further decreased within the range of IFG and diabetes to −25 and −70%, respectively (Fig. 1C). The early-phase insulin release decreased by −6% within the normal range of 2-h PG, and further decreased to −23 and −50% within the range of IGT and diabetes, respectively (Fig. 1D). Age- and BMI-adjusted total insulin release (InsAUC120/GluAUC120) decreased to −13% within the range of IFG, and to −70% within the diabetic range of FPG (Fig. 1E). Total insulin release increased by 14% with higher 2-h PG up to 9.9 mmol/l, and then it decreased to −45% within the diabetic range of 2-h PG (Fig. 1F). The largest decreases in both early-phase (−32 to −50%) and total (−17 to −45%) insulin release were observed within the range of FPG from 7.0 to 7.9 mmol/l (Fig. 1C and E).
Disposition index.
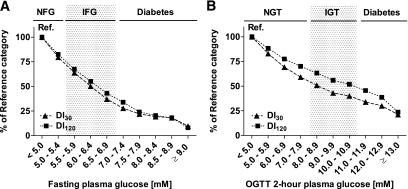
The early-phase DI30 and total DI120 decreased with higher FPG within the normal range by −21 and −18%, respectively. Within the IFG range, the reduction in DI30 and DI120 reached −63 and −57% (Fig. 2A). As a function of 2-h PG, DI30 and DI120 decreased to −41 and −30% in the normal range and further decreased to −60 and −48% in the IGT range (Fig. 2B).
FIG. 2.
Disposition indexes corresponding to early-phase (DI30 = Matsuda ISI × InsAUC30/GluAUC30) and total insulin release (DI120 = Matsuda ISI × InsAUC120/GluAUC120) across the categories of FPG (A) and 2-h PG (B). Cutoff values for different categories of FPG and 2-h PG are as explained in Fig. 1.
Compensatory insulin secretion.
Compensatory insulin secretion was not observed, despite a significant decrease in insulin sensitivity within the normal range of FPG, but, in contrast, the early-phase insulin release started to fall. However, compensatory total insulin secretion already started at low 2-h PG levels, and insulin release increased up to 10 mmol/l and then started to decrease (supplementary Fig. 2). A decrease in DI indexes was already substantial in the normal ranges of FPG and 2-h PG. Our findings remained essentially similar after adjustment for glucose level (analyses based on FPG were adjusted for 2-h PG, and analyses based on 2-h PG were adjusted for FPG) in addition to age and BMI.
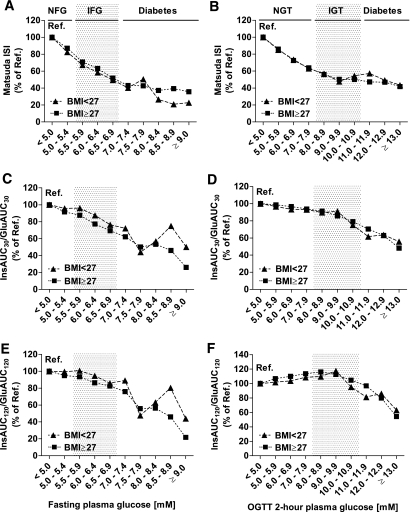
Insulin sensitivity and insulin release according to glucose levels in nonobese and obese individuals.
No significant interaction between BMI and glucose levels in determining insulin sensitivity was found. Obese subjects (BMI ≥27 kg/m2) within the reference categories of FPG and 2-h PG (<5.0 mmol/l) had reduced insulin sensitivity by −45 and −41% compared with nonobese subjects (BMI <27 kg/m2). The decrease in insulin sensitivity with higher FPG and 2-h PG was similar in both nonobese and obese subgroups: −17% (nonobese subjects) and −13% (obese subjects) within the normal range of FPG and −37 and −36%, respectively, within the normal range of 2-h PG (Fig. 3A and B). Obese subjects within the reference categories of FPG and 2-h PG had increased early-phase (+70 and +54%) and total insulin release (+68 and +46%) compared with nonobese subjects. However, changes in both early-phase and total insulin release with higher FPG or 2-h PG were comparable in nonobese and obese subjects (Fig. 3). The interaction between glucose levels and BMI (cutoff point of 27 kg/m2) in determining insulin release was not significant, except for the interaction between 2-h PG and BMI in determining DI120 (P = 0.001).
FIG. 3.
Insulin sensitivity (Matsuda ISI) (A and B), early-phase insulin release (InsAUC30/GluAUC30) (C and D), and total insulin release in an OGTT (InsAUC120/GluAUC120) (E and F) across the categories of FPG and 2-h PG in nonobese (BMI <27 kg/m2; ▲) and obese subjects (BMI ≥27 kg/m2; ■). Bars display insulin sensitivity or insulin release relative to the reference category (FPG <5.0 mmol/l, 2-h PG <5.0 mmol/l). Calculations were based on geometric means, adjusted for age and BMI, with the general linear model (performed separately in subgroups of nonobese and obese subjects). Cutoff values for different categories of FPG and 2-h PG are as explained in Fig. 1. Ref., reference.
Insulin sensitivity and insulin release in categories of glucose tolerance.
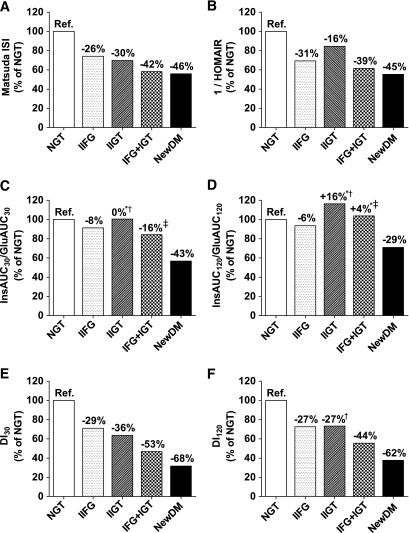
Age- and BMI-adjusted peripheral insulin sensitivity (Matsuda ISI) was significantly decreased by −26% in isolated IFG, by −30% in isolated IGT, by −42% in IFG + IGT, and by −46% in newly diagnosed type 2 diabetes, compared with NGT (Fig. 4A). Matsuda ISI was significantly lower in individuals with isolated IGT than in individuals with isolated IFG (P = 0.0016). A significantly greater decrease in isolated IFG than in isolated IGT (−31 vs. −16%, P = 0.0028) was found when insulin sensitivity was assessed with 1/HOMA-IR. 1/HOMA-IR was reduced by −39% in the IFG + IGT group and by −45% in newly diagnosed type 2 diabetic subjects (Fig. 4B). Categories of glucose tolerance status differed significantly also with respect to other indexes of insulin sensitivity (Table 4). Compared with NGT, the age- and BMI-adjusted early-phase insulin release (InsAUC30/GluAUC30) was significantly decreased by −8% in isolated IFG, not changed in isolated IGT, decreased by −16% in IFG + IGT, and decreased by −43% in newly diagnosed type 2 diabetes (Fig. 4C). The difference between isolated IFG and isolated IGT was not statistically significant (−8 vs. 0%, P = 1.0). The total insulin release (InsAUC120/GluAUC120) was significantly reduced in isolated IFG (−6%) and in newly diagnosed type 2 diabetes (−29%), whereas no significant changes were observed in isolated IGT or in IFG + IGT compared with NGT (Fig. 4D). Individuals with isolated IFG had significantly lower total insulin release than individuals with isolated IGT (−6 vs. +16%, P = 0.001). The HOMA-β index, indicating basal insulin release, was significantly reduced by −17% in isolated IFG (P = 1.2 × 10−26), whereas it was increased by 33% in isolated IGT (P = 1.3 × 10−15), compared with NGT. All categories of glucose tolerance status differed significantly with respect to all other indexes of insulin secretion examined, as shown in Table 4.
FIG. 4.
Insulin sensitivity (Matsuda ISI and 1/HOMA-IR) (A and B), early-phase insulin release (InsAUC30/GluAUC30) (C), total insulin release (InsAUC120/GluAUC120) (D), disposition index for early insulin release (DI30 = Matsuda ISI × InsAUC30/GluAUC30) (E), and disposition index for total insulin release (DI120 = Matsuda ISI × InsAUC120/GluAUC120) (F) in different categories of glucose tolerance. Bars show percentage of each index relative to NGT (reference, 100%). Calculations were based on geometric means, adjusted for age and BMI (ANCOVA). All pairwise comparisons were statistically significant (P < 0.05, Bonferroni post hoc test) except for those marked: *P > 0.05 vs. NGT, †P > 0.05 vs. isolated IFG, ‡P > 0.05 vs. isolated IGT. Ref., reference. IIFG, isolated IFG; IIGT, isolated IGT; NewDM, newly diagnosed diabetes.
TABLE 4.
Surrogate indexes of insulin sensitivity and β-cell function across categories of glucose tolerance
| n | NGT | Isolated IFG | Isolated IGT | IFG + IGT | Newly diagnosed diabetes | P | |
|---|---|---|---|---|---|---|---|
| n | — | 2,154 | 2,831 | 213 | 694 | 467 | — |
| β-Cell function | |||||||
| HOMA-β (mU/l, mmol/l) | 6,359 | 60.0 (44.2–87.3) | 56.4 (40.0–84.3) | 78.2 (51.0–128.7) | 81.7 (51.0–115.3)* | 71.5 (43.3–109.4)† | 9 × 10−60 |
| Insulinogenic index (pmol/mmol) | 6,312 | 100 (62–166) | 96 (58–158) | 75 (44–137) | 84 (51–133)‡ | 54 (30–84) | 9 × 10−129 |
| InsAUC30/GluAUC30 (pmol/mmol) | 6,325 | 24.1 (16.8–37.0) | 24.8 (16.8–37.0) | 24.9 (15.7–45.9)*† | 26.2 (17.7–40.3)‡ | 21.0 (12.4–31.8) | 1 × 10−65 |
| InsAUC120 (nmol/l · min) | 6,321 | 26.1 (17.5–40.2) | 30.1 (20.7–45.4) | 40.6 (24.9–68.8) | 47.3 (30.6–70.1)‡ | 41.2 (25.9–63.7)† | 9 × 10−47 |
| ΔInsAUC120/ΔGluAUC120 (pmol/mmol) | 6,311 | 143 (88–239) | 153 (94–246)* | 99 (59–159) | 114 (73–172)‡ | 75 (43–124) | 2 × 10−149 |
| InsAUC120/GluAUC120 (pmol/mmol) | 6,320 | 33.2 (23.2–50.3) | 34.4 (24.0–50.1) | 42.1 (25.5–67.8)* | 42.5 (28.4–64.2)*‡ | 32.3 (20.0–50.8) | 2 × 10−39 |
| First-phase Stumvoll (pmol/l, mmol/l) | 6,325 | 859 (640–1,185) | 818 (555–1,173) | 786 (560–1,342)*† | 833 (546–1,283) | 612 (270–1,008) | 2 × 10−88 |
| Second-phase Stumvoll (pmol/l, mmol/l) | 6,325 | 234 (187–310) | 232 (174–315) | 221 (172–357)*† | 239 (175–348) | 198 (118–292) | 3 × 10−84 |
| Insulin sensitivity/resistance | |||||||
| Fasting serum insulin (pmol/l) | 6,360 | 30.6 (22.2–45.0) | 41.4 (29.4–63.6) | 40.5 (27.0–69.0)† | 64.2 (40.5–91.8) | 73.2 (43.8–116.4) | 3 × 10−122 |
| HOMA-IR (mU/l, mmol/l) | 6,360 | 1.2 (0.9–1.7) | 1.8 (1.3–2.8) | 1.6 (1.0–2.7) | 2.9 (1.8–4.2) | 3.7 (2.2–6.7) | 6 × 10−266 |
| QUICKI (mU/l, mg/dl) | 6,360 | 0.37 (0.35–0.39) | 0.35 (0.33–0.37) | 0.36 (0.33–0.38) | 0.33 (0.31–0.35) | 0.31 (0.29–0.34) | 8 × 10−255 |
| MCR Stumvoll (pmol/l, mmol/l) | 6,360 | 8.1 (7.6–8.5) | 6.9 (6.0–7.4) | 7.9 (6.8–8.3) | 5.9 (4.7–6.8) | 4.4 (2.2–5.7) | <1 × 10−255 |
| ISI Stumvoll (pmol/l, mmol/l) | 6,353 | 0.11 (0.10–0.12) | 0.10 (0.09–0.11) | 0.07 (0.05–0.09) | 0.06 (0.04–0.08)‡ | 0.04 (0.01–0.06) | <1 × 10−255 |
| Matsuda ISI (mU/l, mg/dl) | 6,320 | 8.2 (5.6–11.9) | 5.8 (3.8–8.2) | 5.0 (3.1–8.0) | 3.2 (2.2–5.0) | 2.7 (1.7–4.4) | 1 × 10−255 |
| Disposition index | |||||||
| DI30 | 6,320 | 195 (157–245) | 139 (112–173) | 123 (100–152) | 88 (73–107) | 55 (44–73) | <1 × 10−255 |
| DI120 | 6,320 | 266 (225–315) | 192 (162–229) | 198 (171–231)† | 145 (124–168) | 95 (68–116) | <1 × 10−255 |
Data are medians (25th–75th percentile). Variables with nonnormal right-skewed distribution were logarithmically transformed before analyses. P values are based on the general linear model adjusted for age and BMI. DI30 = Matsuda ISI × InsAUC30/GluAUC30; DI120 = Matsuda ISI × InsAUC120/GluAUC120. Bonferroni post hoc tests: all pairwise comparisons between the categories of glucose tolerance were significant at P < 0.05, except for:
*P > 0.05 vs. NGT;
†P > 0.05 vs. isolated IFG;
‡P > 0.05 vs. isolated IGT.
Disposition index.
The early-phase DI30 was lower in isolated IGT than in isolated IFG (−36 vs. −29% compared with NGT, P = 0.0003). In the IFG + IGT group, DI30 was decreased by −53%, and in newly diagnosed type 2 diabetic subjects by −68% (Fig. 4E). In contrast, the total DI120 was decreased to the same extent in isolated IFG and isolated IGT (both −27%). DI120 was reduced significantly in the IFG + IGT group by −44% and in newly diagnosed type 2 diabetic subjects by −62% (Fig. 4F).
All statistical analyses were also performed using a cutoff point of 6.1 mmol/l (110 mg/dl) for FPG. All results were essentially similar (supplemental Tables 1 and 2, supplemental Fig. 3).
DISCUSSION
Our study is the largest population-based study focusing on the pathophysiology of pre-diabetes using validated surrogate markers for insulin sensitivity and insulin release. This allowed us to perform a detailed analysis on the changes in insulin sensitivity and insulin secretion from normal glucose levels to the diabetic range of hyperglycemia. We demonstrated that insulin sensitivity was already decreased substantially (by −17 and −37%, respectively) within the normal range of FPG and 2-h PG. Insulin sensitivity further decreased through the IFG and IGT range and reached a reduction of approximately −65 and −53% in the diabetic range of FPG and 2-h PG. In contrast, early-phase insulin release declined only slightly within the normal range of FPG and 2-h PG (by approximately −5%) and reached a substantial decrease in the diabetic range of FPG (∼50–70%) and 2-h PG (∼35–50%). Compensatory insulin secretion was entirely missing when FPG increased from the normal range to the IFG range, but compensatory insulin secretion was observed within the normal range of 2-h PG. We also showed that peripheral insulin resistance is the predominant feature of isolated IGT, whereas impairment in early and total insulin release characterize isolated IFG.
The novel finding of our study was the observation that peripheral insulin sensitivity was already decreased considerably at relatively low glucose levels within the normal range of FPG and 2-h PG. Other studies addressing the same question have been considerably smaller in size. In a study including 188 subjects, Ferrannini et al. (24) reported a decrease in insulin sensitivity (measured by clamp) with higher 2-h PG levels within the NGT group. The increase in insulin resistance was associated with higher BMI, suggesting that obesity is the main factor responsible for the decrease in insulin sensitivity. We found that insulin sensitivity decreased comparably in both nonobese and obese individuals by −37 and −36%, respectively, within the 2-h PG range from 5.0 to 7.9 mmol/l. Thus, our findings suggest that obesity does not affect insulin sensitivity related to hyperglycemia. In another study (n = 148), Ahrén (27) reported decreased insulin sensitivity (∼14%) measured by clamp in postmenopausal normoglycemic women within the highest quartile of FPG.
The mechanisms leading to a decrease in insulin sensitivity within the normal range of FPG and 2-h PG remain unclear. Although high glucose (20 mmol/l) and/or high insulin levels decrease glucose uptake in human adipose cells in vitro (37), it is not known whether this effect could be observed at moderately increased glucose concentrations in vivo. Furthermore, our results show that despite a substantial decrease in peripheral insulin sensitivity (−37%), 2-h PG could be maintained within the normal range by a compensatory increase in total insulin release.
The pattern of changes in early-phase insulin release differed from that of insulin sensitivity. The decrease was small in the normal range (approximately −5%), progressed within the IFG and IGT range, and was substantial within the diabetic range (reaching −70% and −51% for diabetic FPG and 2-h PG, respectively). The defect in early insulin response manifested at lower glucose levels than the defect in total insulin response. Previous studies (13,25,26,28) have not assessed the effect of obesity on the relationship between glucose levels and insulin secretion. We showed that both early-phase and total insulin release were substantially higher in obese subjects than in nonobese subjects within the normal range of FPG and 2-h PG (by ∼50–70%), but there was no significant interaction between obesity and glucose levels in determining insulin release. Therefore, obesity does not seem to have a major impact on further changes in insulin release in hyperglycemia. Accordingly, Camastra et al. (38) have observed that the dynamic aspects of β-cell response to glucose were unaltered in the morbidly obese nondiabetic subjects.
Previous studies have been inconsistent with respect to differences in insulin sensitivity between isolated IFG and isolated IGT. Decreased peripheral insulin sensitivity in subjects with isolated IGT compared with subjects with isolated IFG has been reported in some studies using the clamp method or IVGTT (9,10,12,14), but also similar impairment in insulin action has been found in both isolated IFG and isolated IGT in Pima Indians (13) and obese Chinese subjects (16). In two studies, the decrease in insulin sensitivity in isolated IGT compared with isolated IFG was related to obesity (11,17). In our study, peripheral insulin sensitivity was significantly more reduced in isolated IGT than in isolated IFG (−30 vs. −26%, P = 0.0016). The decrease in insulin sensitivity was quite similar in nonobese and obese subjects (−27 and −31%) with isolated IGT, indicating that the reduction in peripheral insulin sensitivity in isolated IGT was not explained by obesity.
Conflicting findings have been published on 1/HOMA-IR as an index of hepatic insulin sensitivity (11,12,14,18–23). In our study, 1/HOMA-IR was more reduced in isolated IFG than in isolated IGT (−31 vs. −16%). However, HOMA-IR also reflects peripheral insulin sensitivity, and therefore reliable results on hepatic insulin sensitivity can be obtained only by using the tracer technique (13,39).
We observed a small reduction in early-phase insulin release in isolated IFG (−8%) but not in isolated IGT, compared with NGT. Furthermore, isolated IFG and isolated IGT differed significantly in basal and total glucose-stimulated insulin release. Basal and total insulin release were reduced in isolated IFG by −17 and −6%, respectively, but increased in isolated IGT by +33% and +16% compared with NGT. These results suggest that individuals with isolated IFG have impairment in both basal and glucose-stimulated (early and total) insulin release, whereas individuals with isolated IGT have increased basal and total insulin release. Some previous studies using IVGTT or clamp to assess insulin secretion have reported impaired insulin release in individuals with isolated IFG (9,10,13,14,16), whereas others have found impaired insulin release in individuals with IGT (11,15). Studies based on OGTT measurements have reported impaired early-phase insulin secretion (insulinogenic index) in individuals with IGT only (21,23) or in both individuals with IGT or IFG (12,18,22). Few studies have found decreased basal insulin secretion in IFG (11,22).
The currently accepted paradigm is that type 2 diabetes develops when the pancreas is unable to secrete more insulin to compensate for insulin resistance. However, in the nondiabetic range of glucose levels, compensatory total insulin release was observed only for increasing 2-h PG levels (Fig. 1F). In contrast, in the nondiabetic range of FPG levels, this compensatory hyperinsulinemia was entirely missing, and insulin release (early-phase and total) (Fig. 1C and E) linearly decreased with increasing FPG levels and was lowest in the diabetic range, indicating a major defect in insulin secretion. This observation is consistent with recent evidence from genetic studies showing that the insulin secretion defect is very often the primary cause for type 2 diabetes (40). The changes in insulin sensitivity and insulin secretion in response to higher glucose levels in our study suggest that there are two major pathways for the development of type 2 diabetes, one leading to diabetes via the elevation of FPG and another via postprandial hyperglycemia or 2-h PG. Prospective follow-up studies including subjects with isolated IFG and isolated IGT are needed, however, to confirm this hypothesis.
The strengths of this study are large size, homogeneous study population, and validation of the methods used as surrogate markers for insulin sensitivity and secretion. The limitation of the study is that only middle-aged Finnish men were included in the study, and therefore we do not know if the results are valid for women or for other ethnic and racial groups. Previous studies have not reported sex differences in insulin secretion, but in some studies women were more insulin sensitive than men (41–43). Because of the large size of our cohort, we could not use the most accurate methods to evaluate insulin sensitivity (clamp) and insulin secretion (IVGTT or hyperglycemic clamp) or hepatic insulin sensitivity (tracer techniques). However, we validated our OGTT-derived indexes of insulin secretion and insulin sensitivity against the gold standard measures in an independent sample of Finnish subjects. We did not measure 1-h glucose during the OGTT, which has been shown to be a better predictor of future risk of diabetes than FPG and 2-h PG levels in a recent study (44).
In summary, we showed in a large cohort of Finnish men that the impairment of peripheral insulin sensitivity started at relatively low PG levels within the normoglycemic range. In contrast, the impairment of insulin secretion progressed substantially only in the diabetic range of fasting and 2-h glucose levels. Peripheral insulin resistance was a predominant feature of isolated IGT, whereas impaired insulin secretion characterized isolated IFG.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from the Academy of Finland (124243), the Finnish Heart Foundation, the Finnish Funding Agency for Technology and Innovation (TEKES) (1510/31/06), and the Commission of the European Community (LSHM-CT-2004-512013 EUGENE2).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Unwin N, Shaw J, Zimmet P, Alberti KG: Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002; 19: 708– 723 [DOI] [PubMed] [Google Scholar]
- 2.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P; the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26: 3160– 3167, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Dunstan DW, Zimmet PZ, Welborn TA, De Courten MP, Cameron AJ, Sicree RA, Dwyer T, Colagiuri S, Jolley D, Knuiman M, Atkins R, Shaw JE: The rising prevalence of diabetes mellitus and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002; 25: 829– 834 [DOI] [PubMed] [Google Scholar]
- 4.Carnevale Schianca GP, Rossi A, Sainaghi PP, Maduli E, Bartoli E: The significance of impaired fasting glucose versus impaired glucose tolerance: importance of insulin secretion and resistance. Diabetes Care 2003; 26: 1333– 1337 [DOI] [PubMed] [Google Scholar]
- 5.Vaccaro O, Riccardi G: Changing the definition of impaired fasting glucose: impact on the classification of individuals and risk definition. Diabetes Care 2005; 28: 1786– 1788 [DOI] [PubMed] [Google Scholar]
- 6.Qiau Q, Hu G, Tuomilehto J, Balkau B, Bord-Johnsen Kfor the DECODE Study Group: Age and sex specific prevalence of diabetes and impaired glucose regulation in 13 European cohorts. In Proceedings of the 37th Annual Meeting of the European Diabetes Epidemiology Group Oxford, U.K., European Diabetes Epidemiology Group, 2002, p. A37 [Google Scholar]
- 7.Qiao Q, Hu G, Tuomilehto J, Nakagami T, Balkau B, Borch-Johnsen K, Ramachandran A, Mohan V, Iyer SR, Tominaga M, Kiyahara Y, Kato I, Okubo K, Nagai M, Shibazaki S, Yang Z, Tong Z, Fan Q, Wang B, Chew SK, Tan BY, Heng D, Emmanual S, Tajima N, Iwamoto Y, Snehalatha C, Vijay V, Kapur A, Dong Y, Nan H, Gao W, Shi H, Fu F; the DECODE Study Group: Age and sex specific prevalence of diabetes and impaired glucose regulation in 10 Asian cohorts. Diabetes Res Clin Pract 2002; 56: 540. [DOI] [PubMed] [Google Scholar]
- 8.Haffner SM: The importance of hyperglycemia in the nonfasting state to the development of cardiovascular disease. Endocr Rev 1998; 19: 583– 592 [DOI] [PubMed] [Google Scholar]
- 9.Faerch K, Vaag A, Holst JJ, Glümer C, Pedersen O, Borch-Johnsen K: Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia 2008; 51: 853– 861 [DOI] [PubMed] [Google Scholar]
- 10.Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vänttinen M, Stančáková A, Jansson PA, Pellmé F, Holst JJ, Kuulasmaa T, Hribal ML, Sesti G, Stefan N, Fritsche A, Häring H, Pedersen O, Smith U; EUGENE2 Consortium Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 2008; 51: 502– 511 [DOI] [PubMed] [Google Scholar]
- 11.Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J: Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 2006; 29: 1909– 1914 [DOI] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA: Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006; 55: 1430– 1435 [DOI] [PubMed] [Google Scholar]
- 13.Weyer C, Bogardus C, Pratley RE: Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 1999; 48: 2197– 2203 [DOI] [PubMed] [Google Scholar]
- 14.Festa A, D'Agostino R, Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM: Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes 2004; 53: 1549– 1555 [DOI] [PubMed] [Google Scholar]
- 15.Festa A, Williams K, Hanley AJ, Haffner SM: Beta-cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes: comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes 2008; 57: 1638– 1644 [DOI] [PubMed] [Google Scholar]
- 16.Hong J, Gui MH, Gu WQ, Zhang YF, Xu M, Chi ZN, Zhang Y, Li XY, Wang WQ, Ning G: Differences in insulin resistance and pancreatic B-cell function in obese subjects with isolated impaired glucose tolerance and isolated impaired fasting glucose. Diabet Med 2008; 25: 73– 79 [DOI] [PubMed] [Google Scholar]
- 17.van Haeften TW, Pimenta W, Mitrakou A, Korytkowski M, Jenssen T, Yki-Jarvinen H, Gerich JE: Disturbances in beta-cell function in impaired fasting glycemia. Diabetes 2002; 51 ( Suppl 1): S265– S270 [DOI] [PubMed] [Google Scholar]
- 18.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T; Impaired Glucose Tolerance for Atherosclerosis and Diabetes Study Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care 2003; 26: 868– 874 [DOI] [PubMed] [Google Scholar]
- 19.Kim DJ, Lee MS, Kim KW, Lee MK: Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism 2001; 50: 590– 593 [DOI] [PubMed] [Google Scholar]
- 20.Davies MJ, Raymond NT, Day JL, Hales CN, Burden AC: Impaired glucose tolerance and fasting hyperglycaemia have different characteristics. Diabet Med 2000; 17: 433– 440 [DOI] [PubMed] [Google Scholar]
- 21.Melchionda N, Forlani G, Marchesini G, Baraldi L, Natale S: WHO and ADA criteria for the diagnosis of diabetes mellitus in relation to body mass index: insulin sensitivity and secretion in resulting subcategories of glucose tolerance. Int J Obes Relat Metab Disord 2002; 26: 90– 96 [DOI] [PubMed] [Google Scholar]
- 22.Snehalatha C, Ramachandran A, Sivasankari S, Satyavani K, Vijay V: Insulin secretion and action show differences in impaired fasting glucose and in impaired glucose tolerance in Asian Indians. Diabete Metab Res Rev 2003; 19: 329– 332 [DOI] [PubMed] [Google Scholar]
- 23.Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, Groop LC: Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 2000; 49: 975– 980 [DOI] [PubMed] [Google Scholar]
- 24.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005; 90: 493– 500 [DOI] [PubMed] [Google Scholar]
- 25.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA; San Antonio metabolism study Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004; 47: 31– 39 [DOI] [PubMed] [Google Scholar]
- 26.Abdul-Ghani MA, Matsuda M, Jani R, Jenkinson CP, Coletta DK, Kaku K, DeFronzo RA: The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab 2008; 295: E401– E406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahrén B: Insulin secretion and insulin sensitivity in relation to fasting glucose in healthy subjects. Diabetes Care 2007; 30: 644– 648 [DOI] [PubMed] [Google Scholar]
- 28.Godsland IF, Jeffs JA, Johnston DG: Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia 2004; 47: 1157– 1166 [DOI] [PubMed] [Google Scholar]
- 29.Fuller N, Elia M: Potential use of bioelectrical impedance of the ‘whole body’ and of body segments for the assessment of body composition: comparison with densitometry and anthropometry. Eur J Clin Nutr 1989; 43: 779– 791 [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412– 419 [DOI] [PubMed] [Google Scholar]
- 31.Stumvoll M, Van Haeften T, Fritsche A, Gerich J: Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 2001; 24: 796– 797 [DOI] [PubMed] [Google Scholar]
- 32.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, Renn W, Gerich J: Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000; 23: 295– 301 [DOI] [PubMed] [Google Scholar]
- 33.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462– 1470 [DOI] [PubMed] [Google Scholar]
- 34.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B: Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008; 16: 1901– 1907 [DOI] [PubMed] [Google Scholar]
- 35.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE: Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009; 32: 335– 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA: Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 2007; 30: 89– 94 [DOI] [PubMed] [Google Scholar]
- 37.Renström F, Burén J, Svensson M, Eriksson JW: Insulin resistance induced by high glucose and high insulin precedes insulin receptor substrate 1 protein depletion in human adipocytes. Metabolism 2007; 56: 190– 198 [DOI] [PubMed] [Google Scholar]
- 38.Camastra S, Manco M, Mari A, Baldi S, Gastaldelli A, Greco AV, Mingrone G, Ferrannini E: Beta-cell function in morbidly obese subjects during free living: long-term effects of weight loss. Diabetes 2005; 54: 2382– 2389 [DOI] [PubMed] [Google Scholar]
- 39.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA: Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 2007; 56: 1703– 1711 [DOI] [PubMed] [Google Scholar]
- 40.Frayling TM: Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 2007; 8: 657– 662 [DOI] [PubMed] [Google Scholar]
- 41.Moran A, Jacobs DR, Jr, Steinberger J, Steffen LM, Pankow JS, Hong CP, Sinaiko AR: Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation 2008; 117: 2361– 2368 [DOI] [PubMed] [Google Scholar]
- 42.Ferrara CM, Goldberg AP, Nicklas BJ, Sorkin JD, Ryan AS: Sex differences in insulin action and body fat distribution in overweight and obese middle-aged and older men and women. Appl Physiol Nutr Metab 2008; 33: 784– 790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otsuki M, Kasayama S, Saito H, Mukai M, Koga M: Sex differences of age-dependent changes of insulin sensitivity in Japanese nondiabetic subjects. Diabetes Care 2005; 28: 2590– 2591 [DOI] [PubMed] [Google Scholar]
- 44.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L: Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care 2009; 32: 281– 286 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.