Abstract
Adenine nucleotide translocase (Ant) mediates the exchange of ADP and ATP across the inner mitochondrial membrane in eukaryotes. Mice possess three distinct but highly homologous Ant isoforms, encoded by independent genes, whose transcription depends upon tissue type. Ant1 is expressed selectively in heart and skeletal muscle, Ant2 is ubiquitously expressed in most tissues but lower in skeletal muscle and testis, while Ant4 is exclusively expressed in the testis. Of interest, each of these Ant genes contains CpG islands in their proximal promoter regions. We investigated the methylation status of the three Ant genes in various tissues with active and inactive transcription. In contrast to the Ant4 gene in which CpG island methylation is essential for gene repression, the CpG islands of Ant1 and Ant2 are hypomethylated regardless of the gene expression status throughout the tissues of male mice. Despite the tissue specific expression profile of Ant1, CpG methylation is unlikely involved in the regulation of the gene. Consistent with these findings, addition of a CpG-demethylating agent, 5-aza-2’-deoxycitidine, to fibroblasts increased the expression of Ant4 but not Ant1 or Ant2 genes. This study provides insight regarding the differential regulation of Ant isoforms in mammals, whereby both the Ant1 and Ant2 genes are capable of expression, but the Ant4 gene is completely repressed throughout somatic tissues. To the best of our knowledge, this is a first example to clearly demonstrate a differential usage of CpG island methylation within a family of genes.
Keywords: Adenine nucleotide translocase, ATP, gene expression, transcription, CpG island, DNA methylation
Introduction
Adenine nucleotide translocase (Ant), also called ADP/ATP carrier (Aac) is expressed on the inner mitochondrial membrane and facilitates the exchange of ADP and ATP between the mitochondria and the cytosol, and thus plays an essential role for energy metabolism in eukaryotes [1-3]. Under aerobic conditions, ATP produced within the mitochondria through oxidative phosphorylation is exported to the cytosol through Ant to support cellular activities. In exchange, ADP is imported through Ant to provide a substrate for the conversion of ADP to ATP by ATP synthase. In contrast, under anaerobic conditions, glycolytic ATP is imported into mitochondria through Ant. This retro-translocation of ATP is considered necessary for the energy-dependent processes of mitochondria and is critical for maintaining the mitochondrial membrane potential and preventing apoptosis mediated by mitochondrial membrane permeabilization [4,5].
Of interest, all eukaryotic species investigated so far, from single cellular yeast to multicellular organisms, have multiple Ant genes. For example, Saccaromyces cerevisiae has Aac1, 2 and 3, among which Aac1 and Aac3 are expressed almost exclusively in aerobic and anaerobic conditions, respectively [6,7]. Thus, the different Ants are likely utilized in order to efficiently cope with varying external oxygen and nutrient conditions in single cellular organisms. Mice have three Ant genes (Ant1, 2 and 4), and their expression differs depending on the tissue [8-11]. Ant1 (also called Aac1, Slc25a4) is predominantly expressed in heart and skeletal muscle, Ant2 (Aac2, Slc25a5) is present in most tissues with its lowest expression in skeletal muscle and testis, while Ant4 (Aac4, Slc25a31) is expressed exclusively in testicular germ cells [8-10]. It is speculated that different Ants, each with unique kinetic properties, may have developed to adapt to the specific energy requirements in particular tissues including heart, skeletal muscle and male germ cells in multicellular organisms [8, 10, 11]. Disruption of Ant1 leads to mitochondrial myopathy, cardiomyopathy, and severe exercise intolerance in mice [12], which confirms the specific role of the gene in heart and skeletal muscle. Ant4 disruption leads to meiotic arrest of male germ cells and male infertility but no apparent abnormalities in somatic tissue or female reproductive organs [10], also confirming the specific role of Ant4 in testis. It should be noted here that non-rodent mammals including human, cow and dog have an additional Ant gene, Ant3, which is encoded at the pseudoautosomal region of X chromosome and is ubiquitously expressed [8,13, 14, 15].
The Ant genes are made up of distinct promoter sequences [16], supporting the view that their expression is under different regulatory controls. However, mechanisms underlying tissue specific expression of the Ant genes are not fully understood. The human ANT1 gene promoter possesses the OXBOX element which is also seen at the ATP Synβ promoter [17,18]. This element acts as a positive regulatory element in muscle cells [19]; hence, the OXBOX might account for the high ANT1 expression in heart and skeletal muscle [8]. However, the other report indicates that the mouse Ant1 gene promoter lacks the OXBOX element, questioning the role of the element in tissue specific expression [20]. Ant2 expression is ubiquitous but growth-dependent [8,21,22]. The GRBOX (glycolysis regulated box) motif, Sp1-binding elements, and NF1 binding site in the Ant2 gene promoter are proposed to regulate Ant2 expression in a cell cycle-dependent manner and also depending on cellular glycolysis status [4,23-25]. Ant4 expression is exclusively detected in male germ cells and is particularly high during meiosis [10]. The Ant4 gene promoter has an E2F6-binding element, which is essential for repression of the Ant4 in somatic tissue [26].
In addition, the Ant4 gene has a CpG island which spans its proximal promoter region to exon 1 [9, 27]. The CpG island of the Ant4 gene is hypomethylated in testis where Ant4 is highly expressed, whereas it is hypermethylated in somatic tissue where Ant4 expression is undetectable [9, 27]. Moreover, the disruption of DNA methyltransferase or the addition of 5-aza-2’-deoxycitidine resulted in a loss of Ant4 repression in somatic cells [9]. These indicate that DNA methylation is essential for Ant4 repression in somatic tissues. Since Ant1 and Ant2 genes also have CpG islands, here we investigated whether CpG island methylation is involved in the tissue-specific gene expression of other Ants.
Materials and methods
CpG island analysis
The promoter regions of Ant1, Ant2, and Ant4 were analyzed for the presence of CpG islands using the MethPrimer program (http://www.urogene.org/methprimer/index1.html). A CpG island is defined as a region of DNA greater than 200 bp, with a GC content above 0.5 and an observed/expected CpG ratio above 0.6 [28]. Briefly, sequences containing 500 bp upstream of the transcription start site and 212 bp downstream, were entered and searched by MethPrimer for CpG islands in Ant1, Ant2, and Ant4. The predictions were then further confirmed by sequence analysis of these regions.
Bisulfite sequencing and combined bisulfite restriction analysis
DNA was extracted from various tissues of adult C57BL/6 mice (~8 weeks) using the DNA Wizard Genomic DNA purification Kit (Promega, Madison, WI). All mice have been maintained under standard specific-pathogen-free (SPF) conditions, and the procedures performed on the mice were reviewed and approved by the University of Florida Institutional Animal Care and Use Committee (IACUC). Testes were decapsulated, and seminiferous tubules were collected for analysis. Bisulfite reactions were performed using the EZ DNA Methylation Kit (Zymo Research, Orange, CA). Up to 2 μg of genomic DNA was used for conversion with the bisulfite reagent. Approximately 80 ng of bisulfite-converted DNA was used as template for each polymerase chain reaction (PCR) analysis. PCR was performed in 25 μl reaction mixtures containing 2 μl of bisulfite converted DNA (50-100ng), 1 μM of primers, 0.625 U of HotStar Taq (Qiagen, Maryland, USA), 200 μM deoxy-nucleotide triphosphates and 0.25x Q-solution. Primers used for combined bisulfite restriction analysis (COBRA) were Ant1: 5’-GGAAGGGGTGGAAGTTTG and 5’-CTAATCCCCCATACTAAAAACC; Ant2: 5’-GGTTTGATTAGGTGTTAAGGGTAAG and 5’-ACATCTATCATATTAAAAACAAAAA; Ant4: 5’-GTAGTATTTGGTTAGAGTGTGTTTTTTGG and 5’-ACACTAAAAAAAACTAAAAAACC (40 cycles). Following PCR amplification, fragments were purified using QIAquick PCR purification kit (Qiagen) and eluted with a final volume of 35 μl. Digestion of PCR purified fragments was then carried out with HhaI as follows: 35 μl eluted PCR purified fragments, 4.5 μl of NEB buffer 4 (New England Biolabs, Beverly, MA), 1.5 μl of HhaI enzyme (New England Biolabs), 0.25 μl of Bovine serum albumin (New England Biolabs), and 3.75 μl water. Digestion was carried out overnight and products were subsequently subjected to electrophoresis at 100V for 30 min. on 2% agarose gels. Primers used for bisulfite sequencing were Ant1: 5’-TGTTTAGGGATTAGTTTAGTTAATG and 5’-CTAATCCCCCATACTAAAAACC; Ant2: 5’-GGTTTGATTAGGTGTTAAGGGTAAG and 5’-ACATCTATCATATTAAAAACAAAAA; Ant4: 5’-TTGTTGTGTATTGATTGAGTATG and 5’-ACACTAAAAAAAACTAAAAAACC. PCR fragments were cloned into pCRII-TOPO cloning vector (Invitrogen, Carlsbad, CA), and individual clones were sequenced.
Real-time RT-PCR
We isolated total RNA from various tissues using the RNAqueous kit (Ambion, Austin, TX). cDNA was synthesized using the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) using random primers. Real-time PCR reactions were performed using the TaqMan Gene Expression Assay (Applied Biosystems) according to manufacturer’s instructions. Each 20 μL reaction consisted of 10 μL of TaqMan Universal PCR Master Mix, No AmpErase-UNG; 1 μL of TaqMan Gene Expression Assay Mix, for β-actin (VIC-labeled), Ant4 (FAM-labeled), Ant2 (FAM-labeled), or Ant1 (FAM-labeled); and 9 μL of cDNA (25 ng). Reactions (40 cycles of 95°C, 15 sec and 60°C, 1 min) were performed using Applied Biosystems 7900HT Fast Real-Time PCR instrument. Gene expression analysis was performed using the comparative cycle threshold (Ct) method using β-actin for normalization. The relative transcript levels was calculated using the formula, 2ˆ(-ΔCt).
Cell culture
NIH3T3 cells (American Type Culture Collection, Manassas, VA) were maintained in Dulbecco’s Modified Eagle Medium (Invitrogen) containing 10% Fetal Bovine Serum. Freshly prepared 5-aza-2’-deoxycytidine (5-aza-dC; Sigma), and/or trichostatin A (TSA; Wako) were added to culture media at the indicated final concentrations.
Results
Identification of CpG islands at the promoter regions of mouse Ant1, Ant2, and Ant4 genes
In order to determine whether Ant1 and Ant2 are regulated by DNA methylation similarly to Ant4, we initially investigated the proximal promoter regions of Ant1, Ant2, and Ant4 for the presence of CpG rich areas known as CpG islands. CpG islands are short stretches of DNA in which the frequency of the CpG sequence is higher than in other regions. The accepted definition of a CpG island is a region of DNA greater than 200 bp, with a GC content above 0.5 and an observed/expected CpG ratio above 0.6 [28]. Figure 1 illustrates murine Ant1, Ant2 and Ant4 genes regions from 500 bp upstream of the predicted transcription initiation site to 212 bp downstream from the transcription initiation site extending into exon 1. Based on the definition, analysis using MethPrimer program revealed that like Ant4, Ant1 and Ant2 also contained discernable CpG islands between their proximal promoter regions and exon1.
Figure 1. CpG islands of murine Ant1, Ant2 and Ant4 genes.
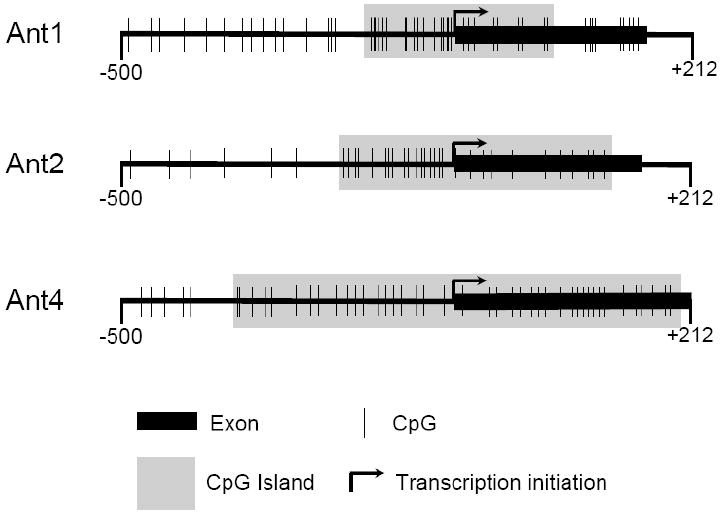
The promoter regions along with exon 1 (-500 to +212) of Ant1, Ant2, and Ant4 were analyzed for the presence of CpG islands using the MethPrimer program. A CpG island is defined as a region of DNA greater than 200 bp, with a GC content above 0.5 and an observed/expected CpG ratio above 0.6.
Analysis of Ant1, Ant2, and Ant4 transcript levels in various tissues
Transcript levels of Ant1, Ant2, and Ant4 in various mouse tissues were then examined using real-time RT-PCR analysis of RNA isolated from male mouse testis, kidney, heart, skeletal muscle, and tail, as well as RNA isolated from female tail. Each tissue was chosen carefully after searching the published data regarding the expression levels of Ant1, 2, and 4 in various tissues [8,9,12]. The goal of the expression analysis was to determine the highest and lowest expressing tissues for each Ant gene. Within the tissues analyzed, Ant1 was expressed highly in heart and skeletal muscle but very low to undetectable expression levels in kidney, liver, tail and testis; Ant2 expression was rather ubiquitously expressed in the tissues examined but lower in skeletal muscle and testis. Ant4 was expressed highly in testis and had no to very low expression in other tissues (Fig. 2).
Figure 2. Tissue specific expression of murine Ant1, Ant2 and Ant4 genes.
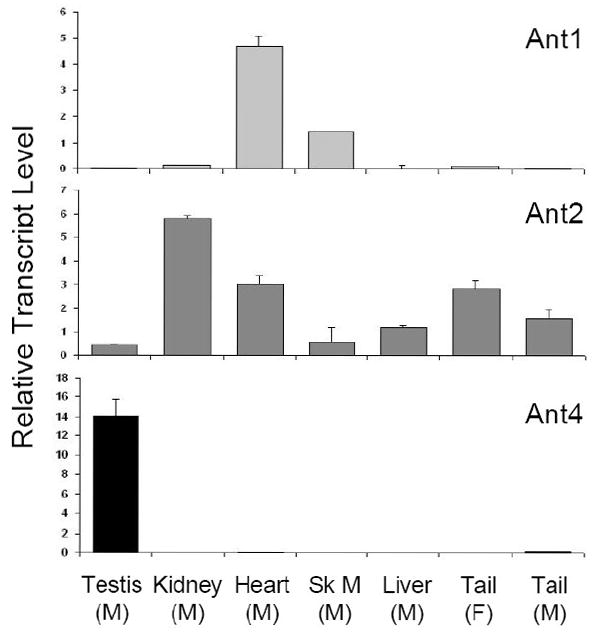
RNA expression of Ant1, Ant2, and Ant4 genes in various tissues of adult C57BL/6 mice (~8 weeks) were analyzed using real-time RT-PCR. Relative expression levels of Ant genes to β-actin using the comparative Ct method are shown. Sk M: Skeletal Muscle, M: Male, F: Female
Methylation analysis of CpG islands of Ant1, Ant2, and Ant4 genes in various tissues
To investigate the methylation status of Ant1, Ant2, and Ant4 genes in various mouse tissues we initially utilized combined bisulfite restriction analysis (COBRA). Using the restriction enzyme HhaI which digests DNA protected from bisulfite conversion by methylation at the sequence GCGC, we were able to quickly determine the methylation status of the promoter regions of Ant1, Ant2, and Ant4 in various tissues. HhaI digestion of Ant1, Ant2, and Ant4 promoter loci revealed an absence of methylation at the Ant1 and Ant2 promoter regions in all male tissues analyzed. Ant4 gene exhibited hypomethylation in testis but hypermethylation in somatic tissues as described previously (Fig. 3) [9]. Interestingly, the Ant2 gene, which is located on the X chromosome, shows a partial methylation pattern in female tissue when analyzed by COBRA. In order to further confirm the methylation status of Ant1, Ant2, and Ant4 genes, we performed bisulfite sequencing analysis. Bisulfite sequencing analysis of the CpG islands was carried out on selected tissues for Ant1, Ant2, and Ant4. For each of Ant1, Ant2, and Ant4 we chose one tissue in which transcript levels were high and also one in which little to no transcript was detectable. This allowed us to determine if there was any differential methylation status present between tissues in which expression of these Ants were present or absent to very low. Bisulfite sequencing analysis of the Ant1 CpG island was performed using heart and liver which express Ant1 at high and very low levels, respectively. For Ant2 analysis, we utilized kidney as the highly expressing tissue and skeletal muscle as the low expressing tissue. We also analyzed female tail to confirm our COBRA data for the partial methylation pattern present. For Ant4, we utilized testis as the tissue expressing the gene and kidney as the tissue with little or no detectable expression. Bisulfite sequencing analysis confirmed our COBRA data that there is no significant increase in methylation at Ant1 and Ant2 CpG islands in all the male tissues examined regardless of gene expression status (Fig 4). Female tail showed hypermethylation of the Ant2 CpG island among approximately half samples examined. In contrast to Ant1 and Ant2, the Ant4 gene demonstrated a distinct methylation profile between tissues with high expression and those without. The Ant4 CpG island was hypomethylated in testis but hypermethylated in kidney, which was consistent with our COBRA data.
Figure 3. CpG methylation of murine Ant1, Ant2 and Ant4 genes (COBRA).
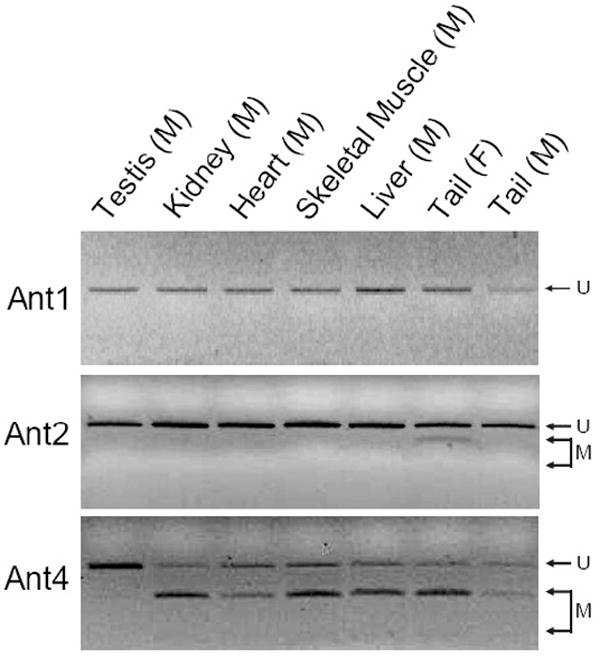
CpG methylation status of Ant1, Ant2, and Ant4 gene promoters in various tissues was analyzed using combined bisulfite restriction analysis (COBRA) as described in Materials and Methods. “U” represents unmethylated DNA fragments, while “M” represents methylated DNA fragments.
Figure 4. CpG methylation of murine Ant1, Ant2 and Ant4 genes (Bisulfite Sequencing).
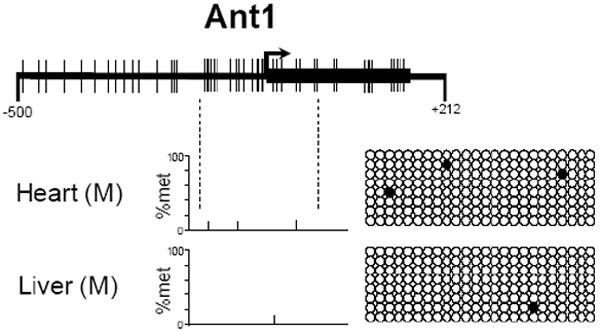
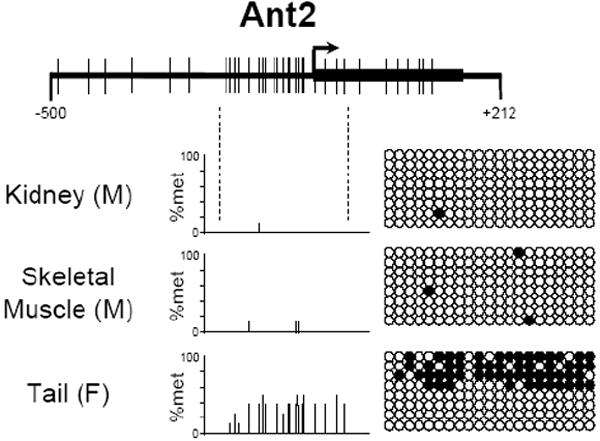
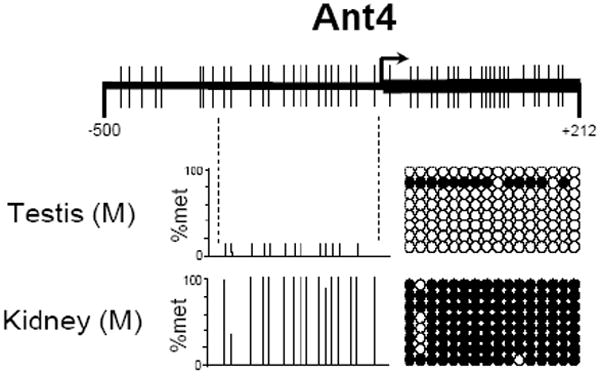
CpG methylation status of (A) Ant1, (B) Ant2, and (C) Ant4 gene promoters in various tissues was analyzed using bisulfite sequencing analysis as described in Materials and Methods. % Methylation (% Met) for each CpG site investigated is demonstrated in the bar graphs. Actual CpG methylation status of the region in eight sequenced clones is also shown to the right of the graph. The open circles represents unmethylated CpG residues, while the closed circles represents methylated CpG residues.
5-aza-2’-deoxycytidine increased the expression of Ant4 but not Ant1 or Ant2 gene in fibroblasts
To further investigate the differential role of CpG methylation in Ant1, Ant2, and Ant4 genes, we examined the effects of DNA demethylation on gene expression. NIH3T3 mouse fibroblast cells were treated with a DNA-demethylating agent, 5-aza-2’-deoxycitidine (5-aza-dC) [29], a histone deacetylase (HDAC) inhibitor, trichostatin A (TSA) [30], or both for 48 hours. As shown in Fig. 5, Ant4 expression was derepressed by 5-aza-dC but not TSA, indicating that DNA methylation, but not histone deacetylation is the primary repression mechanism for the gene. In contrast, Ant1 or Ant2 gene expression was not affected by either 5-aza-dC or TSA, supporting the idea that neither the Ant1 nor the Ant2 gene is likely epigenetically controlled.
Figure 5. The effects of 5-aza-2’-deoxycitidine and trichostatin A on Ant1, Ant2 and Ant4 gene expression.
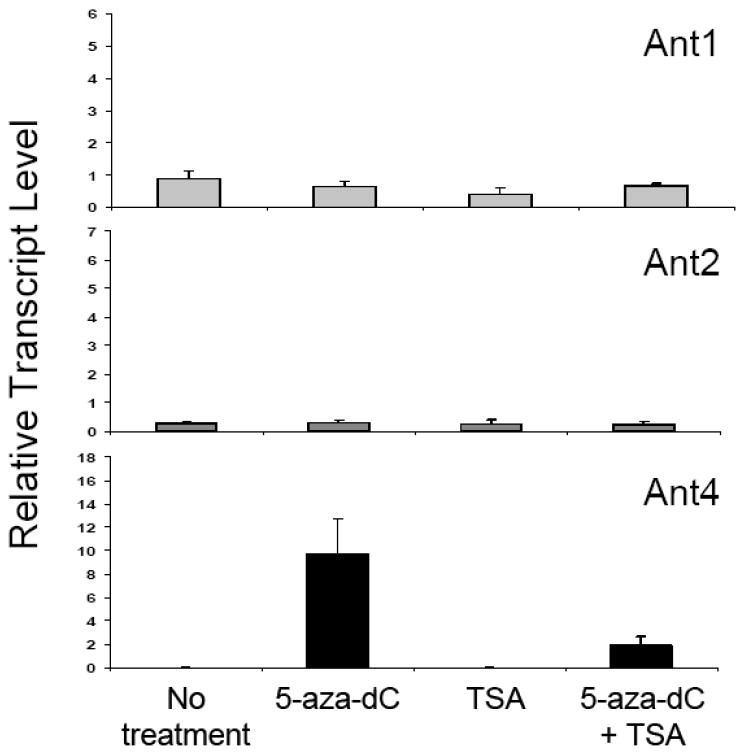
NIH3T3 mouse fibroblasts were treated with 5-aza-2’-deoxycitidine (5-aza-dC, 5 μM), trichostatin A (TSA, 200 nM) or both for 48 hrs. RNA expression of Ant1, Ant2, and Ant4 genes was analyzed using real-time RT-PCR as described above in Fig. 2. Relative expression levels of Ant genes compared to β-actin using the comparative Ct method are shown. The mean values for the Ant4 transcript are: No treatment (1.21 × 10-3), 5-aza-dC (9.71), TSA (1.53 × 10-3) and 5-aza-dC+TSA (1.89).
Discussion
Our data demonstrate that all mouse Ant genes: Ant1, Ant2, Ant4, similarly posses CpG islands spanning their proximal promoter regions to first exons. However, their methylation status was dissimilar between the family of genes. Ant1 and Ant2 promoter loci showed no differential methylation pattern throughout tissues regardless of their gene expression status (Table 1). This is in contrast to the Ant4 gene which is hypermethylated in tissues lacking expression. Consistent with these findings, addition of a CpG-demethylating agent, 5-aza-deoxycitidine, to fibroblasts increased gene expression of Ant4 but not Ant1 or Ant2. The present data indicate that, unlike Ant4, DNA methylation does not likely play a role in tissue specific expression of Ant1 and Ant2. To the best of our knowledge, this is a first example to clearly demonstrate a differential usage of CpG island methylation within a family of genes.
Table 1.
| Ant1 | Ant2 | Ant4 | ||||
|---|---|---|---|---|---|---|
| Met | Exp | Met | Exp | Met | Exp | |
| Testes (M) | ○ | - | ○ | ± | ○ | ++ |
| Kidney (M) | ○ | - | ○ | ++ | ● | - |
| Heart (M) | ○ | ++ | ○ | + | ● | - |
| Sk M(M) | ○ | + | ○ | ± | ● | - |
| Liver (M) | ○ | - | ○ | + | ● | - |
| Tail (F) | ○ | - | ◑ | + | ● | - |
| Tail (M) | ○ | - | ○ | + | ● | - |
Met - Methylation Status
○ - unmethylated
● - methylated
◑ - mixture
Exp - Expression level
++ high
+ intermediate
± low
- very low to absent
The state of CpG methylation is believed to regulate chromatin structure and the accessibility of transcriptional machinery to gene promoters [31]. High CpG content is enriched at the proximal promoter regions of genes, and in particular most of the “housekeeping” genes have CpG islands at their promoters [32]. In contrast, genes associated with more specific functions and those expressed in tissue-specific manner typically have low CpG content in their promoters [32]. In this context, in contrast to the previous data that Ant(s) are expressed in a tissue-dependent manner, Ant1 and Ant2 genes may be more categorized with the “housekeeping” side as are most other mitochondrial protein-encoding genes which have high CpG content at the promoters [32]. Although tissue-selective expression is seen to a degree particularly with Ant1, it may not be necessary to completely repress the gene in other tissues. In eukaryotic evolution, it is thought that different Ants have developed to cope with differential external oxygen and nutrient conditions [33]. Human ANT1 gene contains the OXBOX/REBOX element while both human ANT2 and mouse Ant2 contain the GRBOX element in their promoters [17-20], indicating that the genes are likely still under the control of external oxygen and nutrient conditions. Thus, tissue selective Ant1 and Ant2 expression may not be absolute but more flexible in each tissue. Leaving the gene capable of expression may be important for the whole body to cope with various external conditions. The present data are also consistent with the notion that the X-linked Ant2 gene is methylated in one allele in female tissues due to somatic X chromosome inactivation (see review, for example, [34]). This methylation, due to X chromosome inactivation, is unlikely to be regulating expression of Ant2.
In contrast to Ant1 and Ant2, the Ant4 gene appears to follow the DNA methylation pattern of germ cell-specific genes. As seen in many other germline genes [35,36], the Ant4 CpG island becomes methylated during early embryonic development [9] and is completely hypermethylated in somatic tissues. The reason for complete repression of Ant4 in somatic tissue is currently unknown. Human ANT4 was shown to have very unique affinity for and kinetics of ADP/ATP exchange when compared to somatic ANTs [11], which may render Ant4 expression detrimental to somatic tissues. Alternatively, the Ant4 gene may be under the control of a yet-unrevealed universal gene repression mechanism common to germline genes in soma, and repression of the Ant4 gene itself may not be critical in somatic tissues. In conclusion, the present study provided us with further insights regarding differential regulation of Ant isoforms in mammals, which would help increase the understanding of how the genes developed throughout evolution and currently function in mammals.
Acknowledgments
We thank Drs. Keith D. Robertson, James L. Resnick, (U. Florida) and members of Terada’s lab for critical reading of the manuscript. This work was supported in part by National Institutes of Health grants, DK59699 and RR17001 (to N. T.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klingenberg M. Molecular aspects of the adenine nucleotide carrier from mitochondria. Arch Biochem Biophys. 1989;270:1–14. doi: 10.1016/0003-9861(89)90001-5. [DOI] [PubMed] [Google Scholar]
- 2.Fiore C, Trézéguet V, Le Saux A, Roux P, Schwimmer C, Dianoux AC, Noél F, Lauquin GJ, Brandolin G, Vignais PV. The mitochondrial ADP/ATP carrier: structural, physiological and pathological aspects. Biochimie. 1998;80:137–150. doi: 10.1016/s0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DR, Felix CM, Swanson JM. Highly conserved charge-pair networks in the mitochondrial carrier family. J Mol Biol. 1998;277:285–308. doi: 10.1006/jmbi.1997.1594. [DOI] [PubMed] [Google Scholar]
- 4.Giraud S, Bonod-Bidaud C, Wesolowski-Louvel M, Stepien G. Expression of human ANT2 gene in highly proliferative cells: GRBOX, a new transcriptional element, is involved in the regulation of glycolytic ATP import to mitochondria. J Mol Biol. 1998;281:409–418. doi: 10.1006/jmbi.1998.1955. [DOI] [PubMed] [Google Scholar]
- 5.Chevrollier A, Loiseau D, Chabi B, Renier G, Douay O, Malthiery Y, Stepien G. ANT2 isoform required for cancer cell glycolysis. J Bioenerg Biomembr. 2005;37:307–316. doi: 10.1007/s10863-005-8642-5. [DOI] [PubMed] [Google Scholar]
- 6.Kolarov J, Kolarova N, Nelson N. A third ADP/ATP translocator gene in yeast. J Biol Chem. 1990;265:12711–12716. [PubMed] [Google Scholar]
- 7.Sabova L, Zeman I, Supek F, Kolarov J. Transcriptional control of AAC3 gene encoding mitochondrial ADP/ATP translocator in Saccharomyces cerevisiae by oxygen, heme and ROX1 factor. Eur J Biochem. 1993;213:547–553. doi: 10.1111/j.1432-1033.1993.tb17793.x. [DOI] [PubMed] [Google Scholar]
- 8.Stepien G, Torroni A, Chung AB, Hodge JA, Wallace DC. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem. 1992;267:14592–14597. [PubMed] [Google Scholar]
- 9.Rodić N, Oka M, Hamazaki T, Murawski MR, Jorgensen M, Maatouk DM, Resnick JL, Li E, Terada N. DNA methylation is required for silencing of Ant4, an adenine nucleotide translocase selectively expressed in mouse embryonic stem cells and germ cells. Stem Cells. 2005;23:1314–1323. doi: 10.1634/stemcells.2005-0119. [DOI] [PubMed] [Google Scholar]
- 10.Brower JV, Rodić N, Seki T, Jorgensen M, Fliess N, Yachnis AT, McCarrey J, Oh SP, Terada N. Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem. 2007;282:29658–29666. doi: 10.1074/jbc.M704386200. [DOI] [PubMed] [Google Scholar]
- 11.Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005;579:633–637. doi: 10.1016/j.febslet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 13.Houldsworth J, Attardi G. Two distinct genes for ADP/ATP translocase are expressed at the mRNA level in adult human liver. Proc Natl Acad Sci USA. 1988;85:377–381. doi: 10.1073/pnas.85.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiebel K, Weiss B, Wöhrle D, Rappold G. A human pseudoautosomal gene, ADP/ATP translocase, escapes X-inactivation whereas a homologue on Xq is subject to X-inactivation. Nat Gen. 1993;3:82–97. doi: 10.1038/ng0193-82. [DOI] [PubMed] [Google Scholar]
- 15.Dietzel S, Stiegel K, Little G, Edelmann P, Rappold GA, Eils R, Cremer C, Cremer T. The 3D positioning of ANT2 and ANT3 genes within female X chromosome territories correlates with gene activity. Exp Cell Res. 1999;252:363–375. doi: 10.1006/excr.1999.4635. [DOI] [PubMed] [Google Scholar]
- 16.Ku D, Kagan J, Chen S, Chang C, Baserga R, Wurzel J. The human fibrobroblast adenine nucleotide translocator gene: Molecular cloning and sequence. J Biol Chem. 1990;265:16060–16063. [PubMed] [Google Scholar]
- 17.Li K, Warner CK, Hodge JA, Minoshima S, Kudoh J, Fukuyama R, Maekawa M, Shimizu Y, Shimizu N, Wallace DC. A human muscle adenine nucleotide translocator gene has four exons, is located on chromosome 4, and is differentially expressed. J Biol Chem. 1989;264:13998–14004. [PubMed] [Google Scholar]
- 18.Neckelmann N, Warner CK, Chung A, Kudoh J, Minoshima S, Fukuyama R, Maekawa M, Shimizu Y, Shimizu N, Liu JD, Wallace DC. The human ATP synthase beta subunit gene: sequence analysis, chromosome assignment, and differential expression. Genomics. 1989;5:829–843. doi: 10.1016/0888-7543(89)90125-0. [DOI] [PubMed] [Google Scholar]
- 19.Li K, Hodge JA, Wallace DC. OXBOX, a positive transcriptional element of the heart-skeletal muscle ADP/ATP translocator gene. J Biol Chem. 1990;265:20585–20588. [PubMed] [Google Scholar]
- 20.Levy SE, Chen YS, Graham BH, Wallace DC. Expression and sequence analysis of the mouse adenine nucleotide translocase 1 and 2 genes. Gene. 2000;254:57–66. doi: 10.1016/s0378-1119(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 21.Battini R, Ferrari S, Kaczmarek L, Calabretta B, Chen ST, Baserga R. Molecular cloning of a cDNA for a human ADP/ATP carrier which is growth-regulated. J Biol Chem. 1987;262:4355–4359. [PubMed] [Google Scholar]
- 22.Barath P, Luciakova K, Hodny Z, Li R, Nelson BD. The growth-dependent expression of the adenine nucleotide translocase-2 (ANT2) gene is regulated at the level of transcription and is a marker of cell proliferation. Exp Cell Res. 1999;248:583–588. doi: 10.1006/excr.1999.4432. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Hodny Z, Luciakova K, Barath P, Nelson BD. Sp1 activates and inhibits transcription from separate elements in the proximal promoter of the human adenine nucleotide translocase 2 (ANT2) gene. J Biol Chem. 1996;271:18925–18930. doi: 10.1074/jbc.271.31.18925. [DOI] [PubMed] [Google Scholar]
- 24.Barath P, Poliakova D, Luciakova K, Nelson BD. Identification of NF1 as a silencer protein of the human adenine nucleotide translocase-2 gene. Eur J Biochem. 2004;271:1781–1788. doi: 10.1111/j.1432-1033.2004.04090.x. [DOI] [PubMed] [Google Scholar]
- 25.Luciakova K, Kollarovic G, Barath P, Nelson BD. Growth-dependent repression of human adenine nucleotide translocator-2 (ANT2) transcription: evidence for the participation of Smad and Sp family proteins in the NF1-dependent repressor complex. Biochem J. 2008;412:123–130. doi: 10.1042/BJ20071440. [DOI] [PubMed] [Google Scholar]
- 26.Kehoe SM, Oka M, Hankowski KE, Reichert N, Garcia S, McCarrey JR, Gaubatz S, Terada N. A conserved E2F6-binding element in murine meiosis-specific gene promoters. Biol Reprod. 2008 doi: 10.1095/biolreprod.108.067645. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki M, Sato S, Arai Y, Shinohara T, Tanaka S, Greally JM, Hattori N, Shiota K. A new class of tissue-specifically methylated regions involving entire CpG islands in the mouse. Genes Cells. 2007;12:1305–1314. doi: 10.1111/j.1365-2443.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 28.Gardiner-Garden M, Frommer M. CpG Islands in vertebrate genomes. J Mol Biol. 1987;196:261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 29.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 31.Bird AP, Wolffe AP. Methylation-induced repression--belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 32.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci USA. 2006;103:1412–1417. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santamaria M, Lanave C, Saccone C. The evolution of adenine nucleotidetranslocase family. Gene. 2004;222:51–59. doi: 10.1016/j.gene.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Chow JC, Yen Z, Ziesche SM, Brown CJ. Silencing of the mammalian X chromosome. Annu Rev Genomics Hum Genet. 2005;6:69–92. doi: 10.1146/annurev.genom.6.080604.162350. [DOI] [PubMed] [Google Scholar]
- 35.Shen L, Kondo Y, Guo Y, Zhang J, Zhang LL, Ahmed S, Shu J, Chen X, Waterland RA, Issa JP. Genome-wide profiling of DNA methylation reveals a class of normally methylated CpG island promoters. PLoS Genet. 2007;3:2023–2036. doi: 10.1371/journal.pgen.0030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]


