Abstract
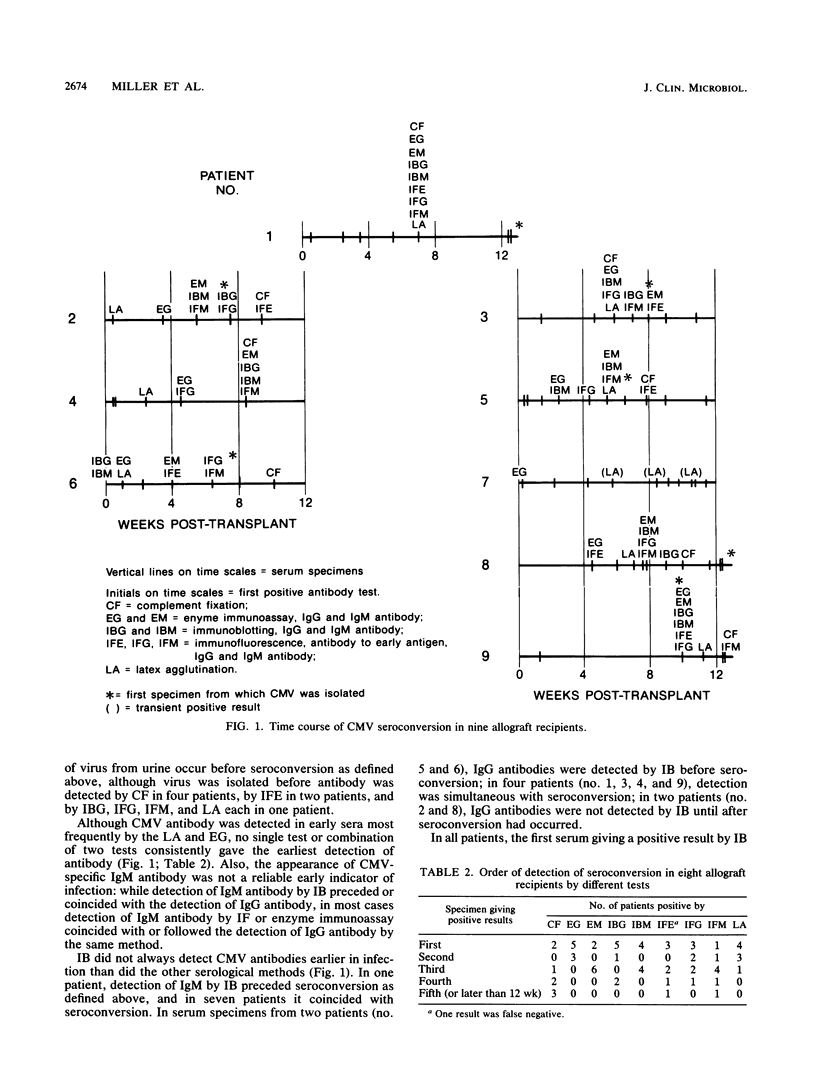
Sequential specimens from nine allograft recipients were examined by using a variety of methods to detect primary cytomegalovirus (CMV) infection as rapidly as possible posttransplantation. Sera were examined for immunoglobulin G (IgG) and IgM antibodies by immunoblotting, enzyme immunoassay, and immunofluorescence and also by complement fixation, latex agglutination, and an immunofluorescence test for antibody to CMV early antigen. Urine and occasionally blood, tissue, and other specimens were centrifuged onto cell cultures to enhance CMV infectivity. Eight of the nine patients showed laboratory evidence of primary CMV infection, and CMV was isolated from seven of the eight: in no case was virus isolated before seroconversion had become evident. However, serological tests differed in their abilities to detect antibody response to CMV infection in different patients; while immunoblotting, latex agglutination, and enzyme immunoassay for IgG antibodies generally detected seroconversion before complement fixation, this was not invariably the case. At present, optimal laboratory detection of CMV infections in these patients can be achieved only by a combination of serological methods and virus isolation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., McVoy M., Biro V. G., Britt W. J., Hider P., Marshall D. Detection of cytomegalovirus antibody with latex agglutination. J Clin Microbiol. 1985 Jul;22(1):68–70. doi: 10.1128/jcm.22.1.68-70.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert G., Mazeron M. C., Colimon R., Plotkin S. Rapid detection of human cytomegalovirus in the urine of humans. J Infect Dis. 1985 Sep;152(3):631–633. doi: 10.1093/infdis/152.3.631. [DOI] [PubMed] [Google Scholar]
- BRADSTREET C. M., TAYLOR C. E. Technique of complementfixation test applicable to the diagnosis of virus diseases. Mon Bull Minist Health Public Health Lab Serv. 1962 May;21:96–104. [PubMed] [Google Scholar]
- Braun D. K., Pereira L., Norrild B., Roizman B. Application of denatured, electrophoretically separated, and immobilized lysates of herpes simplex virus-infected cells for detection of monoclonal antibodies and for studies of the properties of viral proteins. J Virol. 1983 Apr;46(1):103–112. doi: 10.1128/jvi.46.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Faix R. G. Cytomegalovirus antigenic heterogeneity can cause false-negative results in indirect hemagglutination and complement fixation antibody assays. J Clin Microbiol. 1985 Nov;22(5):768–771. doi: 10.1128/jcm.22.5.768-771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Comparison of standard tube and shell vial cell culture techniques for the detection of cytomegalovirus in clinical specimens. J Clin Microbiol. 1985 Feb;21(2):217–221. doi: 10.1128/jcm.21.2.217-221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984 Jun;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. J., Alvey B., Smith D. J., Wreghitt T. G. Evaluation of a commercial latex agglutination test for detecting antibodies to cytomegalovirus in organ donors and transplant recipients. J Virol Methods. 1987 May;16(1-2):13–19. doi: 10.1016/0166-0934(87)90026-7. [DOI] [PubMed] [Google Scholar]
- Landini M. P. Antibody response against early antigens in Herpesviridae infections. Eur J Epidemiol. 1985 Mar;1(1):62–66. doi: 10.1007/BF00162314. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Michelson S. Human cytomegalovirus proteins. Prog Med Virol. 1988;35:152–185. [PubMed] [Google Scholar]
- Landini M. P., Re M. C., Mirolo G., Baldassarri B., La Placa M. Human immune response to cytomegalovirus structural polypeptides studied by immunoblotting. J Med Virol. 1985 Dec;17(4):303–311. doi: 10.1002/jmv.1890170403. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Rossier E., Schmitz H. Antibodies to human cytomegalovirus structural polypeptides during primary infection. J Virol Methods. 1988 Dec;22(2-3):309–317. doi: 10.1016/0166-0934(88)90113-9. [DOI] [PubMed] [Google Scholar]
- McHugh T. M., Casavant C. H., Wilber J. C., Stites D. P. Comparison of six methods for the detection of antibody to cytomegalovirus. J Clin Microbiol. 1985 Dec;22(6):1014–1019. doi: 10.1128/jcm.22.6.1014-1019.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh T. M., Casavant C. H., Wilber J. C., Stites D. P. Comparison of six methods for the detection of antibody to cytomegalovirus. J Clin Microbiol. 1985 Dec;22(6):1014–1019. doi: 10.1128/jcm.22.6.1014-1019.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Bankier A. T., Landini M. P., Brown C. M., Barrell B. G., Rüger B., Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988 Jul;62(7):2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani D. D., Ball M. G., Berry N. J., Wimperis J. Z., Blacklock H. A., Prentice H. G., Hoffbrand A. V., Griffiths P. D. Virological and serological diagnosis of cytomegalovirus infection in bone marrow allograft recipients. J Med Virol. 1985 Aug;16(4):357–365. doi: 10.1002/jmv.1890160409. [DOI] [PubMed] [Google Scholar]
- Pass R. F., Griffiths P. D., August A. M. Antibody response to cytomegalovirus after renal transplantation: comparison of patients with primary and recurrent infections. J Infect Dis. 1983 Jan;147(1):40–46. doi: 10.1093/infdis/147.1.40. [DOI] [PubMed] [Google Scholar]
- Phipps P. H., Grégoire L., Rossier E., Perry E. Comparison of five methods of cytomegalovirus antibody screening of blood donors. J Clin Microbiol. 1983 Dec;18(6):1296–1300. doi: 10.1128/jcm.18.6.1296-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripalti A., Landini M. P., Mocarski E. S., La Placa M. Identification and preliminary use of recombinant lambda gt11 fusion proteins in human cytomegalovirus diagnosis. J Gen Virol. 1989 May;70(Pt 5):1247–1251. doi: 10.1099/0022-1317-70-5-1247. [DOI] [PubMed] [Google Scholar]
- Rossier E., Dimock K., Taylor D., Larose Y., Phipps P. H., Brodeur B. Sensitivity and specificity of enzyme immunofiltration and DNA hybridization for the detection of HCMV-infected cells. J Virol Methods. 1987 Feb;15(2):109–120. doi: 10.1016/0166-0934(87)90054-1. [DOI] [PubMed] [Google Scholar]
- Scholl B. C., Von Hintzenstern J., Borisch B., Traupe B., Bröker M., Jahn G. Prokaryotic expression of immunogenic polypeptides of the large phosphoprotein (pp150) of human cytomegalovirus. J Gen Virol. 1988 Jun;69(Pt 6):1195–1204. doi: 10.1099/0022-1317-69-6-1195. [DOI] [PubMed] [Google Scholar]
- Shibata D., Martin W. J., Appleman M. D., Causey D. M., Leedom J. M., Arnheim N. Detection of cytomegalovirus DNA in peripheral blood of patients infected with human immunodeficiency virus. J Infect Dis. 1988 Dec;158(6):1185–1192. doi: 10.1093/infdis/158.6.1185. [DOI] [PubMed] [Google Scholar]
- Sorbello A. F., Elmendorf S. L., McSharry J. J., Venezia R. A., Echols R. M. Rapid detection of cytomegalovirus by fluorescent monoclonal antibody staining and in situ DNA hybridization in a dram vial cell culture system. J Clin Microbiol. 1988 Jun;26(6):1111–1114. doi: 10.1128/jcm.26.6.1111-1114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckl E., Popow-Kraupp T., Heinz F. X., Mühlbacher F., Balcke P., Kunz C. Potential of in situ hybridization for early diagnosis of productive cytomegalovirus infection. J Clin Microbiol. 1988 Dec;26(12):2536–2540. doi: 10.1128/jcm.26.12.2536-2540.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele G. M., Bicak M. S., Young A., Kinsey J., White R. J., Purtilo D. T. Rapid detection of cytomegalovirus by tissue culture, centrifugation, and immunofluorescence with a monoclonal antibody to an early nuclear antigen. J Virol Methods. 1987 Jul;16(4):327–338. doi: 10.1016/0166-0934(87)90018-8. [DOI] [PubMed] [Google Scholar]
- Weir M. R., Irwin B. C., Maters A. W., Genemans G., Shen S. Y., Charache P., Williams G. M. Morbid outcome of cytomegalovirus-negative transplant recipients receiving cytomegalovirus-positive kidneys. Transplant Proc. 1987 Feb;19(1 Pt 3):2137–2141. [PubMed] [Google Scholar]
- van der Bij W., Schirm J., Torensma R., van Son W. J., Tegzess A. M., The T. H. Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J Clin Microbiol. 1988 Dec;26(12):2531–2535. doi: 10.1128/jcm.26.12.2531-2535.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]