Abstract
OBJECTIVE
Perturbations to the prenatal environment have been associated with the development of adult chronic disease, findings that gave rise to the “Barker Hypothesis” or the “developmental origins of adult disease” concept. In this study, we used an animal model to determine the metabolic consequences of maternal prenatal stress and high-fat feeding on the developing offspring.
RESEARCH DESIGN AND METHODS
Pregnant female Sprague-Dawley rats were maintained on standard chow or 60% high-fat diet throughout gestation and lactation. Half of each group were exposed to a novel variable stress paradigm during the 3rd week of gestation, whereas control dams were left undisturbed. Body weight, body composition, glucose tolerance, and endocrine parameters were measured in offspring through early adulthood.
RESULTS
Male and female pups from dams that experienced prenatal stress and/or were on a high-fat diet weighed more beginning on postnatal day 7 compared with standard chow–control pups. Access to high-fat diet at weaning increased the body weight effect through early adulthood and was attributable to greater adiposity. Pups weaned onto standard chow diet showed no significant difference in glucose clearance or insulin secretion. However, pups weaned onto high-fat diet had impaired glucose tolerance if their dams were on a high-fat diet, experienced prenatal stress, or both.
CONCLUSIONS
Our data demonstrate that prenatal stress and/or high-fat diet during the intrauterine or postnatal environment affects offspring in a manner that increases their susceptibility to diet-induced obesity and leads to secondary adverse metabolic consequences.
Obesity is a worldwide public health problem and a major contributor to the increased incidence of coronary artery disease, hypertension, and type 2 diabetes (1,2). In addition, and perhaps more disturbing, there is an escalating prevalence of overweight and obesity among infants and children worldwide (3–5). The trend toward greater obesity in the young raises concern because infant or childhood obesity alone significantly increases susceptibility to adult chronic diseases, including cardiovascular disease, hypertension, and diabetes (6). Although it is recognized that genetics plays a role in the development of obesity, genetic factors alone cannot account for the tripling in the prevalence of overweight and obesity over the past 3 decades (7).
The intrauterine environment has a significant influence on the health of offspring, and exposure to suboptimal in utero conditions can predispose offspring to adult chronic disease. This concept, originally termed the “Barker Hypothesis,“ derives from human and animal studies demonstrating that, for example, exposure to limited resources in utero produces offspring who show maladaptive responses to the ample postnatal nutritional environment and will develop obesity and diabetes (8). Poor maternal nutrition during the Dutch famine resulted in an increased incidence of obesity in men (9). Although undernutrition and growth restriction caused by famine is not a health issue in modern Western societies, these conditions do occur with hyperemesis gravidarum, high-altitude pregnancy, and pregnancy in women with eating disorders. Based on this, models of low birth weight or IUGR (intrauterine growth restriction) have been studied and are now well characterized (10,11). Animal models of altered nutritional conditions in dams during pregnancy (e.g., caloric restriction, low-protein diet, and gestational diabetes) result in low birth weights and eventually lead to adult conditions such as obesity, hypertension, and diabetes reminiscent of observations reported in human epidemiological studies, particularly when exposed to a high-energy diet after birth (12). The mismatched prenatal versus postnatal nutritional environments result in adverse consequences for the offspring as proposed by the “predictive adaptive hypothesis” (13).
Undernutrition and growth restriction are not the only conditions that impact the long-term health of the offspring. Maternal diets have changed such that dietary fat intake among pregnant mothers has increased in the U.S. (14). Alterations in maternal diet have led to a twofold increase in the incidence of maternal overweight and obesity over the last 20 years. Overnutrition or consumption of Western diets that contain a high amount of dietary fat during pregnancy can also result in metabolic syndrome in offspring (15,16).
Another aspect of the maternal environment that may have significant effects on the developing fetus is stress arising from socioeconomic or psychosocial factors. Psychosocial and socioeconomic challenges activate the hypothalamic-pituitary-adrenal (HPA) axis, causing hypersecretion of cortisol, and this in turn has been associated with the development of obesity-related conditions including excessive visceral fat deposition, insulin resistance, dyslipidemia, hypertension, and cardiovascular disease in humans (17). Increased glucocorticoid levels and its associated conditions during pregnancy can have significant long-term effects on the developing fetus. Prenatal stress in rodent and primate models has been implicated in altered stress responsivity (18), increased anxiety-like behavior (19), schizophrenia (20,21), cognitive impairments (20), and reduced neurogenesis (22). Although exogenous glucocorticoid administration to pregnant rats during gestation has been linked to later development of hypertension, hyperglycemia, and features of the metabolic syndrome (23,24), there are a limited number of studies that have directly examined the consequences of prenatal stress on energy homeostasis (25–27).
The neural pathways that regulate stress responses are also involved in maintaining metabolic homeostasis, and the available literature strongly suggests interactions between the two systems (28). In light of the current dietary and stress environment that many humans live in, it is important to address whether prenatal stress, in addition to a high-fat diet, may exacerbate the effects of the high-fat diet alone. Animal models provide the ability to study the long-term consequences of maternal diet or prenatal stress on offspring while allowing for control over variables that human studies do not afford. We hypothesize that prenatal stress or maternal high-fat diet consumption will predispose offspring to metabolic side effects that will be exacerbated by weaning on to a high-fat diet.
RESEARCH DESIGN AND METHODS
Timed-pregnant (second parity) female Sprague-Dawley rats (Charles River, Kingston, NY) were received on gestation day 2. Animals were individually housed in conventional tub cages with ad libitum access to food and water in an environmentally controlled room maintained on a 12-h light/dark cycle with light onset at 0600. All animal procedures were approved by the animal care and use committee of the Johns Hopkins University School of Medicine.
Pregnant rats were assigned to two diet groups: standard chow diet (Harlan Teklad 2018, 17% kcal from fat; n = 21) or high-fat diet (Research Diets, D12492, 60% kcal from fat, n = 21). All dams remained on their respective diets from gestation day 2 throughout gestation and lactation. Dams were weighed, and food intake was measured daily throughout gestation.
Variable stress.
Beginning on gestation day 14, 10 dams from each diet group (n = 10, standard chow–stress group and high-fat–stress group) were subjected to an 8-day schedule of variable stress (Table 1) (20). Each stressor was applied during the light cycle unless noted otherwise. We selected a variable stress paradigm for our studies to prevent the animals from habituating to the stress, as has been documented using predictable stressors such as repeated restraint stress (29). Stress was restricted to the 3rd week of gestation because the neural circuits that regulate the HPA axis and energy homeostasis, including the hypothalamus, cortex, and hippocampus, are rapidly developing during this period (30).
TABLE 1.
Schedule of variable stress during gestation
| Gestational day | Time of day |
||
|---|---|---|---|
| Morning | Noon | Afternoon | |
| 14 | Restraint: 60 min | Swim: 15 min at room temperature | Restraint: 60 min |
| 15 | Cold exposure (4°C): 6 h | ||
| 16 | Swim: 15 min | Restraint: 60 min | Swim: 15 min |
| 17 | Swim: 15 min | Lights on during dark phase | |
| 18 | Social stress: group housing: 12 h | ||
| 19 | Restraint: 60 min | Swim: 15 min | Restraint: 60 min |
| 20 | Cold exposure (4°C): 6 h | ||
| 21 | Swim: 15 min | Restraint: 60 min | Swim: 15 min |
The remaining control dams were exposed to normal animal room husbandry practices in the animal facility (n = 11, standard chow–control group and high-fat–control group). Tail blood samples were collected from all dams on the morning of gestation days 14 (prestress) and 21 (poststress) for measurement of basal plasma corticosterone, leptin, and insulin.
The day a litter was found was designated postnatal day 0. On postnatal day 1, litters were culled to 10 pups each (5 male and 5 female). Only litters that contained ≥10 pups were included.
Body weight.
Pups and dams were weighed once weekly beginning on postnatal day 1. Pups were weaned on postnatal day 21 and housed in groups of two to three by sex and treatment group. Half of each group (n = 4–5 pups) were weaned onto either standard chow or high-fat diet.
Glucose tolerance test.
On postnatal day 22 and postnatal day 70, rats were food-deprived overnight for 16 h with only water available. A baseline blood sample was taken via a small tail nick for determination of plasma insulin. Baseline fasted blood glucose was determined at the same time by a handheld glucose meter (Freestyle; TheraSense, Alameda, CA). An oral gavage of glucose (2.0 g/kg body wt, 20% glucose in sterile water solution) was then administered. Blood samples were collected at 15, 30, 45, 60, and 120 min after glucose gavage to determine plasma insulin levels. Blood glucose was determined at each time point using the glucometer.
Death.
Male pups (n = 1 per litter) were killed on postnatal day 1, 7, 14, 21, and 90 by decapitation. Blood was collected into a heparinized microcentrifuge tube, centrifuged at 4°C to collect plasma, and stored at −80°C for hormone analysis. Fat pads (dorsosubcutaneous, inguinal, and retroperitoneal) were unilaterally dissected and weighed. The entire subscapular brown adipose tissue fat pad was removed and weighed.
Endocrine assays.
Plasma hormone concentrations were determined by commercially available radioimmunoassay kits for corticosterone (for rats and mice; MP Biomedicals, Solon, OH) and for leptin and insulin (both for rat; Millipore, Billerca, MA). Inter- and intra-assay variability for each assay, respectively, were as follows: corticosterone, 6.5–7.1% and 4.4–10.3%; leptin, 3.0–5.7% and 2.0–4.6%; and insulin, 8.5–9.4% and 1.4–4.6%.
Statistical analysis.
Data were analyzed by Statistica 7.0 (Systat, Tulsa, OK) by ANOVA, repeated-measures ANOVA, or Student's t tests for independent samples as appropriate. Subsequent comparisons between groups used Newman-Keuls procedures. Data are presented as the means ± SEM.
RESULTS
Pregnant dams.
There were no significant differences in maternal body weight among the groups before stress exposure (Table 2). However, compared with beginning body weight on gestation day 2, dams maintained on high-fat diet gained significantly more weight during the first 2 weeks of gestation compared with dams fed standard chow diet (P < 0.05). The greater weight gain in high-fat dams may be attributable to significantly higher caloric intake of dams fed high-fat diet during gestation days 2–14 (P < 0.05). There were no significant differences in plasma corticosterone among the groups before stress.
TABLE 2.
Maternal body weight, food intake, and endocrine measures during gestation
| Standard chow–control group | Standard chow–stress group | High-fat–control group | High-fat–stress group | |
|---|---|---|---|---|
| n | 11 | 10 | 11 | 10 |
| Gestation day 2–14 (prestress) | ||||
| Body weight | ||||
| Gestation day 2 (g) | 301.4 ± 12.1 | 308.3 ± 20.2 | 300.0 ± 14.2 | 316.9 ± 16.9 |
| Gestation day 14 (g) | 386.0 ± 14.9 | 401.9 ± 17.1 | 408.4 ± 17.3 | 424.7 ± 19.7 |
| Body weight gain (g) | 84.7 ± 5.0 | 93.6 ± 7.3 | 108.4 ± 7.3* | 107.8 ± 4.7* |
| Food intake (kcal/day) | 79.6 ± 4.7 | 86.2 ± 4.5 | 109.3 ± 6.6† | 111.2 ± 5.8† |
| Corticosterone, gestation day 14 (ng/ml) | 100.5 ± 13.9 | 126.0 ± 33.0 | 91.1 ± 20.0 | 125.4 ± 7.5 |
| Gestation day 15–21 (stress period) | ||||
| Body weight, gestation day 21 (g) | 474.9 ± 18.3 | 475.1 ± 21.4 | 488.8 ± 21.1 | 488.6 ± 21.1 |
| Body weight gain (g) | 88.9 ± 4.1 | 73.2 ± 8.2 | 80.4 ± 5.9 | 63.9 ± 2.9* |
| Food intake (kcal/day) | 82.5 ± 3.2 | 85.0 ± 4.0 | 95.1 ± 5.6 | 93.5 ± 5.0 |
| Corticosterone, gestation day 21 (ng/ml) | 186.2 ± 31.8 | 389.6 ± 78.0* | 269.8 ± 74.2 | 400.5 ± 61.8* |
| Leptin, gestation day 21 (ng/ml) | 4.6 ± 0.6 | 3.4 ± 0.6 | 10.3 ± 2.0† | 7.6 ± 1.3 |
| Insulin, gestation day 21 (ng/ml) | 2.0 ± 0.3 | 2.0 ± 0.3 | 2.6 ± 0.4 | 2.3 ± 0.4 |
*P < 0.05 vs. standard chow–control group;
†P < 0.05 vs. standard chow–control group and standard chow–stress group.
Stress during the last week of gestation (gestation days 14–21) resulted in less body weight gain in stress groups (Table 2). Post hoc analysis revealed that dams in the high-fat–stress group gained significantly less body weight compared with standard chow–control group. However, body weight on the final day of gestation (gestation day 21) was not different among the groups. Caloric intake was not affected by stress (gestation days 15–21). To assess the effect of stress and consumption of high-fat diet, we measured plasma corticosterone, leptin, and insulin on gestation day 21. At this time point, plasma corticosterone was significantly higher in the variable stress groups compared with nonstressed controls (P < 0.05), indicating that exposure to variable stress resulted in HPA axis activation and increased circulating glucocorticoid levels. There was an overall effect of diet on plasma leptin levels after 20 days of high-fat diet feeding (P < 0.05). Leptin was significantly elevated in the high-fat–control group compared with standard chow–fed groups. Because leptin is secreted by adipocytes and is highly correlated to adiposity, these data suggest that even though they were not significantly heavier, the high-fat diet dams had greater body adiposity compared with the standard chow–fed dams. Blood glucose (data not shown) and plasma insulin were not different among the groups, suggesting that high-fat feeding did not result in symptoms of gestational diabetes in high-fat–fed dams.
Neonatal offspring.
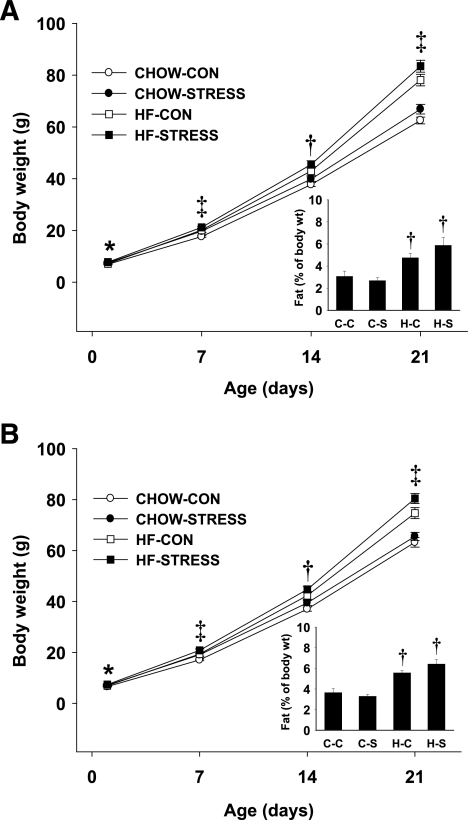
Among the groups, there were no significant differences in litter size (standard chow–control, 15.3 ± 0.7; standard chow–stress, 15.3 ± 0.9; high-fat–control, 14.6 ± 0.9; and high-fat–stress, 14.5 ± 0.7) or male-to-female ratios (standard chow–control, 0.51 ± 0.05; standard chow–stress, 0.48 ± 0.03; high-fat–control, 0.45 ± 0.04; and high-fat–stress, 0.50 ± 0.03). There was an overall effect of maternal stress resulting in higher birth weight of both males (P < 0.05) and females (P < 0.05). By postnatal day 21, there were significant effects of both maternal diet and stress on body weight of male and female pups (Fig. 1A and B). Fat pad (retroperitoneal and subcutaneous pads) weight as a percentage of body weight was reliably higher in both male (P < 0.01) and female (P < 0.001) pups from dams that were fed high-fat diet during gestation and lactation (Fig. 1A and B, inset). Thus, these data suggest that the increase in body weight on postnatal day 21 in pups from dams on high-fat diet is attributable, at least in part, to increased adiposity. Subscapular brown adipose tissue was not different among the groups (data not shown).
FIG. 1.
A and B: Body weight and fat as a percentage of body weight (inset graphs) of offspring through postnatal day 21. A: Male offspring in each litter were weighed on postnatal days 1, 7, 14, and 21. B: Female offspring in each litter were weighed on postnatal days 1, 7, 14, and 21. Fat as a percentage of body weight was determined on postnatal day 21 (inset graphs). Groups shown are: standard chow–control (CHOW-CON; n = 11 litters), standard chow–stress (CHOW-STRESS; n = 10 litters), high-fat–control (HF-CON; n = 11 litters), and high-fat–stress (HF-STRESS; n = 10 litters). *Main effect of stress, P < 0.05 vs. control group; †main effect of high-fat diet, P < 0.05 vs. standard chow; ‡main effect of high-fat diet and stress, P < 0.05. C-C, standard chow–control; C-S, standard chow–stress group; H-C, high-fat–control group; H-S, high-fat–stress group.
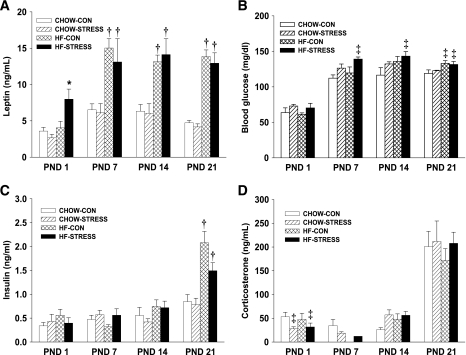
Plasma leptin was elevated at birth in male pups from dams in the high-fat–stress group (P < 0.05) (Fig. 2A). By postnatal day 7, maternal high-fat diet resulted in significantly higher plasma leptin levels compared with pups of standard chow–fed dams, and this effect persisted throughout the preweaning period (P < 0.05). Pups from dams in the high-fat–stress group had significantly greater blood glucose levels on postnatal days 7 and 14, and by postnatal day 21, both high-fat–control and high-fat–stress groups had elevated blood glucose compared with the standard chow–control group (P < 0.05) (Fig. 2B). There was no difference in plasma insulin from postnatal day 1 through 14; however, by postnatal day 21, insulin was significantly greater in pups from high-fat diet–fed dams (high-fat–control group, P < 0.01; high-fat–stress group, P < 0.05) (Fig. 2C). Plasma corticosterone was significantly lower on postnatal day 1 in pups from stressed dams (P < 0.05), regardless of maternal diet, but there were no differences among the groups for the remainder of the preweaning period (Fig. 2D).
FIG. 2.
A–D: Endocrine parameters in male offspring postnatal days (PND) 1–21. A: Plasma leptin. B: Blood glucose. C: Plasma insulin. D: Plasma corticosterone. Groups shown are: standard chow–control (CHOW-CON; n = 11), standard chow–stress (CHOW-STRESS; n = 10), high-fat–control (HF-CON; n = 11), and high-fat–stress (HF-STRESS; n = 10). *P < 0.05 vs. standard chow–control, standard chow–stress, and high-fat–control groups; †P < 0.05 vs. standard chow–control and standard chow–stress groups; ‡P < 0.05 vs. standard chow–control group.
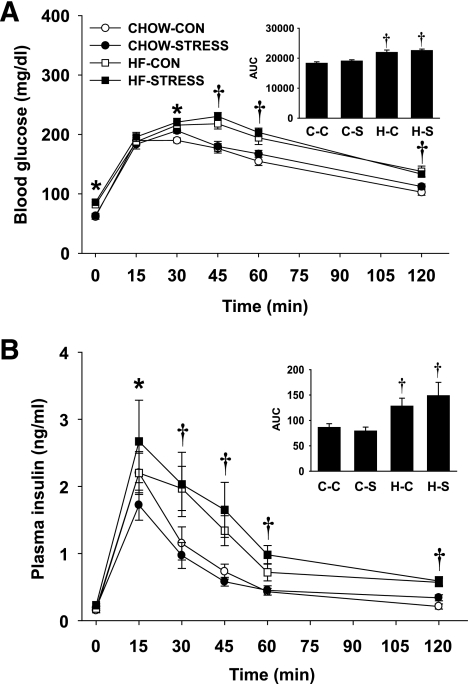
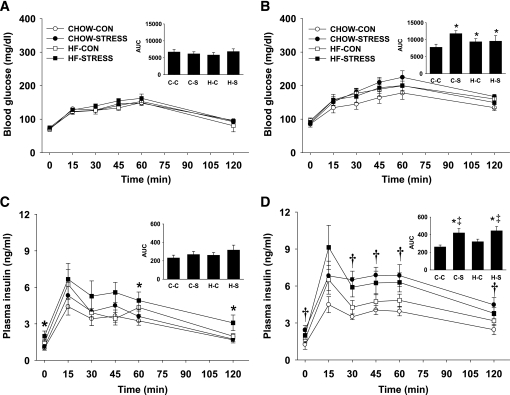
At weaning, male and female pups were challenged with a glucose tolerance test (GTT). There were no significant differences between males and females within each group in any of the measures associated with the GTT; therefore, the data have been combined in Fig. 3. After a 16-h food deprivation period, there was an overall effect of high-fat diet in elevating baseline blood glucose (Time 0). Although there was no difference in blood glucose at 15 min, it remained elevated in the high-fat–control and high-fat–stress pups at 30, 45, 60, and 120 min. The glucose area under the curve (AUC) was higher in high-fat diet pups compared with standard chow pups, indicating that pups from dams fed high-fat diet cleared the glucose load slower than pups from dams fed standard chow diet (P < 0.001) (Fig. 3A). There was no significant effect of stress on glucose clearance at weaning. There was a main effect of maternal high-fat diet on insulin secretion in response to the glucose load. At 30, 45, 60, and 120 min, plasma insulin remained higher in both high-fat–control (P < 0.05) and high-fat–stress (P < 0.01) pups compared with standard chow pups. Insulin AUC was higher in both high-fat–control (P < 0.05) and high-fat–stress (P < 0.01) pups compared with pups from standard chow dams (Fig. 3B). Together, these data suggest that maternal high-fat diet resulted in offspring that cleared glucose more slowly and required greater insulin to do so.
FIG. 3.
A and B: Glucose tolerance test (2.0 g/kg, oral gavage) for male and female pups on postnatal day 23. A: Blood glucose after oral administration of glucose. B: Plasma insulin after oral administration of glucose. The integrated AUC was determined for glucose and insulin using the trapezoidal method. Groups shown are: standard chow–control (CHOW-CON; n = 8), standard chow–stress (CHOW-STRESS; n = 8), high-fat–control (HF-CON; n = 8), and high-fat–stress (HF-STRESS; n = 8). *P < 0.05 standard chow–stress, high-fat–control, and high-fat–stress vs. standard chow–control groups; †P < 0.05 high-fat–control and high-fat–stress vs. standard chow–control and standard chow–stress groups. C-C, standard chow–control; C-S, standard chow–stress; H-C, high-fat–control; H-S, high-fat–stress.
Adult offspring.
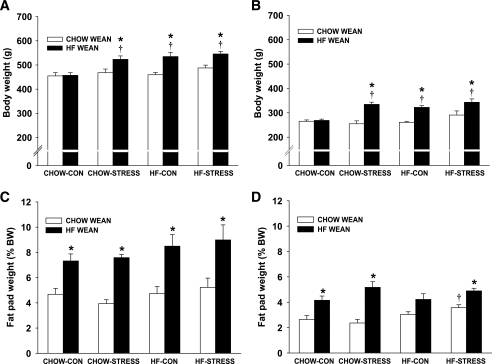
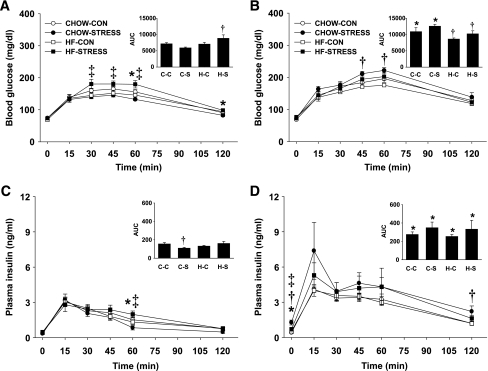
On postnatal day 21, half of the male and female offspring were weaned onto standard chow diet and half on to high-fat diet. When pups were weaned onto standard chow diet, there were no longer any significant differences in body weight among the groups in either males or females at postnatal day 70 (Fig. 4A and B). Body composition was not different among males, but there was a significant increase in percent body fat in high-fat–stress females compared with the standard chow–control group (P < 0.05) (Fig. 4C and D). Plasma insulin after an overnight fast was elevated in the high-fat–stress offspring (P < 0.05), but there were no significant differences among the groups in plasma leptin or fasted blood glucose (Table 3). When adult male offspring (postnatal day 70) were again challenged with a GTT, there were no differences among the groups in glucose clearance (Fig. 5A). Offspring from high-fat–fed dams had higher fasting insulin and showed a prolonged elevation of insulin at 60 and 120 min, although there was no overall effect on insulin AUC (Fig. 5C). However, among females, the high-fat–stress group cleared glucose slower and had greater glucose AUC compared with standard chow–control and standard chow–stress groups (P < 0.05) (Fig. 6A). The standard chow–stress group had lower insulin AUC compared with the standard chow–control group, suggesting that this group may be more efficient in clearing the glucose load (Fig. 6C).
FIG. 4.
A–D: Body weight and fat as a percentage of body weight for adult male (A) and female (B) offspring. Fat as a percentage of body weight for males (C) and females (D) is expressed as the weight of dorsosubcutaneous, inguinal, and retroperitoneal fat pads as a percentage of body weight. Males weaned on standard chow (CHOW WEAN) are: standard chow–control (CHOW-CON; n = 6), standard chow–stress (CHOW-STRESS; n = 4), high-fat–control (HF-CON; n = 4), and high-fat–stress (HF-STRESS; n = 4). Males weaned on high-fat diet (HF WEAN) are: standard chow–control (n = 4), standard chow–stress (n = 4), high-fat–control (n = 5), and high-fat–stress (n = 4). Females weaned on standard chow are: standard chow–control (n = 5), standard chow–stress (n = 4), high-fat–control (n = 4), and high-fat–stress (n = 4). Females weaned on high-fat diet are: standard chow–control (n = 4), standard chow–stress (n = 4), high-fat–control (n = 5), and high-fat–stress (n = 4). *P < 0.05 vs. weaned on standard chow; †P < 0.05 vs. standard chow–control group.
TABLE 3.
Endocrine measures in adult male offspring
| Weaned onto standard chow | Weaned onto high-fat diet | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Leptin (ng/ml) | Fasted glucose (mg/dl) | Fasted insulin (ng/ml) | n | Leptin (ng/ml) | Fasted glucose (mg/dl) | Fasted insulin (ng/ml) | |
| Standard chow–control group | 6 | 14.5 ± 2.5 | 69.3 ± 5.5 | 1.01 ± 0.17 | 3 | 16.4 ± 5.8 | 85.5 ± 9.1† | 1.25 ± 0.39 |
| Standard chow–stress group | 4 | 14.4 ± 3.8 | 72.8 ± 3.9 | 1.13 ± 0.20 | 4 | 25.8 ± 3.2† | 87.3 ± 5.4† | 2.43 ± 0.37*† |
| High-fat–control group | 4 | 17.6 ± 3.7 | 71.0 ± 4.3 | 1.48 ± 0.25 | 5 | 30.7 ± 4.6*† | 95.8 ± 7.9† | 1.68 ± 0.30 |
| High-fat–stress group | 4 | 18.2 ± 3.3 | 75.0 ± 2.8 | 1.98 ± 0.42* | 4 | 23.1 ± 3.6 | 91.1 ± 5.3† | 1.99 ± 0.31 |
*P < 0.05 vs. standard chow–control group;
†P < 0.05 vs. corresponding weaned on standard chow.
FIG. 5.
A–D: Glucose tolerance test in males on postnatal day 70. Blood glucose (A and B) and plasma insulin (C and D) were determined for 2 h after oral administration of glucose for male offspring weaned onto standard chow (A and C) or high-fat diet (B and D). Males weaned on standard chow are: standard chow–control (CHOW-CON; n = 6), standard chow–stress (CHOW-STRESS; n = 4), high-fat–control (HF-CON; n = 4), and high-fat–stress (HF-STRESS; n = 4). Males weaned on high-fat are: standard chow–control (n = 4), standard chow–stress (n = 4), high-fat–control (n = 5), and high-fat–stress (n = 4). *High-fat diet main effect, P < 0.05; †stress main effect, P < 0.05; ‡high-fat diet and stress interaction, P < 0.05. A–D Insets: The integrated AUC was determined for glucose and insulin using the trapezoidal method. *P < 0.05 vs. corresponding standard chow–weaned group; †P < 0.05 vs. standard chow–control and standard chow–stress groups; ‡P < 0.05 vs. standard chow–control and high-fat–control groups. C-C, standard chow–control; C-S, standard chow–stress; H-C, high-fat–control; H-S, high-fat–stress.
FIG. 6.
A–D: Glucose tolerance test in females on postnatal day 70. Blood glucose (A and B) and plasma insulin (C and D) were determined for 2 h after oral administration of glucose for female offspring weaned onto standard chow (A and C) or high-fat diet (B and D). Females weaned onto standard chow are: standard chow–control (CHOW-CON; n = 5), standard chow–stress (CHOW-STRESS: n = 4), high-fat–control (HF-CON; n = 4), and high-fat–stress (HF-STRESS; n = 4). Females weaned onto high-fat are: standard chow–control (n = 4), standard chow–stress (n = 4), high-fat–control (n = 5), and high-fat–stress (n = 4). *High-fat diet main effect, P < 0.05; †stress main effect, P < 0.05. A–D Insets: The integrated AUC was determined for glucose and insulin using the trapezoidal method. *P < 0.05 vs. corresponding standard chow WEAN group; †P < 0.05 vs. standard chow–control and standard chow–stress groups; ‡P < 0.05 vs. standard chow–control and high-fat–control groups. C-C, standard chow–control; C-S, standard chow–stress; H-C, high-fat–control; H-S, high-fat–stress.
When male and female pups were weaned onto a high-fat diet on postnatal day 21, those pups that were from dams in the high-fat diet or stress groups or combination high-fat–stress group gained more weight compared with pups from the standard chow–control dams (Fig. 4A and B). The increase in body weight could be attributed to significantly greater subcutaneous and retroperitoneal fat pad weights (P < 0.05) (Fig. 4C and D), and this was associated with elevated plasma leptin levels in standard chow–stress and high-fat–control groups (P < 0.05) (Table 3).
When male offspring were challenged with a GTT at postnatal day 70 after being weaned onto a high-fat diet, those from dams in the high-fat diet or stress groups or combination high-fat–stress group cleared glucose more slowly relative to male offspring from the same groups weaned onto standard chow diet (Fig. 5B) (P < 0.05). Overall, male offspring from the stress group required greater insulin to clear the glucose load, regardless of maternal diet, compared with those pups weaned onto a standard chow diet (P < 0.05) (Fig. 5D).
Among female offspring that were weaned onto high-fat diet, the standard chow–control and standard chow–stress groups cleared glucose slower than high-fat–control and high-fat–stress groups (P < 0.05) (Fig. 6B). Although the offspring weaned onto high-fat diet had greater insulin responses compared with those weaned onto standard chow, there was no significant difference among the high-fat–weaned groups in insulin AUC (Fig. 6D).
DISCUSSION
The intrauterine environment is critical to fetal development, and perturbations of this environment can have significant short- and long-term consequences on the offspring. A growing body of epidemiological data demonstrates that obesity and other metabolic disease may have developmental origins, and determination of mechanisms contributing to those conditions may lead to treatment strategies and early interventions to prevent these disorders. We hypothesized that exposure to prenatal stress or maternal high-fat diet in utero would predispose offspring to develop obesity and that weaning onto a high-fat diet would exacerbate this effect. Our data suggest that exposure to maternal prenatal stress or high-fat diet feeding results in offspring that gain more weight and have greater adiposity than controls. Consistent with their body composition, offspring of stressed or high-fat diet–fed dams are hyperleptinemic and hyperinsulinemic at weaning. Males and females are similarly affected by maternal prenatal stress and high-fat diet, resulting in impaired glucose tolerance at weaning. Although weaning on standard chow diet appears to normalize early obesity, the combination of maternal prenatal stress and high-fat diet continued to have some effects on glucose tolerance in both male and female offspring. Weaning onto high-fat diet resulted in obesity and impaired glucose tolerance in offspring exposed to prenatal stress, high-fat diet, or both. Together, the data suggest that maternal prenatal stress or high-fat diet alters susceptibility of offspring to diet-induced obesity and its metabolic consequences.
Although high-fat diet–fed dams were not heavier, their plasma leptin levels were elevated on gestation day 21, suggesting that they had greater adiposity. The offspring that were born to dams on high-fat diet were heavier at birth, remained heavier throughout the suckling period, and had impaired glucose tolerance at weaning, suggesting that they were developing insulin resistance. Pups of high-fat diet–fed dams also were hyperleptinemic and hyperinsulinemic by the time they were weaned. Prior studies that have attempted to identify the effects of prolonged maternal high-fat diet consumption have been difficult to interpret because of concurrence of high-fat diet exposure and maternal obesity (31). Maternal obesity is usually associated with other comorbid conditions, such as gestational diabetes, that also have a significant influence on the phenotype of the offspring independent of dietary fat consumption during gestation. Indeed, offspring of rodents with gestational diabetes show increased adiposity, impaired pancreatic function, impaired glucose tolerance, and altered hypothalamic development (32–34). For these reasons, the dams in our study were provided with high-fat diet only during gestation and lactation. Although dams in the current study were hyperleptinemic, indicating that they had greater adiposity after 3 weeks on high-fat diet, there were no differences in plasma concentrations of insulin or glucose, suggesting that they had not developed signs of gestational diabetes. It is unclear from this experiment whether maternal high-fat diet consumption during gestation or lactation had a greater influence because high-fat dams remained on high-fat diet throughout these periods. Future studies with cross-fostered control groups will be required to make this distinction.
Baseline corticosterone was elevated in dams in the stress group, suggesting that exposure to variable stress during the 3rd week of gestation was effective. Maternal prenatal stress in our study resulted in higher birth weights in both male and female offspring, and this occurred in the absence of significant differences in litter size or male-to-female ratios. By postnatal day 21, both male and female offspring of the stress group had greater body weight and showed signs of impaired glucose tolerance, suggesting that prenatal stress has early consequences for the offspring. In contrast to the effect of high-fat diet on endocrine measures, the only hormone that was significantly different in the stress offspring was plasma corticosterone, which was lower in the stress group compared with control pups at birth. This may be a compensatory response resulting from the stressed dams' elevated plasma corticosterone at parturition. It is not known whether negative feedback control of corticosterone secretion is operational at this early age.
Prior studies examining the effects of prenatal stress on birth weights have produced mixed results. Some report decreased birth weights resulting from prenatal stress (26,35,36), and others show increased birth weights (27) or no difference compared with controls (25). One possible explanation to account for some of the difference is that those studies reporting lower birth weights used the immobilization stress paradigm, which also results in decreased food intake and body weight gain in the dams. Because stress-induced maternal undernutrition and decreased body weight gain during pregnancy has consequences similar to those of intrauterine growth restriction, parceling out the direct effects of stress is difficult in these models. The variable stress schedule that we used did not significantly affect maternal food intake of dams during the stress period. Epidemiological reports in humans indicate that the birth weight–to–adult fat mass picture has developed into a U-shaped curve, such that being very light or very heavy at birth may predispose offspring to cardiovascular disease, diabetes, obesity, and some types of cancer (15,37). Therefore, although the findings regarding the effects of prenatal stress on birth weight are variable, the different models may operate through different mechanisms depending on the type, intensity, and timing of stress during gestation (38).
Maternal obesity and high-fat diet feeding have been associated with hyperphagia and obesity in offspring, particularly when challenged postnatally with a hypercaloric diet (39). We hypothesized that prenatal stress against a background of high-fat diet feeding would result in an additive or synergistic effect on the offspring. The data at the time points examined suggest that there is no increased effect of the combination of stress and high-fat diet. An alternative possibility was that maternal high-fat diet would have a “beneficial” effect in attenuating the stress response of the dam and, in turn, lessen its impact on the developing fetus. Dallman et al. (40) have demonstrated that high-fat diet can attenuate the HPA axis response to stress. We did not note either a detrimental or beneficial effect of maternal high-fat diet on top of the effects of stress.
In adulthood, offspring exposed to stress or high-fat diet or both gained significantly more body weight and adiposity, but only if they were weaned onto a high-fat diet. Consistent with increased body weight and adiposity on high-fat diet in adulthood, animals from stressed or high-fat diet–fed dams also continued to have impaired glucose tolerance. In contrast, when offspring were weaned on to standard chow, body weight, body composition, and glucose tolerance differences among the groups were no longer as pronounced. It is important to note that the data reported here are from young adult offspring and do not preclude the possibility that metabolic disorders may occur more slowly, emerging later in life.
Overall, our data suggest that prenatal conditions can alter the susceptibility of offspring to future metabolic challenge, in this case high-fat diet resulting in obesity. This concept has been proposed as the “two-hit” model and suggests that genetic or environmental factors disrupt early development and produce increased susceptibility to disease, including Parkinson's disease (41,42) and schizophrenia (43,44). The “first hit” disruptions that occur during early development set the stage for long-term vulnerability to a “second hit” that occurs later in life and leads to pathology. Thus, it appears that prenatal stress or maternal high-fat diet is not the direct cause of increased body weight or disturbances in energy homeostasis, but instead increases future susceptibility. Identification of the pathways and mechanisms that produce long-term vulnerability in response to early environmental disruption will facilitate development of clinical intervention and prevention strategies to reduce the incidence of disease. Using these animal models, we are now able to extend our studies to determine the potential mechanisms through which prenatal stress or high-fat diet may program systems that regulate body weight and control food intake. There is evidence that hormones such as corticosterone, insulin, and leptin can cross the placental-fetal barrier to potentially affect fetal development (45–47), and thus it is likely that fetuses have hormone levels that mirror those of their dams. The significant changes in endocrine parameters in offspring throughout the early postnatal period reported here represent reasonable candidates for “metabolic programming” that occurs in response to high-fat diet consumption or maternal stress (48). Both leptin and insulin are trophic factors that act during the pre- and postnatal periods and can significantly affect development of neural systems important in the maintenance of energy homeostasis (49). Similarly, stress and excess glucocorticoids may have an effect prenatally by programming increased susceptibility to stress in adulthood. Although placental 11β-hydroxysteroid dehydrogenase (11β-HSD) serves to buffer the developing fetus from excess glucocorticoids in response to acute stress, its upregulation of 11β-HSD is often insufficient during chronic stress (50). Future studies are required to explore the role of 11β-HSD in the phenotypes we observed.
The intrauterine period is a critical time of development, and evidence shows that disturbances to the fine balance in utero can have significant and persistent consequences for the offspring. The development of relevant animal models is required to examine the etiology of metabolic disorders and to determine the mechanisms for greater predisposition to those disorders.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants K99HD055030 (to K.L.K.T.), R01DK077623 (to T.H.M.), and R01MH073826 (to J.I.K.).
No potential conflicts of interest relevant to this article were reported.
The authors acknowledge Dr. Nicholas T. Bello, Karen A. Scott, R. Ryan Behles, and Matthew H. Kemm for their assistance with this study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL: Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002; 288: 1723– 1727 [DOI] [PubMed] [Google Scholar]
- 2.Procopiou M, Philippe J: The metabolic syndrome and type 2 diabetes: epidemiological figures and country specificities. Cerebrovasc Dis 2005; 20 ( Suppl. 1:) 2– 8 [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Flegal KM: Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin North Am 2003; 32: 741– 760 [DOI] [PubMed] [Google Scholar]
- 4.Tremblay MS, Katzmarzyk PT, Willms JD: Temporal trends in overweight and obesity in Canada, 1981–1996. Int J Obes Relat Metab Disord 2002; 26: 538– 543 [PubMed] [Google Scholar]
- 5.Jebb SA, Rennie KL, Cole TJ: Prevalence of overweight and obesity among young people in Great Britain. Public Health Nutr 2004; 7: 461– 465 [DOI] [PubMed] [Google Scholar]
- 6.Caprio S, Tamborlane WV: Metabolic impact of obesity in childhood. Endocrinol Metab Clin North Am 1999; 28: 731– 747 [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM: Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295: 1549– 1555 [DOI] [PubMed] [Google Scholar]
- 8.Hales CN, Barker DJ: Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35: 595– 601 [DOI] [PubMed] [Google Scholar]
- 9.Painter RC, Roseboom TJ, Bleker OP: Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 2005; 20: 345– 352 [DOI] [PubMed] [Google Scholar]
- 10.Ross MG, Desai M: Gestational programming: population survival effects of drought and famine during pregnancy. Am J Physiol Regul Integr Comp Physiol 2005; 288: R25– R33 [DOI] [PubMed] [Google Scholar]
- 11.Ozanne SE, Fernandez-Twinn D, Hales CN: Fetal growth and adult diseases. Semin Perinatol 2004; 28: 81– 87 [DOI] [PubMed] [Google Scholar]
- 12.Bertram CE, Hanson MA: Animal models and programming of the metabolic syndrome. Br Med Bull 2001; 60: 103– 121 [DOI] [PubMed] [Google Scholar]
- 13.Gluckman PD, Hanson MA: Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res 2004; 56: 311– 317 [DOI] [PubMed] [Google Scholar]
- 14.Kral JG: Preventing and treating obesity in girls and young women to curb the epidemic. Obes Res 2004; 12: 1539– 1546 [DOI] [PubMed] [Google Scholar]
- 15.Taylor PD, Poston L: Developmental programming of obesity in mammals. Exp Physiol 2007; 92: 287– 298 [DOI] [PubMed] [Google Scholar]
- 16.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L: Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 2003; 41: 168– 175 [DOI] [PubMed] [Google Scholar]
- 17.Rosmond R, Bjorntorp P: Psychosocial and socio-economic factors in women and their relationship to obesity and regional body fat distribution. Int J Obes Relat Metab Disord 1999; 23: 138– 145 [DOI] [PubMed] [Google Scholar]
- 18.Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S: Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol 1994; 6: 341– 345 [DOI] [PubMed] [Google Scholar]
- 19.Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S: Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci 1997; 17: 2626– 2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL: Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res 2005; 156: 251– 261 [DOI] [PubMed] [Google Scholar]
- 21.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI: Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res 2007; 1156: 152– 167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E: Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry 2003; 54: 1025– 1034 [DOI] [PubMed] [Google Scholar]
- 23.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR: Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 1998; 101: 2174– 2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR: Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia 1996; 39: 1299– 1305 [DOI] [PubMed] [Google Scholar]
- 25.D'Mello AP, Liu Y: Effects of maternal immobilization stress on birth weight and glucose homeostasis in the offspring. Psychoneuroendocrinology 2006; 31: 395– 406 [DOI] [PubMed] [Google Scholar]
- 26.Lesage J, Del-Favero F, Leonhardt M, Louvart H, Maccari S, Vieau D, Darnaudery M: Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J Endocrinol 2004; 181: 291– 296 [DOI] [PubMed] [Google Scholar]
- 27.Mueller BR, Bale TL: Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav 2006; 88: 605– 614 [DOI] [PubMed] [Google Scholar]
- 28.Nieuwenhuizen AG, Rutters F: The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav 2008; 94: 169– 177 [DOI] [PubMed] [Google Scholar]
- 29.Barnum CJ, Blandino P, Jr, Deak T: Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. J Neuroendocrinol 2007; 19: 632– 642 [DOI] [PubMed] [Google Scholar]
- 30.Weinstock M: Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 2001; 65: 427– 451 [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS: Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab 2006; 291: E792– E799 [DOI] [PubMed] [Google Scholar]
- 32.Fahrenkrog S, Harder T, Stolaczyk E, Melchior K, Franke K, Dudenhausen JW, Plagemann A: Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr 2004; 134: 648– 654 [DOI] [PubMed] [Google Scholar]
- 33.Jones AP, Olster DH, States B: Maternal insulin manipulations in rats organize body weight and noradrenergic innervation of the hypothalamus in gonadally intact male offspring. Brain Res Dev Brain Res 1996; 97: 16– 21 [DOI] [PubMed] [Google Scholar]
- 34.Jones AP, Pothos EN, Rada P, Olster DH, Hoebel BG: Maternal hormonal manipulations in rats cause obesity and increase medial hypothalamic norepinephrine release in male offspring. Brain Res Dev Brain Res 1995; 88: 127– 131 [DOI] [PubMed] [Google Scholar]
- 35.Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O: Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab 2007; 292: E1526– E1533 [DOI] [PubMed] [Google Scholar]
- 36.O'Regan D, Welberg LL, Holmes MC, Seckl JR: Glucocorticoid programming of pituitary-adrenal function: mechanisms and physiological consequences. Semin Neonatol 2001; 6: 319– 329 [DOI] [PubMed] [Google Scholar]
- 37.Baker JL, Olsen LW, Sorensen TI: Weight at birth and all-cause mortality in adulthood. Epidemiology 2008; 19: 197– 203 [DOI] [PubMed] [Google Scholar]
- 38.Koenig JI: Schizophrenia: a unique translational opportunity in behavioral neuroendocrinology. Horm Behav 2006; 50: 602– 611 [DOI] [PubMed] [Google Scholar]
- 39.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD: Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 2000; 279: E83– E87 [DOI] [PubMed] [Google Scholar]
- 40.Dallman MF, Pecoraro NC, la Fleur SE: Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun 2005; 19: 275– 280 [DOI] [PubMed] [Google Scholar]
- 41.Barlow BK, Cory-Slechta DA, Richfield EK, Thiruchelvam M: The gestational environment and Parkinson's disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol 2007; 23: 457– 470 [DOI] [PubMed] [Google Scholar]
- 42.Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM: Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol 2006; 199: 499– 512 [DOI] [PubMed] [Google Scholar]
- 43.Maynard TM, Sikich L, Lieberman JA, LaMantia AS: Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull 2001; 27: 457– 476 [DOI] [PubMed] [Google Scholar]
- 44.Bayer TA, Falkai P, Maier W: Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis.” J Psychiatr Res 1999; 33: 543– 548 [DOI] [PubMed] [Google Scholar]
- 45.Menon RK, Cohen RM, Sperling MA, Cutfield WS, Mimouni F, Khoury JC: Transplacental passage of insulin in pregnant women with insulin-dependent diabetes mellitus: its role in fetal macrosomia. N Engl J Med 1990; 323: 309– 315 [DOI] [PubMed] [Google Scholar]
- 46.Takahashi LK, Turner JG, Kalin NH: Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology 1998; 23: 571– 581 [DOI] [PubMed] [Google Scholar]
- 47.Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP: Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 2001; 142: 1692– 1702 [DOI] [PubMed] [Google Scholar]
- 48.Levin BE: Metabolic imprinting on genetically predisposed neural circuits perpetuates obesity. Nutrition 2000; 16: 909– 915 [DOI] [PubMed] [Google Scholar]
- 49.Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS: Development of metabolic systems. Physiol Behav 2005; 86: 646– 660 [DOI] [PubMed] [Google Scholar]
- 50.Welberg LA, Thrivikraman KV, Plotsky PM: Chronic maternal stress inhibits the capacity to upregulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J Endocrinol 2005; 186: R7– R12 [DOI] [PubMed] [Google Scholar]