Abstract
Purpose
To determine the correlation function between the steady-state free precession (SSFP) and fast gradient echo (FGRE) cine MRI pulse sequences for measuring the myocardial mass and volumes.
Materials and Methods
Cardiac cine MRI examinations were acquired in 50 individuals (female: 35, male: 15, mean age 64.1 ± 9.1 years, range 48–83) using SSFP and FGRE cardiac pulse sequences.
Results
The mean (standard deviation [SD]) left ventricular end diastolic volume measured by SSFP was significantly larger (4.5%) than by FGRE (p < 0.001); this was also the case for end systolic volume (15.0%, p < 0.001). The relationship between SSFP and FGRE measures were linear and highly correlated (p < 0.001) for both left ventricular end diastolic and end systolic volumes (r2 = 0.90 vs. 0.91, respectively). We determined linear regression models to estimate the SSFP values based on the FGRE measures. Slope (intercept) for ejection fraction, stroke volume, and cardiac output were 0.99 (−2.79), 0.77 (17.5), and 0.76 (1.29), respectively.
Conclusion
Linear relationships exist for key LV function parameters when comparing SSFP and FGRE cine MRI. These results indicate that existing databases and normal values for FGRE LV function may be converted to corresponding LV function values for SSFP MRI.
Keywords: magnetic resonance imaging, normal cardiac function, steady state free precession, fast gradient echo
Due to its large field of view (FOV), operator independence, no exposure to radiation, and lack of geometric assumptions to estimate heart size, cardiac MRI is considered a standard of reference for evaluation of cardiac mass, volume, and function (1–3). The traditional fast gradient echo cine sequence (FGRE, also known as FLASH) has been proven to be accurate in the assessment of ventricular volume, mass, and function (4). However, steady-state free precession (SSFP) cine MRI has inherent advantages in speed and contrast-to-noise ratio compared to FGRE/FLASH cine MRI. SSFP images have greater contrast at the endocardial border with less blood flow dependence and greater fat-to-myocardial contrast at the epicardial border. These properties lead to significant differences between mass and volume measures between the two methods (5,6).
More than 10 years of data in the literature used the older FGRE technique to establish databases of normal individuals as well as that of patients for myocardial size and function by MRI. It will therefore be important to determine the relationship between the two MRI methods in order to adjust the older data and relate it to cardiac size and function measured with SSFP MRI. The purpose of this study was to determine the relationship between myocardial mass, volumes, and function when determined by FGRE versus SSFP MRI.
MATERIALS AND METHODS
Study Population
Fifty consecutive participants of the Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis (ESCAPE) study were recruited for the purpose of this study (Table 2). The ESCAPE study is one of the prospective ancillary studies of the Multi Ethnic Study of Atherosclerosis (MESA) (7). Participants in the ESCAPE study did not have a history or sign and symptoms of cardiovascular disease at the base of recruitment. However, if a participant had a cardiovascular problem (mostly arrhythmia) during the scan that could interfere with gating and image acquisition, that participant was excluded from the MRI study. Other exclusion criteria for MRI study were the presence of claustrophobia and ferromagnetic implants.
Table 2.
Characteristics of the Population
| N (%) | |
|---|---|
| Female | 35 (70) |
| Male | 15 (30) |
| Age (year) ± SD | 64.08 ± 9.1 |
| Range (year) | 48–83 |
| BMI (kg/m2) ± SD | 26.9 ± 4.4 |
| BSA (m2) ± SD | 1.8 ± 0.2 |
BMI, body mass index; BSA, body surface area; SD, standard deviation.
Image Acquisition
All subjects were imaged on a using a 1.5T whole-body MRI system (Signa CVi, General Electric Medical Systems, Waukesha, WI) with 40 mT/m gradients using a four-element phased-array coil for signal reception. Three plane scout images were acquired and used to verify coil positioning and to localize the heart. To determine the planes needed for the short axis images, an electrocardiogram-gated, 2D, single slice, cardiac four-chamber view cine SSFP sequence was performed with temporal resolution 48 msec, retrospectively reconstructed at overlapping 20–35-msec intervals over the cardiac cycle. SSFP and FGRE cardiac cine MRI were then acquired in the short axis plane in the same MRI session.
FGRE imaging parameters were: echo time 4.5 msec, repetition time 8.1 msec, flip angle 20°, matrix size 256 × 160, slice thickness 6 mm at 10-mm intervals (4 mm gap between adjacent slices), flow compensation and FOV 360 × 360 cm. SSFP imaging parameters were: echo time 1.6 msec, repetition time 3.7 msec, flip angle 45°, matrix size 256 × 160, slice thickness 8 mm at 10-mm intervals (2 mm gap between adjacent slices), and FOV 360 × 360 cm. These parameters are shown in Table 1 for comparison. Thinner slices were used with the FGRE sequence than SSFP because sequence optimization showed improved blood/myocardium contrast for thinner slices related to the time of flight effect for blood signal.
Table 1.
FGRE and SSFP Pulse Sequence Parameters
| FGRE | SSFP | |
|---|---|---|
| Echo time | 4.5 | 1.6 |
| Repetition time | 8.1 | 3.7 |
| Flip angle | 20 | 45 |
| Matrix size | 256*160 | 256*160 |
| Field of view | 360*360 | 360*360 |
| Slice thickness | 6 | 8 |
| Slice gap | 4 | 2 |
SSFP, steady-state free precession; FGRE, fast gradient recalled echo.
Image Analysis
Short axis endocardial and epicardial contours were determined using semiautomated analysis for the left ventricle by a single trained technologist using software QMASS MR 6.1.6 (Medis Medical System, Leiden, Netherlands). The technologist had prior mentored experience in the analysis of more than 2000 cardiac function studies. All studies were then checked by a cardiac MRI physician with 12 years of experience in cardiac MRI interpretation. In order to be consistent, the technologist followed the same analyzing protocol for both imaging methods (8). The end diastolic (ED) phase was defined as the largest ventricular volume and end systolic (ES) phase was defined as the smallest left ventricular volume. Papillary muscles in the left ventricle were excluded from the endocardial ventricular border definition and included in the ventricular volume. Endocardial and epicardial contouring was performed in the basal slices only if more than 50% of the blood pool was surrounded by the myocardium. For apical slices, only the epicardial contours were done if the lumen was not visible (Fig. 1). The contours were checked regularly on adjacent images for consistency in the analysis. End diastolic and systolic volumes were calculated using Simpson’s rule. Stroke volume (SV) was calculated by subtraction of ED volume from ES volume. Ejection fraction (EF) was calculated as EF = SV/EDV × 100. Cardiac output (CO) was calculated by the product of stroke volume and heart rate measured at the time of the MRI acquisition. End diastolic and systolic mass were measured according to the following equation: ventricular mass = 1.05 × [epicardial volume − endocardial volume].
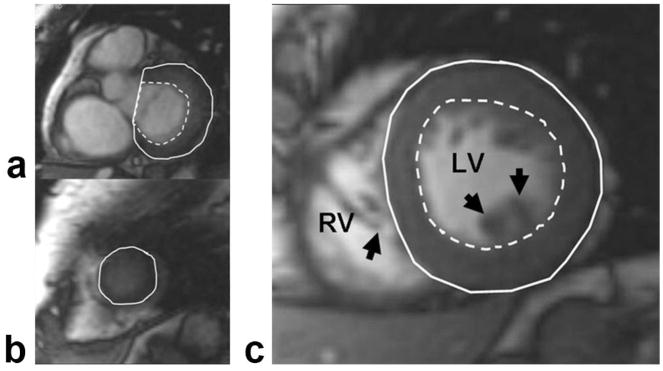
Figure 1.
The endocardial (dashed line) and epicardial (solid line) contours were determined using semiautomated software. The phases of the basal slice, in which more than 50% of the blood pool was surrounded by myocardium, were analyzed (a). Epicardial border was defined in apical slices without visible lumen (b), and papillary muscles (arrows) were excluded from the endocardial border and right ventricular side of the septum (c). RV, Right ventricle; LV, left ventricle.
Statistical Analysis
Statistical analyses were performed using SPSS (v. 14.0 for Windows; Chicago, IL). Data are expressed as mean values with standard deviations (SDs). Paired Student t-tests performed to evaluate the difference between the sequences and unpaired t-tests were used to assess the difference between the independent datasets. Linear regression models provide correlation estimates as well as slope and intercept estimates with FGRE predicting SSFP measures. A two-way mixed model was used to estimate the intraclass correlation coefficient between the groups and average measures were reported. The limits of agreement (defined as ± SD from the mean difference) between the FGRE and SSFP measured volume and function of the left ventricle were compared using the Bland–Altman analysis (9). P-values less than 0.05 were considered statistically significant.
RESULTS
Of 50 participants of this study, 35 (70%) were female and the mean age was 64.1 ± 9.1 years (range, 48–83 years) (Table 2).
Differences of Ventricular Dimensions
The mean ED and ES volumes were significantly higher for the SSFP method compared to FGRE (mean difference: 5.6 and 5.3 mL, respectively, p < 0.001). Stroke volume and cardiac output were not significantly different between the two sequences (mean differences: 0.25 mL and 0.04 mL/min, respectively). However, the EF was significantly lower for SSFP compared to FGRE (mean absolute difference: −3%, p < 0.001). The left ventricular mass was significantly lower using SSFP, both at end-systole and end-diastole (mean difference: 4.8 and 6.0 g, respectively, p < 0.001) (Table 3).
Table 3.
Paired Comparison of the Images by SSFP and FGRE Sequences
| Sequence | Mean | SD | Mean Difference (% of FGRE Value) |
t | Significance (2 - Tailed) |
|
|---|---|---|---|---|---|---|
| ED LV volume (mL) | SSFP | 120.8 | 29 | 5.3 (4.5%) | 3.971 | <0.001 |
| FGRE | 115.5 | 30 | ||||
| ES LV volume (mL) | SSFP | 42.2 | 15 | 5.5 (15.0%) | 7.713 | <0.001 |
| FGRE | 36.7 | 12 | ||||
| EF (%) | SSFP | 65.4 | 7 | −3.0 (−4.4%) | −6.378 | <0.001 |
| FGRE | 68.4 | 6 | ||||
| SV (mL) | SSFP | 78.5 | 18 | −0.3 (−0.4%) | −0.184 | 0.8 |
| FGRE | 78.8 | 20 | ||||
| CO (mL/min) | SSFP | 5.38 | 1 | −0.04 (−0.74%) | −0.456 | 0.6 |
| FGRE | 5.42 | 2 | ||||
| ED LV mass (g) | SSFP | 109.8 | 25 | −6 (−5.2%) | −6.588 | <0.001 |
| FGRE | 115.8 | 27 | ||||
| ES LV mass (g) | SSFP | 105.7 | 24 | −4.8 (−4.3%) | −4.515 | <0.001 |
| FGRE | 110.5 | 28 |
SSFP, Steady-state free precession; FGRE, fast gradient recalled echo; ED, end diastolic; ES, end systolic; LV, left ventricle; SV, stroke volume; CO, cardiac output; EF, ejection fraction; SD, standard deviation.
Correlation Between the Methods
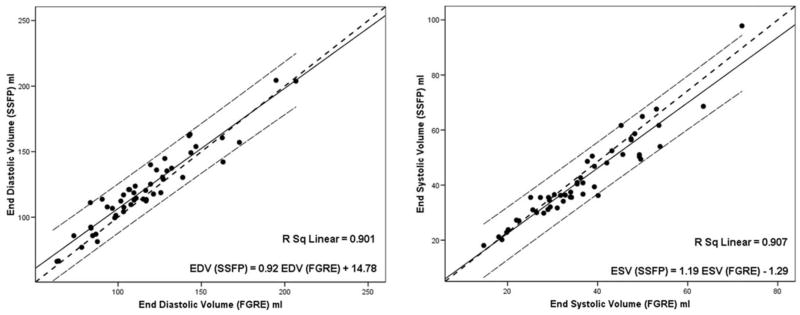
End-diastolic and end-systolic volumes for SSFP and FGRE were strongly correlated (r2: 0.91 and 0.90, respectively) (Figs. 2, 3). Cardiac output was also correlated for the two methods (r2: 0.82). The correlation between the stroke volume and EF was moderate (r2: 0.79 and 0.75, respectively). The end-diastolic masses by two methods were strongly correlated (r2: 0.95). The slopes and intercepts derived from the linear regression models between key variables related FGRE and SSFP are shown in Table 4.
Figure 2.
Relationship between FGRE and SSFP parameters for cine MRI. Data is shown for end diastolic volume (a), end systolic volume (b). The solid line represents the fitted line and the dotted lines represent the 95% individual confidence interval lines. All the correlations were statistically significant. SSFP, steady state free precession; FGRE, fast gradient recalled echo; EDV, end diastolic volume; ESV, end systolic volume.
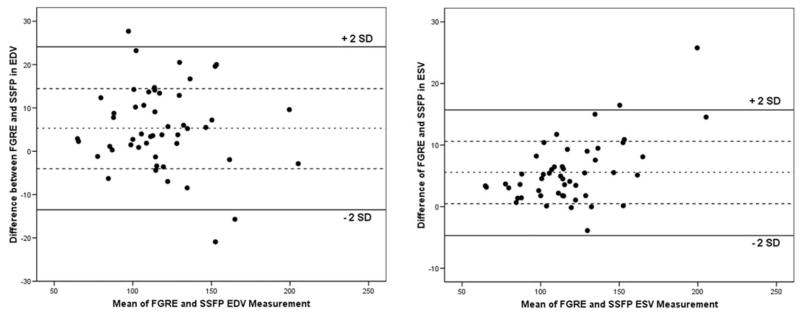
Figure 3.
Bland–Altman plots show the agreement between the SSFP and FGRE in determination of the EDV (a) and ESV (b). Bold lines indicate 2 SD from the middle dotted line, which indicates the mean difference between SSFP and FGRE measures. All the correlations were statistically significant. SSFP, steady state free precession; FGRE, fast gradient recalled echo; EDV, end diastolic volume; ESV, end systolic volume.
Table 4.
Linear Regression Model Analysis With FGRE Measured Values as Independent Variables and SSFP Assessed Variables as the Dependent Variables
| Slopea | Intercept (P Value) |
ICCb | |
|---|---|---|---|
| ED LV volume (mL) | 0.92 (0.067) | 14.78 (0.007) | 0.97 |
| ES LV volume (mL) | 1.19 (0.001) | −1.29 (0.54) | 0.96 |
| EF (%) | 0.99 (0.969) | −2.79 (0.62) | 0.92 |
| SV (mL) | 0.77 (<0.001) | 17.50 (0.001) | 0.93 |
| CO (mL/min) | 0.76 (<0.001) | 1.29 (<0.001) | 0.94 |
| ED LV mass (g) | 0.88 (<0.001) | 7.73 (0.03) | 0.98 |
ED, end diastolic; ES, end systolic; LV, left ventricle; SV, stroke volume; CO, cardiac output; EF, ejection fraction; ICC, intraclass correlation.
Provide P-value estimates corresponding to a test of slope, 1.
All P-values less than 0.001.
DISCUSSION
In this study we demonstrated that end diastolic and end systolic volumes were ≈5–6 mL greater using SSFP compared to FGRE MRI. Left ventricular mass was ≈5–6 gm lower using SSFP versus FGRE. There was no significant difference in cardiac output/stroke volume between the two methods, although the ejection fraction was about 3% lower using SSFP. Importantly, over a broad range of ventricular masses and volumes the volume and mass relationships between the two pulse sequences were well explained by linear modeling.
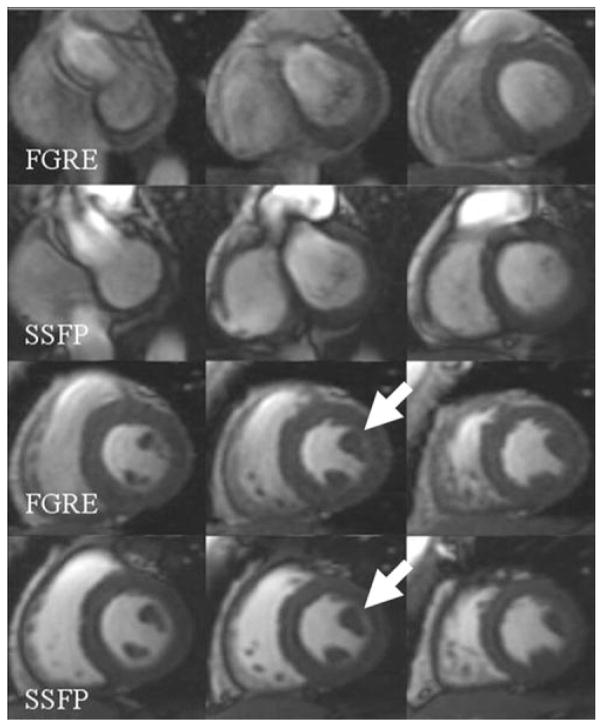
Segmented FGRE sequences have been well validated for cardiac cine MR imaging. The increased blood signal in FGRE compared to the myocardium is largely due to flowing blood, and therefore signal in the blood pool varies during the cardiac cycle (10). With SSFP, high blood signal is due to the longer T2* time of blood relative to the myocardium. Endocardial and epicardial borders are more distinct using SSFP compared to FGRE (6,10) (Fig. 4), which yields a more reproducible and accurate LV mass and function (12) and facilitates automated contour detection (6).
Figure 4.
Comparison of the FGRE and SSFP images. Note the brighter blood pool in SSFP images and higher contrast between blood and the myocardium (arrows), which help to delineate the papillary muscles more clearly.
The data in the literature on comparison of the FGRE and SSFP methods on left ventricular dimensions and function is presented in Table 5. The percent difference between the end diastolic volume measurements varies less than the end systolic volume measurements. This is due to compaction of the papillary muscles in the ventricular lumen, probably leading to underestimation of the end systolic ventricular volume in the FGRE imaging. As a consequence, the ejection fraction is overestimated by FGRE imaging in all previous studies in comparison to SSFP. In addition, all prior studies also demonstrate that left ventricular mass is greater with FGRE compared to SSFP. This is due to low contrast at the endocardial and epicardial border in FGRE compared to SSFP (5,11–16). It should be mentioned that the variation of the EDV and ESV could be due to methodological differences in image analysis and method of imaging itself. However, since CO, SV, and EF are derivates of the heart volumes (discussed in Materials and Methods), the ratio of these volumes has a minimal effect in the outcome calculated functional value (Table 5).
Table 5.
Comparison of Left Ventricle Dimensions and Functional Measurements by SSFP and FGRE in the Literature
| Current Study | Plein et al (11) | Alfakih et al (13) | Thiele et al (14) | Li et al (16) | Moon et al (5)a | Natori et al (8)b | |
|---|---|---|---|---|---|---|---|
| Number of subjects | 50 | 41 | 60 | 25 | 11 | 10 | 800 |
| M:F | 15:35 | 23:18 | 30:30 | 23:2 | 5:6 | 4:6 | 400:400 |
| Average age (range) | 64.1 (48–83) | 53.1 (32–77) | 42.5 (20–65) | 57 (28–77) | 42.8 (20–81) | 32 (27–44) | 60.7 (45–85) |
| Papillary muscles included in LV mass | No | Yes | Yes | No | No | Yes | No |
| Absolute difference between SSFP and FGRE (percentage difference) | |||||||
| ED LV volume (mL) | 5.3 (4.5) | 13.2 (8.0) | 14.3 (10.4) | 13.7 (7.3) | 9.8 (10.2) | 18 (13) | 4.8 (3.8) |
| ES LV volume (mL) | 5.5 (15) | 12.2 (17.5) | 8.2 (17.7) | 18.1 (20.3) | 10.2 (21.6) | 9 (17) | 6.1 (15.7) |
| EF (%) | −3.0 (−4.4) | −3.8 (−5.9) | −1.6 (−2.4) | −5.8 (−10.1) | −5.3 (−9.9) | −2 (−3) | −3.4 (−4.9) |
| SV (mL) | −0.3 (−0.4) | 0.7 (0.7) | 5.7 (6.2) | −4.4 (−4.5) | −0.4 (−0.8) | — | −2.3 (−2.6) |
| CO (mL/min) | −0.04 (−0.74) | — | — | — | — | — | −0.04 (−0.57) |
| ED LV mass (g) | −6.0 (−5.2) | −21.5 (−13.8) | −21.5 (−16.1) | — | −13.9 (−19.2) | −25 (−19) | −8.8 (−6.3) |
The data are presented in the absolute difference between the SSFP and FGRE measured LV volumes, mass, and function. The percentage differences were calculated by dividing the absolute difference by FGRE measured value of the same variable.
M, Male; F, female; LV, left ventricle; ED, end diastolic; ES, end systolic; FGRE, fast gradient recalled echo; SSFP, steady state free precession.
Only the healthy participants are demonstrated.
SSFP measurements were calculated based on the conversion functions of the current study.
In a study of 800 participants (females: 400, males: 400) of the MESA cohort, free of symptomatic cardiac problems at baseline recruitment, Natori et al (8) presented the range of the left ventricular indices based on gender, age, and ethnicity. The results of this large sample size study is in line with previous smaller samples (5,11–16). In comparison to previous studies the dimension of the heart in our study was smaller, both in mass and volume. The age of the patients in our study was slightly higher than other studies (Table 5). However, this is not expected to alter the relationships between the volumetric measurements on SSFP versus FGRE.
An important finding in the current study is the high linear correlation between the SSFP and FGRE sequences (Table 4). The correlation coefficients ranged from 0.9 to 0.95 for the primary measurements (LV volumes and mass) and decreased slightly for the calculated variables (stroke volume and ejection fraction). Since the analytic methods in this study were identical to those of the study by Natori et al (8), Tables 6 and 7 present the Natori et al data for FGRE and the converted SSFP values based on the regression models in this study. The results of the converted variables are close to the largest previously reported SSFP and FGRE imaging normal values (60 participants; females: 30, males: 30) by Alfakih et al (13). The results of both FGRE and SSFP measured volumes and functions for Natori et al are lower than the other study. The size of the heart was shown to be inversely related to age (8). Since the average age of participants in the Alfakih et al study is 42.5 years compared to 60.7 in the Natori et al study (Table 5), the difference between the measurements of the two studies can be attributed to age difference. Interestingly, when Alfakih et al (13) selected an older population (40–65 years), the differences between the two studies become minimal, especially for calculated values (EF and SV).
Table 6.
Normal Calculated Values for Male Population Based on FGRE Images of the Study by Natori et al (8)
| Male Participants | FGREa Mean (SD) |
SSFP Mean (SD) |
|---|---|---|
| ED LV volume (mL) | 142 (34) | 146 (31) |
| ED LV volume/BSA (mL/m2) | 74 (15) | 83 (14) |
| ES LV volume (mL) | 47 (19) | 55 (23) |
| ES LV volume/BSA (mL/m2) | 25 (9) | 28 (11) |
| EF (%) | 67 (7) | 64 (7) |
| SV (mL) | 95 (21) | 91 (16) |
| SV/BSA (mL/mm2) | 49 (10) | 56 (8) |
| ED LV mass (g) | 164 (36) | 152 (32) |
| ED LV mass/BSA (g/m2) | 85 (15) | 83 (13) |
The data are presented as mean (SD).
SSFP, Steady-state free precession; FGRE, fast gradient recalled echo; EF, ejection fraction; SV, stroke volume; BSA, body surface area; ED, end diastolic; ES, End systolic; LV, left ventricular.
FGRE data are from Natori et al (8).
Table 7.
Normal Calculated Values for Female Population Based on FGRE Images of the Study by Natori et al (8)
| Female Participants | FGREa Mean (SD) |
SSFP Mean (SD) |
|---|---|---|
| ED LV volume (mL) | 109 (22) | 115 (21) |
| ED LV volume/BSA (mL/m2) | 65 (11) | 74 (10) |
| ES LV volume (mL) | 31 (9) | 36 (11) |
| ES LV volume/BSA (mL/m2) | 18 (5) | 20 (6) |
| EF (%) | 72 (6) | 68 (6) |
| SV (mL) | 78 (17) | 78 (13) |
| SV/BSA (mL/m2) | 46 (8) | 53 (6) |
| ED LV mass (g) | 114 (24) | 108 (21) |
| ED LV mass/BSA (g/m2) | 67 (11) | 67 (10) |
The data are presented as mean (SD).
SSFP, Steady-state free precession; FGRE, fast gradient recalled echo; EF, ejection fraction; SV, stroke volume; BSA, body surface area; ED, end diastolic; ES, end systolic; LV, left ventricular.
FGRE data are from Natori et al (8).
There are some limitations to this study. Fifty subjects were included for analysis for derivation of the correlation curves. We initially analyzed the results after the first 20 subjects, and the results were not significantly different after the additional subjects, so we concluded that the sample size was sufficient. Our methods included the papillary muscles in the ventricular volume rather than as part of the ventricular mass, similar to prior studies and Natori et al. The motivation for this was based on inter- and intrareader studies (not shown) that showed greater reproducibility for this approach. Other laboratories may choose an equally valid method of including the papillary muscles as part of the ventricular mass. A previous study by Vogel-Claussen et al (17) has shown that the size of the papillary muscle has a direct relationship with the size of the LV mass (r: 0.81) and end diastolic volume (r: 0.56). The results of this study showed a smaller difference between the two methods compared to previous studies, since all the scans were acquired on GE scanners; the results of this study may not be applicable to other studies using other scanners.
In conclusion, myocardial mass, volume, and function are related to progression of cardiovascular diseases and are frequently requested for serial testing (2). FGRE cine MRI had been the standard method for evaluation of cardiac function since its introduction for cardiac imaging (18). Changes in MRI technique from FGRE to SSFP will result in altered myocardial mass and volumes independent of the patient’s disease status. The results of this study will allow conversion of previous FGRE data to the more current and reliable SSFP method.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Contract grant sponsor: National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH-NIAMS); Contract grant number: AR050026; Contract grant sponsor: National Heart, Lung, and Blood Institute; Contract grant numbers: N01-HC-95159 through N01-HC-95165 and N01-HC-95168
Footnotes
Published online in Wiley InterScience (www.interscience.wiley.com).
References
- 1.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 2.Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–228. doi: 10.1016/0895-7061(94)00178-E. [DOI] [PubMed] [Google Scholar]
- 3.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 4.Pattynama PM, Lamb HJ, van der Velde EA, van der Wall EE, de Roos A. Left ventricular measurements with cine and spin-echo MR imaging: a study of reproducibility with variance component analysis. Radiology. 1993;187:261–268. doi: 10.1148/radiology.187.1.8451425. [DOI] [PubMed] [Google Scholar]
- 5.Moon JC, Lorenz CH, Francis JM, Smith GC, Pennell DJ. Breath-hold FLASH and FISP cardiovascular MR imaging: left ventricular volume differences and reproducibility. Radiology. 2002;223:789–797. doi: 10.1148/radiol.2233011181. [DOI] [PubMed] [Google Scholar]
- 6.Barkhausen J, Ruehm SG, Goyen M, Buck T, Laub G, Debatin JF. MR evaluation of ventricular function: true fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: feasibility study. Radiology. 2001;219:264–269. doi: 10.1148/radiology.219.1.r01ap12264. [DOI] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 8.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 9.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 10.Carr JC, Simonetti O, Bundy J, Li D, Pereles S, Finn JP. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology. 2001;219:828–834. doi: 10.1148/radiology.219.3.r01jn44828. [DOI] [PubMed] [Google Scholar]
- 11.Plein S, Bloomer TN, Ridgway JP, Jones TR, Bainbridge GJ, Sivananthan MU. Steady-state free precession magnetic resonance imaging of the heart: comparison with segmented k-space gradient-echo imaging. J Magn Reson Imaging. 2001;14:230–236. doi: 10.1002/jmri.1178. [DOI] [PubMed] [Google Scholar]
- 12.Thiele H, Nagel E, Paetsch I, et al. Functional cardiac MR imaging with steady-state free precession (SSFP) significantly improves endocardial border delineation without contrast agents. J Magn Reson Imaging. 2001;14:362–367. doi: 10.1002/jmri.1195. [DOI] [PubMed] [Google Scholar]
- 13.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 14.Thiele H, Paetsch I, Schnackenburg B, et al. Improved accuracy of quantitative assessment of left ventricular volume and ejection fraction by geometric models with steady-state free precession. J Cardiovasc Magn Reson. 2002;4:327–339. doi: 10.1081/jcmr-120013298. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa Y, Sakuma H, Kitagawa K, et al. Evaluation of left ventricular volumes and ejection fraction using fast steady-state cine MR imaging: comparison with left ventricular angiography. J Cardiovasc Magn Reson. 2003;5:333–342. doi: 10.1081/jcmr-120019422. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Stern JS, Mai VM, Pierchala LN, Edelman RR, Prasad PV. MR assessment of left ventricular function: quantitative comparison of fast imaging employing steady-state acquisition (FIESTA) with fast gradient echo cine technique. J Magn Reson Imaging. 2002;16:559–564. doi: 10.1002/jmri.10197. [DOI] [PubMed] [Google Scholar]
- 17.Vogel-Claussen J, Finn JP, Gomes AS, et al. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr. 2006;30:426–432. doi: 10.1097/00004728-200605000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson DJ, Edelman RR. Cineangiography of the heart in a single breath hold with a segmented turboFLASH sequence. Radiology. 1991;178:357–360. doi: 10.1148/radiology.178.2.1987592. [DOI] [PubMed] [Google Scholar]