Abstract
Objective
The serum C-reactive protein (CRP) concentration is commonly used in rheumatoid arthritis (RA) as a surrogate marker of systemic inflammation, presumably induced by synovitis. However, other tissues, such as adipose tissue, can induce CRP production. This study was undertaken to explore the associations between measures of adiposity and CRP levels in RA.
Methods
One hundred ninety-six men and women with RA underwent anthropometric assessment and total body dual-energy x-ray absorptiometry for measurement of total and regional body fat and lean mass. The associations between measures of fat and lean mass and serum levels of CRP and interleukin-6 (IL-6) were determined in analyses stratified by sex, with adjustment for pertinent demographic, lifestyle, and RA disease and treatment covariates as well as for the potential modifying effects of articular activity and biologic pharmacotherapeutic agents.
Results
All measures of adiposity were significantly associated with the level of CRP in women, but not in men. In women, the measure of adiposity that showed the strongest association with the CRP level was truncal fat, in which, in adjusted analyses, each kilogram increase was associated with a 0.101-unit increase in the logarithmically transformed CRP level (P < 0.001). Neither the level of articular activity nor the use of biologic agents significantly modified this association in women. However, in men, elevated articular involvement was associated with a decreasing CRP level as truncal fat increased. For all analyses, substitution of IL-6 for CRP produced similar findings.
Conclusion
Adiposity is independently associated with CRP levels in women with RA, and thus may confound the estimation of RA disease activity when serum CRP concentration is used as a surrogate for systemic inflammation.
Over the last few decades, the serum C-reactive protein (CRP) concentration has been widely adopted as a marker of systemic inflammation. In rheumatoid arthritis (RA), the level of CRP is frequently used in conjunction with assessments of articular swelling and tenderness to estimate the level of disease activity and, in addition to the erythrocyte sedimentation rate, is a component of the American College of Rheumatology core set for measuring clinical response in RA trials (1), is a component of the Disease Activity Score in 28 joints (DAS28) (2), and has been proposed as a biomarker of structural damage in RA trials (3).
CRP is a pentamer of 23-kd subunits that is synthesized and secreted by hepatocytes when stimulated by a variety of inflammatory cytokines, including tumor necrosis factor α (TNFα), interleukin-1 (IL-1), and especially IL-6 (4). Under these conditions, serum CRP levels can rise ≥100-fold over baseline levels (4). In addition to being a sensitive surrogate marker of the acute-phase response, an increasing level of CRP may also have direct downstream proinflammatory effects, including activation of complement and induction of tissue factor (5).
In RA, inflamed synovium and circulating monocytes are sources of the cytokines that induce CRP production. However, other tissues are known sources of these cytokines. Adipose tissue is a potent source of inflammatory cytokines, including TNFα and IL-6, that induce hepatic production of CRP. In studies of the general population, an association between body fat mass and circulating CRP levels has been confirmed (6). In particular, visceral adipose tissue, distributed within the intraabdominal cavity and surrounding the mesentery and omentum, has been shown to be the adipose depot with the greatest contribution to systemic inflammation (7). Interventions that reduce adipose tissue mass (e.g., weight loss) are associated with a decline in CRP production (8).
To investigate the potential contribution of adiposity to CRP levels in RA, we compared the relative contributions of anthropometric and dual-energy x-ray absorptiometry (DXA)–derived measures of body fat, along with RA disease characteristics, demographic and lifestyle factors, and cardiovascular risk factors, to the serum CRP concentration in men and women with RA. We also investigated whether the levels of articular swelling and tenderness modified the association between body fat and CRP.
PATIENTS AND METHODS
Study subjects
Subjects were participants in the ESCAPE RA trial (Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis), a cohort study investigating the prevalence, progression, and risk factors for subclinical cardiovascular disease in RA. Subjects were included if they met the American College of Rheumatology (formerly, the American Rheumatism Association) 1987 classification criteria for RA (9) and were 45–84 years of age. Subjects were excluded if they reported any physician-diagnosed cardiovascular events prior to enrollment, including myocardial infarction, congestive heart failure, coronary artery bypass grafting, angioplasty (with or without arterial stent placement), peripheral vascular disease or peripheral arterial vascular procedures, implanted pacemaker or defibrillator devices, and current atrial fibrillation. Subjects weighing more than 300 pounds were also excluded due to the weight limitations of the cardiovascular imaging equipment used in the study.
ESCAPE RA participants were recruited from among patients being followed up at the Johns Hopkins Arthritis Center, and by referral from local rheumatologists. The study was approved by the Institutional Review Board of the Johns Hopkins Hospital, with all subjects providing written informed consent prior to enrollment. Enrollment began in October 2004 and concluded in May 2006.
Assessments
Body composition
Subjects underwent total body DXA scanning on a Lunar Prodigy DXA scanner (GE/Lunar Radiation, Madison, WI), with results analyzed using Prodigy software (version 05.60.003). DXA was used to analyze and measure body fat, lean mass, and bone mass in the total body (minus the head) and per body region (arms, legs, and trunk). Automated quantification of the total and regional body tissue masses, using the manufacturer’s validated software, was in real-time, with the entire acquisition and analysis process being accomplished by the scanner and technician at the time of subject scanning. Each subject was scanned on the same DXA scanner, and quality control and calibration procedures were performed daily in accordance with the standard protocol provided by the manufacturer.
All subjects underwent measurements of height (in cm) and weight (in kg) with a wall-mounted stadiometer and a Detecto scale, respectively, with the subject placed in a standing position and wearing light indoor clothing and no shoes. Body mass index (BMI) was calculated as body weight (in kg) divided by height (in m2). Waist and hip circumferences were measured with a Gulick II anthropometric measuring tape, using a standard protocol. Waist circumference was measured at the level of the umbilicus, and hip circumference was measured at the maximal circumference of the buttocks.
Other characteristics
Demographic, lifestyle, and RA disease characteristics were assessed on the same day as the body composition assessment. Each of these characteristics was determined by trained examiners using a structured interview in formalized questionnaires.
Disease characteristics
Forty-four joints were examined by a single trained assessor for swelling, tenderness, deformity, and surgical replacement or fusion. The duration of RA was assessed by patient self-report from the date of diagnosis. The severity of RA was estimated using the sum of the number of joints exhibiting deformity or requiring surgical replacement/fusion (10). RA disease activity was calculated using the DAS28 with the CRP measurement included (2). For analyses with CRP as the dependent variable, disease activity was estimated using the swollen joint count or the sum of the swollen joint count and the tender joint count (swollen plus tender joints). Functional limitation was assessed with the Stanford Health Assessment Questionnaire (HAQ) (11), modified to include a subcategory score for subjects who reported requiring assistance from others or using assistive devices. Current use and past use of glucocorticoids and biologic and nonbiologic disease-modifying antirheumatic drugs (DMARDs) were ascertained by detailed, examiner-administered questionnaires.
Lifestyle characteristics
Subjects were classified as current smokers if they reported a current habit of smoking and the total number of cigarettes smoked in their lifetime was at least 100. Physical activity was assessed using the 7-day Physical Activity Recall questionnaire (12). Subjects were classified as engaging in regular exercise if the daily average of the weekly total of self-reported physical activity for intentional exercise activities (moderate or brisk walking for exercise, and moderate or vigorous individual or team sports and conditioning activities) amounted to ≥30 minutes per day on ≥5 days per week, consistent with current recommendations for physical activity (13). In addition, subjects reported the duration of daily television watching, a measure of sedentary activity.
Cardiovascular risk factors
Subjects were classified as having diabetes if the serum fasting glucose level was ≥126 mg/dl or they were currently taking medications for diabetes. Subjects were classified as having dyslipidemia if the serum concentration of high-density lipoprotein (HDL) cholesterol was <50 mg/dl for women or <40 mg/dl for men, if the serum triglyceride concentration was >150 mg/dl, or if the subject was taking lipid-lowering medications.
Laboratory assessments
The CRP level was determined with a high-sensitivity nephelometry method (Dade Behring, Deerfield, IL), and IL-6 was determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT) from fasting morning samples. The serum glucose level was measured by rate reflectance spectrophotometry, levels of HDL cholesterol were measured using the cholesterol oxidase method (Roche Diagnostics, Laval, Quebec, Canada), and triglyceride levels were determined using Triglyceride GB reagent (Roche Diagnostics) at the Collaborative Studies Clinical Laboratory at Fairview University Medical Center (Minneapolis, MN). The presence of rheumatoid factor (RF) was assessed using ELISA, in the clinical laboratory of the Johns Hopkins Hospital (Baltimore, MD), with RF seropositivity defined as ≥40 units.
Statistical analysis
In groups stratified by sex, continuous variables were compared using the unpaired t-test, with results expressed as the mean ± SD. For comparison of nonnormally distributed variables, the Kruskal-Wallis test was used, with results expressed as the median and interquartile range. For categorical variables (swollen and tender joint counts and percentages), results were compared using the chi-square goodness-of-fit test or Fisher’s exact test, as appropriate.
Simple linear regression models were constructed to explore the univariate associations between the log CRP (i.e., the CRP level logarithmically transformed to normality using the natural log) and subjects’ demographic, lifestyle, RA disease, and body composition characteristics, in analyses stratified by sex. Adjustment for height was performed in all regression analyses involving lean or fat mass, either total or truncal. Since truncal fat was determined to be the body composition component with the greatest association with the log CRP, truncal fat mass was retained as the independent variable of interest in subsequent multivariable modeling.
Multivariable linear regression models were then used to further explore the association between the log CRP and truncal fat mass, with adjustments for pertinent demographic and RA disease characteristics. A simple model (model 1) was constructed using only variables that were significantly associated with the dependent variable in univariate comparisons. A second model (model 2) was constructed using the independent variable of interest (truncal fat) adjusted for demographic, RA disease, and treatment characteristics only. A third model (model 3) included further adjustment for lifestyle characteristics, cardiovascular risk factors, physical activity, and menopausal status (in women only). The total variability in log CRP explained by the variables in the model was estimated using the coefficient of determination (R2). To corroborate the findings observed, analyses were repeated using IL-6 instead of CRP as the dependent variable, in analyses using the same protocols as described above.
We next investigated whether the association of the log CRP with truncal fat differed according to the level of disease activity. Because the CRP is a core component of the DAS index, swollen joint counts or the sum of swollen plus tender joint counts were used as alternate definitions of disease activity. The swollen joint counts and swollen plus tender joint counts were plotted against the log CRP to determine the level of articular disease activity at which a significant association with the CRP was obtained, yielding cutoff values (for “high” versus “low” articular disease activity, both in men and in women in this sample) of 10 swollen joints and 16 swollen plus tender joints. Statistical interaction models were constructed to assess whether elevated counts of swollen joints or of swollen plus tender joints significantly modified the log CRP–truncal fat relationship, in analyses stratified by sex. Similar interaction models were constructed according to current use of biologic DMARDs.
Statistical calculations were performed using Inter-cooled Stata 9 (StataCorp, College Station, TX). In all tests, a 2-tailed α value of 0.05 was defined as the level of statistical significance.
RESULTS
Comparison of subjects’ characteristics by sex
One-hundred ninety-six subjects (118 women and 78 men) were studied. The characteristics of the subjects at baseline are summarized in Table 1. Compared with male subjects, female subjects had significantly higher swollen joint counts, tender joint counts, HAQ scores, and total joint deformity. The median levels of CRP did not differ significantly between the sexes. More female subjects than male subjects were receiving treatment with biologic DMARDs; however, current use of glucocorticoids and nonbiologic DMARDs did not differ by sex. Although the mean BMI and the distribution of subjects among the 3 BMI categories did not differ by sex, female subjects had significantly higher total fat mass and a significantly higher body fat percentage compared with male subjects. Despite these differences, the truncal fat mass did not differ significantly between the sexes.
Table 1.
Characteristics of the study subjects*
| Characteristic | Male (n = 78) | Female (n = 118) | P |
|---|---|---|---|
| Demographic | |||
| Age, mean ± SD years | 59.3 ± 9.9 | 59.5 ± 7.8 | 0.90 |
| Caucasian | 69 (88.5) | 101 (85.6) | 0.73 |
| Current smoker | 13 (16.7) | 10 (8.5) | 0.087 |
| Diabetes | 7 (9.0) | 6 (5.1) | 0.28 |
| Dyslipidemia | 50 (64.1) | 59 (50.0) | 0.052 |
| RA | |||
| Duration of RA, mean ± SD years | 11.6 ± 10.8 | 13.0 ± 10.5 | 0.38 |
| Swollen joint count (range 0–42), mean ± SD† | 6.0 ± 4.9 | 8.3 ± 5.0 | 0.002 |
| Tender joint count (range 0–44), mean ± SD† | 7.0 ± 8.7 | 10.7 ± 9.5 | 0.006 |
| CRP, mg/liter | |||
| Mean ± SD | 8.04 ± 17.06 | 6.57 ± 11.90 | 0.48 |
| Median (IQR) | 2.46 (1.09–7.80) | 2.62 (1.18–7.00) | 0.98 |
| IL-6, median (IQR) pg/ml | 4.06 (1.75–8.38) | 3.87 (1.79–7.52) | 0.85 |
| HAQ score (range 0–3), mean ± SD† | 0.55 ± 0.67 | 1.02 ± 0.74 | <0.001 |
| Joint deformity (range 0–42), mean ± SD† | 3.8 ± 6.4 | 6.1 ± 7.9 | 0.035 |
| Current glucocorticoid use | 31 (39.7) | 53 (44.9) | 0.43 |
| Current nonbiologic DMARD use | 67 (85.9) | 98 (83.1) | 0.84 |
| Current biologic DMARD use | 27 (34.6) | 62 (53.0) | 0.009 |
| Body composition | |||
| Body mass index | |||
| Mean ± SD kg/m2 | 28.6 ± 4.9 | 28.2 ± 5.6 | 0.57 |
| Distribution by category | |||
| <25 kg/m2 | 18 (23.1) | 38 (32.2) | 0.35 |
| 25–29.99 kg/m2 | 31 (39.7) | 42 (35.6) | |
| ≥30 kg/m2 | 30 (38.5) | 38 (32.2) | |
| Body fat | |||
| Body fat percentage, mean ± SD | 30.4 ± 7.7 | 42.2 ± 7.1 | <0.001 |
| Total fat mass, mean ± SD kg | 27.4 ± 10.2 | 31.5 ± 10.7 | 0.009 |
| Truncal fat mass, mean ± SD kg | 16.6 ± 6.4 | 16.0 ± 6.1 | 0.48 |
| Total lean mass, mean ± SD kg | 57.1 ± 8.2 | 39.3 ± 6.9 | <0.001 |
| Physical activity | |||
| Intentional exercise >30 minutes per day | 44 (56.4) | 61 (51.7) | 0.52 |
| Sedentary activity, mean ± SD hours‡ | 2.5 ± 1.7 | 2.2 ± 1.5 | 0.27 |
Except where indicated otherwise, values are the number (%) of subjects. RA = rheumatoid arthritis; CRP = C-reactive protein; IQR = interquartile range; IL-6 = interleukin-6; HAQ = Health Assessment Questionnaire; DMARD = disease-modifying antirheumatic drug.
The joints evaluated for tenderness, swelling, and deformity were as follows: 10 metacarpal phalangeals, 10 proximal interphalangeals, 2 wrists, 2 elbows, 2 shoulders, 2 knees, 2 tibiotalar, 2 subtalar, and 10 metatarsal phalangeals. Two hips were examined for tenderness.
Defined as duration of daily television watching.
Association of adiposity with levels of CRP and levels of IL-6 in the RA cohorts by sex
Univariate associations of subjects’ characteristics with the log CRP are summarized in Table 2. Among male subjects, significant univariate associations with the log CRP were observed for increasing age, increasing duration of RA, higher swollen joint counts, and greater total joint deformity. Moreover, the current use of biologic DMARDs was observed to have a significant negative association with the log CRP in men. However, no measure of body composition was significantly associated with the log CRP in men.
Table 2.
Univariate regression coefficients for associations of the log CRP with clinical and demographic characteristics among male and female subjects*
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Characteristic | β | P | R2 | β | P | R2 |
| Demographic | ||||||
| Age | 0.037 | 0.017 | 0.073 | −0.002 | NS | <0.001 |
| Caucasian | −0.547 | NS | 0.018 | −0.722 | 0.043 | 0.035 |
| Current smoker | 0.218 | NS | 0.004 | −0.299 | NS | 0.004 |
| Diabetes | 0.175 | NS | 0.001 | −0.529 | NS | 0.007 |
| Dyslipidemia | 0.549 | NS | 0.024 | 0.362 | NS | 0.150 |
| RA | ||||||
| Duration of RA, years | 0.049 | <0.001 | 0.142 | 0.017 | NS | 0.008 |
| Swollen joint count | 0.078 | 0.014 | 0.076 | 0.020 | NS | 0.005 |
| Tender joint count | 0.010 | NS | 0.004 | −0.015 | NS | 0.012 |
| HAQ score | 0.363 | NS | 0.029 | 0.224 | NS | 0.015 |
| Joint deformity | 0.049 | 0.043 | 0.053 | 0.024 | NS | 0.019 |
| Glucocorticoid use | 0.101 | NS | 0.012 | 0.015 | NS | 0.009 |
| Nonbiologic DMARD use | 0.169 | NS | 0.002 | 0.031 | NS | <0.001 |
| Biologic DMARD use | −0.696 | 0.032 | 0.059 | −0.322 | NS | 0.014 |
| Body composition | ||||||
| Weight | −0.002 | NS | 0.004 | 0.013 | <0.001 | 0.104 |
| Body mass index† | −0.033 | NS | 0.014 | 0.096 | <0.001 | 0.152 |
| Body fat percentage | −0.006 | NS | 0.001 | 0.084 | <0.001 | 0.192 |
| Total fat mass† | −0.006 | NS | 0.002 | 0.052 | <0.001 | 0.164 |
| Truncal fat mass† | −0.023 | NS | 0.024 | 0.106 | <0.001 | 0.226 |
| Total lean mass† | −0.036 | NS | 0.042 | 0.041 | 0.062 | 0.039 |
| Physical activity | ||||||
| Daily exercise | −0.256 | NS | 0.009 | −0.684 | 0.006 | 0.063 |
| Hours of sedentary activity | 0.092 | NS | 0.012 | 0.189 | 0.027 | 0.041 |
Values for the regression coefficient represent the unit change in the log C-reactive protein (CRP) level (logarithmically transformed to normality using the natural log) per unit change in characteristic (for age, duration of rheumatoid arthritis [RA], swollen joint count, tender joint count, Health Assessment Questionnaire [HAQ] score, joint deformity, all body composition measures, and hours of sedentary activity) or the difference in the mean CRP level for subjects with versus those without the characteristic (for Caucasian, current smoker, diabetes, dyslipidemia, use of glucocorticoids or of either type of disease-modifying antirheumatic drugs [DMARDs], or daily exercise). Sedentary activity is defined as duration of daily television watching. Statistically significant interactions of sex in the body composition–log CRP association were detected in all univariate and multivariate models; thus, all analyses are presented stratified by sex. R2 = coefficient of determination; NS = not statistically significant.
Regression coefficient adjusted for height.
Among female subjects, the only demographic characteristic significantly associated with the log CRP was Caucasian race, in which there was a mean difference of 2.06 mg/liter between Caucasians and non-Caucasians, with Caucasians having a lower mean CRP level. In women, no RA disease characteristic or treatment characteristic was significantly associated, either positively or negatively, with the log CRP. In contrast, all measures of adiposity were highly associated with the log CRP in women, with truncal fat demonstrating the greatest magnitude of association. Unlike that observed in male subjects, women were observed to have significant associations between physical activity factors and the log CRP, with higher frequency of regular exercise having a negative association with the outcome and greater sedentary activity having a positive association with the outcome.
The results from multivariable models exploring the associations between truncal fat mass and the log CRP are summarized in Table 3 (listed for women only, since no measure of adiposity was significantly associated with the CRP levels in men). In the simplest model (model 1), which was adjusted for only the covariates that showed significant univariate associations with the log CRP, each kilogram increase in truncal fat mass was associated with a 0.098-unit increase in the log CRP (P < 0.001). This relationship was maintained in the model that was adjusted for demographics, height, and RA disease and treatment characteristics (model 2), in which each kilogram increase in truncal fat mass was associated with a 0.109-unit increase in the log CRP (P < 0.001).
Table 3.
Multivariable regression coefficients for associations of the log CRP with clinical and demographic characteristics among female subjects*
| Model 1 |
Model 2† |
Model 3‡ |
||||
|---|---|---|---|---|---|---|
| Variable | β | P | β | P | β | P |
| Truncal fat mass | 0.098 | <0.001 | 0.109 | <0.001 | 0.101 | <0.001 |
| Age | 0.008 | 0.59 | 0.017 | 0.33 | ||
| Caucasian | −0.731 | 0.024 | −0.594 | 0.083 | −0.668 | 0.072 |
| Current smoker | −0.271 | 0.52 | ||||
| Premenopausal | 0.334 | 0.41 | ||||
| Diabetes | −0.557 | 0.32 | ||||
| Dyslipidemia | −0.069 | 0.79 | ||||
| Swollen joint count | 0.040 | 0.13 | 0.044 | 0.11 | ||
| Tender joint count | −0.023 | 0.088 | −0.025 | 0.088 | ||
| Glucocorticoid use | 0.086 | 0.70 | 0.036 | 0.88 | ||
| Nonbiologic DMARD use | −0.350 | 0.12 | −0.336 | 0.18 | ||
| Biologic DMARD use | −0.026 | 0.93 | 0.052 | 0.87 | ||
| Daily exercise | −0.262 | 0.27 | −0.346 | 0.19 | ||
| Hours of sedentary activity | 0.066 | 0.40 | 0.072 | 0.40 | ||
| R2§ | 0.277 | 0.319 | 0.350 | |||
Values for the regression coefficient represent the unit change in the log C-reactive protein (CRP) level (logarithmically transformed to normality using the natural log) per unit change in characteristic (for truncal fat mass, age, swollen joint count, tender joint count, and hours of sedentary activity) or the difference in the mean CRP level for subjects with versus subjects without the characteristic (for current smoker, premenopausal, diabetes, dyslipidemia, use of glucocorticoids or disease-modifying antirheumatic drugs [DMARDs], or daily exercise). Sedentary activity is defined as duration of daily television watching.
Regression coefficient adjusted for height, duration of rheumatoid arthritis, joint deformity, and Health Assessment Questionnaire score.
Regression coefficient also adjusted for lifestyle, cardiovascular risk, and physical activity factors as well as menopausal status.
Coefficient of determination.
There was little change in the magnitude of the association of truncal fat mass with the log CRP in the fully adjusted model (model 3), which included the same covariates adjusted for in model 2 along with menopausal status, cardiovascular risk factors, and physical activity, with each kilogram increase in truncal fat mass associated with a 0.101-unit increase in the log CRP (P < 0.001). This translates into an expected difference in the CRP level, solely related to the effect of the difference in truncal fat, of 4.79 mg/liter when we compared a woman with 20 kg of truncal fat (the 75th percentile for women with RA in this cohort) with a woman with 10 kg of truncal fat (the 25th percentile in this cohort). Similarly, when we compared the woman with the highest truncal fat mass in our cohort (34.5 kg) with the woman with the lowest truncal fat mass (2.4 kg), the expected difference in CRP level, solely related to the difference in truncal fat, was 31.32 mg/liter (actual difference of 26.04 mg/liter).
In the adjusted models, only truncal fat was independently associated with the log CRP in women. None of the demographic, lifestyle, or RA disease or treatment characteristics, including swollen or tender joint counts, were significantly associated with the log CRP after adjustment.
Although truncal fat mass was the measure of adiposity with the greatest magnitude of association with the log CRP in women, other measures of adiposity were also found to be independently associated with the log CRP. Substituting BMI for truncal fat mass in multivariable model 3 revealed that each unit increase in BMI was associated with a 0.084-unit increase in the log CRP (P = 0.001). When using other, similar substitutions in the fully adjusted models in women, we found that each 1-cm increase in waist circumference was associated with a 0.027-unit increase in the log CRP (P < 0.001), and each kilogram increase in total fat mass was associated with a 0.047-unit increase in the log CRP (P < 0.001). This translates into an expected difference in the CRP level, solely based on the difference in BMI, of 6.22 mg/liter when we compared a woman with a BMI of 30 kg/m2 (the point of transition from “overweight” to “obese,” based on the BMI) with a woman with a BMI of 21.75 kg/m2 (the midpoint for the “healthy weight” BMI category). Likewise, the expected difference in the CRP level between a woman with a waist circumference of 98 cm (the 75th percentile for women in this cohort) and a woman with a waist circumference of 81 cm (the 25th percentile) would be 5.19 mg/liter.
Substitution of the log IL-6 for the log CRP yielded similar relationships, both in men with RA and in women with RA (data not shown).
Interaction of articular symptoms in the truncal fat–CRP relationship
In models stratified by swollen joint count (≥10 swollen joints versus <10 swollen joints) or swollen plus tender joint count (≥16 swollen plus tender joints versus <16 swollen plus tender joints) (Figure 1), the positive linear relationship between increasing truncal fat and the log CRP did not significantly differ in women with elevated swollen joint counts (P for interaction = 0.118) or elevated swollen plus tender joint counts (P for interaction = 0.686) compared with women with lower levels of articular involvement.
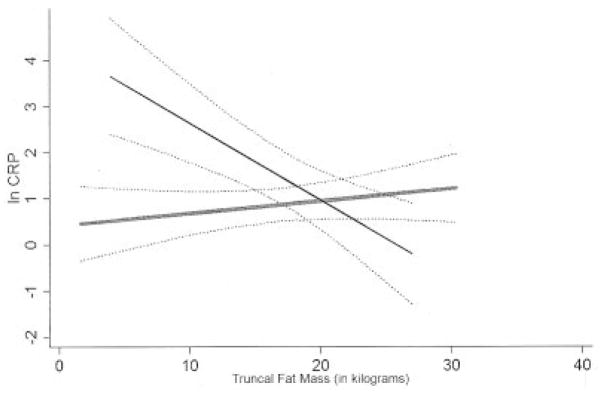
Figure 1.
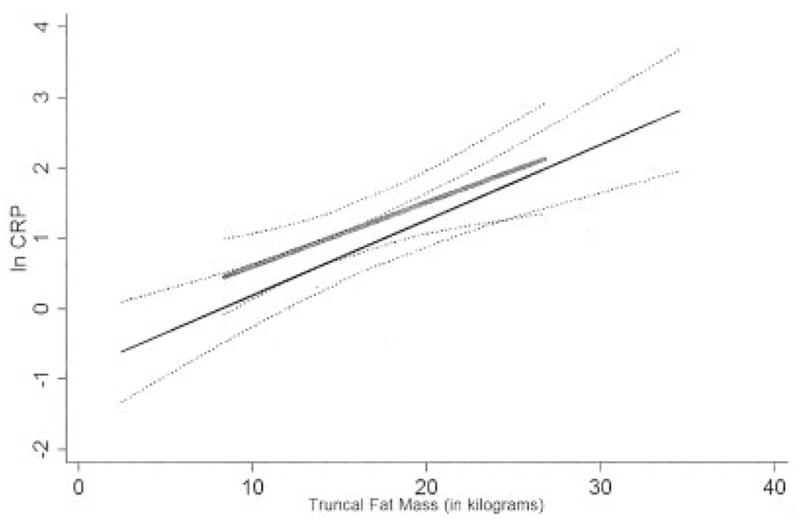
Least squares linear regression analysis of the interaction of the levels of swollen plus tender joints in the relationship between the logarithmically transformed C-reactive protein level (ln CRP) and truncal fat in women with rheumatoid arthritis. Broken lines indicate the 95% confidence intervals of the estimates. Female subjects are divided according to the level of swollen plus tender joints, with “high” articular activity (thin solid line) and “low” articular activity (thick shaded line) defined as a swollen plus tender joint count of ≥16 and <16, respectively. The P value for interaction of high articular activity (versus low articular activity) in the log CRP–truncal fat relationship was 0.686.
In contrast, in men, the relationship between truncal fat and the log CRP differed by level of articular activity (Figure 2). A significant negative linear relationship between truncal fat and the log CRP was observed in men with elevated swollen joint counts, with each kilogram increase in truncal fat mass associated with a 0.171-unit decrease in the log CRP in men in this group (P for interaction = 0.004) compared with no significant relationship between truncal fat and the log CRP in men with lower levels of articular swelling (P for interaction = 0.006). Similar results were observed in men stratified by elevated swollen plus tender joint counts (Figure 2).
Figure 2.
Least squares linear regression analysis of the interaction of the levels of swollen plus tender joints in the relationship between the logarithmically transformed C-reactive protein level (ln CRP) and truncal fat in men with rheumatoid arthritis. Broken lines indicate the 95% confidence intervals of the estimates. Male subjects are divided according to the level of swollen plus tender joints, with “high” articular activity (thin solid line) and “low” articular activity (thick shaded line) defined as a swollen plus tender joint count of ≥16 and <16, respectively. The P value for interaction of high articular activity (versus low articular activity) in the log CRP–truncal fat relationship was 0.004.
Interaction of biologic DMARD use in the truncal fat–CRP relationship
Current use of biologic DMARDs did not significantly modify the relationship between truncal fat and the log CRP or the log IL-6 in either women with RA or men with RA (data not shown).
DISCUSSION
In this investigation of the association of adiposity with CRP levels in RA patients, we observed significant associations between all measures of adiposity, particularly truncal fat, and CRP levels in women. Relationships were not modified by the level of articular activity or the use of biologic DMARDs in women. In contrast, a significant relationship between truncal fat and CRP levels was observed in men with elevated swollen plus tender joint counts. However, this relationship was the opposite of that observed in women, since men in this subgroup had increasing truncal fat in association with a decreasing CRP level. Similar findings were demonstrated when IL-6 levels were used as a substitute for the CRP levels, suggesting that the effects observed may be mediated by production of adipose-derived IL-6.
In a recent report (14), investigators identified an association between waist circumference and CRP levels in RA patients. Our study differs from this previous study in that we used direct quantification, by DXA, of total body fat mass and regional body fat mass, and we used a larger sample of RA patients, which facilitated the detection of the striking sex-specific differences in the adiposity–CRP association.
These results suggest that body fat, in particular truncal fat, should be considered when interpreting the significance of an elevated CRP level in women with RA, and imply that a fat-associated elevation in CRP may confound clinical diagnostic and therapeutic decision making in a variety of settings. For example, when using serum CRP concentration as a diagnostic tool, a fat-associated elevation in the CRP in a woman with noninflammatory musculoskeletal symptoms could prompt an inaccurate diagnosis of seronegative RA. In other settings, such as the use of serum CRP concentration to guide therapeutic decision making, a fat-associated elevation in the CRP may be misinterpreted as a reflection of persistent synovitis.
Ironically, obesity can make the joint examination, and thus the accurate assessment of synovitis, difficult, and the CRP level may be more readily relied upon to gauge RA disease activity. Thus, a fat-associated elevation in the CRP level may prompt the practitioner to unnecessarily accelerate immunomodulating therapy. In clinical trials, the efficacy of a pharmacotherapeutic agent could be underestimated when the serum CRP concentration is used as part of the efficacy outcome measure. Indeed, it has been suggested that women have lower response rates to treatment despite similar levels of articular symptoms (15,16). Fat-associated inflammation may be, at least in part, a source of this sex discrepancy.
Our study identified striking sex differences in the relationship between body fat and CRP levels. The basis of these differences is not immediately evident but could involve several possibilities. First, the influence of hormones could modify the association, since an inverse relationship between testosterone and CRP levels has been reported (17). Estrogen deficiency, such as in polycystic ovarian syndrome, has also been associated with elevated CRP levels, independent of an association with fat (18).
Another reason for the sex differences could lie in the ability to detect the inflammatory potential of depots of adiposity. Although all adipose tissue has been implicated in inflammatory cytokine production, visceral fat appears to have the greatest potential to express cytokines that induce hepatic CRP expression (7). Both subcutaneous and visceral fat are quantified by DXA as truncal fat mass. Thus, it is possible that using methods that specifically measure visceral fat (i.e., abdominal computed tomography) might allow the detection of a relationship between visceral fat and the CRP level in men.
However, other sex-associated differences in the predictors of an elevated CRP level have been noted, suggesting fundamental differences between men and women in the mechanisms of CRP induction. Predictors of an elevated CRP level that are characteristic of the RA disease process (for example, duration of disease or swollen joint counts) were seen in men, whereas in women, measures of RA disease activity and severity were not as robustly associated with the CRP level, despite many of these predictors being generally more prevalent in women.
On the basis of these fundamental differences, we explored the possibility that the observed associations between fat mass and CRP were modified by the level of RA disease activity, with the hypothesis that as the level of swollen and tender joints increased, the influence of fat mass on the CRP level would be blunted. This was not the case in women, in whom no significant difference in the association between truncal fat mass and CRP was found between women with low levels of swollen and tender joints and women with elevated levels of swollen and tender joints, emphasizing the profound influence of adiposity on CRP levels in women at all levels of articular disease activity.
The level of RA articular activity did modify the relationship between truncal fat and the CRP level in men, with increasing truncal fat mass associated with decreasing CRP levels in men with elevated swollen and tender joint counts. This finding was unexpected and not readily explainable, but highlights, in previously unexplored ways, the potential for body fat to influence the dynamics of CRP expression in RA patients. It is possible that elevated levels of articular inflammation in men with high disease activity overwhelm the effects of fat-associated inflammation on systemic CRP levels, or that articular inflammation may directly or indirectly inhibit the production of inflammatory cytokines by adipose tissue in men. It will be of interest to confirm this association in additional populations.
Similarly, we sought to explore whether the use of biologic DMARDs, with their profound capability to suppress the production of synovial inflammatory cytokines, could also modify the association of adiposity with the CRP level. We did not find any effect modification of biologic agents in either men or women, suggesting that biologic therapies may not have an effect on suppression of inflammatory cytokines in adipose tissue in RA patients. This finding is in contrast to that obtained in prior short-term interventional studies (in non-RA patient cohorts), in which it was shown that a TNF inhibitor (etanercept) was able to suppress systemic CRP levels in obese patients with metabolic syndrome or type 2 diabetes (19,20).
The discrepancy between our findings and those from prior studies could be a by-product of our cross-sectional design, since the association between adiposity and CRP level could have differed between TNF inhibitor–treated and untreated patients prior to the initiation of biologic therapy, but with extended use of the TNF inhibitor, the strength of the association could have equalized. Another possibility is that RA and non-RA patient populations differ in terms of the ability of TNF inhibitors to suppress fat-associated inflammation, perhaps related to the prolonged exposure to the biologic agent. Interestingly, other studies have identified transient effects of TNF inhibitors on metabolic end points (21), suggesting that compensatory mechanisms may override the beneficial effect of TNF inhibition on extraarticular tissues, in conditions of extended exposure to these agents.
CRP is not directly expressed by adipose tissue, but its synthesis in the liver is induced by a number of adipose-derived cytokines, principally IL-6 (4). We sought to confirm the possible intermediary effect of IL-6 on the CRP level by exploring the association of fat mass with serum IL-6 levels. The finding that the relationships between fat mass and IL-6 levels were similar to those between fat mass and CRP levels suggests that IL-6 is a mediator of the fat mass–CRP association. Other adipose-derived cytokines, such as TNFα, are difficult to measure in patients receiving TNF inhibitors. Analyses of adipose-derived hormones, such as leptin and adiponectin, could also reveal links between fat mass and the CRP level in RA patients (22), and further investigation of these associations is currently underway.
We did not identify any relationship between measures of lean mass and levels of CRP, either in men with RA or in women with RA. Prior studies in RA patients have noted associations of low body cell mass with elevated levels of TNFα induced in peripheral blood monocytes (23,24). In one study, a simple correlation of low lean mass with the CRP level was identified (25); however, the possible confounding effects of body fat on the CRP level were not considered in that study.
In our study, truncal fat mass quantified by total body DXA was the measure of adiposity with the greatest magnitude of association with the CRP level. However, other measures of adiposity with high correlations to truncal fat (i.e., waist circumference, total fat mass, and BMI) were also found to be significantly associated with the CRP level. In the absence of DXA scanning, which may not be feasible for use on all patients in clinical practice, the practitioner could use waist circumference or BMI as a reasonable proxy for truncal fat to estimate the contribution of truncal fat to systemic inflammation.
Additional limitations of the current investigation should be acknowledged. Subjects were participants in a study in which patients whose weight exceeded 300 pounds and/or patients who had a history of cardiovascular disease were excluded. Based on the identification of links between adiposity, the CRP level, and cardiovascular disease (7,26), it is possible that patients with the most robust associations between adiposity and the CRP level could have been excluded. However, inclusion of these subjects would likely strengthen, not reduce, the magnitude of the association observed between adiposity and the CRP level.
Furthermore, the cross-sectional design of our study limits the ability to ascribe causal relationships to the associations detected. However, because there is no biologically tenable hypothesis to indicate that elevations in the CRP level induce the acquisition of truncal fat, it is more likely that truncal fat induces an elevation in the CRP level. Whether this effect occurs directly through adipose-derived IL-6 or occurs through other mechanisms cannot be systematically established through our study.
In summary, we demonstrated a strong association between body fat, in particular truncal fat, and the serum CRP concentration in women with RA. Neither the level of articular activity nor the use of biologic DMARDs modified the association. Men with RA did not exhibit the same association of body fat with the CRP level, suggesting that there is a fundamental sex-related difference in the interplay between body fat and CRP induction that warrants further investigation. These findings suggest that body fat may confound the commonly ascribed associations between RA disease–related factors and serum CRP concentration in certain patients, and body fat should be considered when using the level of CRP for diagnostic and therapeutic decision making in RA patients.
Acknowledgments
Supported in part by NIH grants (National Institute of Arthritis and Musculoskeletal and Skin Diseases AR-050026-01 and 1K23-AR-054112-01) and the Johns Hopkins Bayview Medical Center General Clinical Research Center. Dr. Giles’ work was also supported by a Clinical Investigator Fellowship award from the Research and Education Foundation of the American College of Rheumatology.
We would like to thank the staff of the Johns Hopkins Bayview Medical Center General Clinical Research Center for providing support for the DXA scanning used in this study. We are also indebted to the ESCAPE RA staff (Marilyn Towns, Michelle Jones, Patricia Jones, Marissa Hildebrandt, and Shawn Franckowiak) for their dedication and hard work. In addition, we would like to thank Drs. Uzma Haque, Clifton Bingham III, Carol Ziminski, Jill Ratain, Ira Fine, Joyce Kopicky-Burd, David McGinnis, Achini Perera, Peter Holt, Megan Clowse, and Gordon Lam, among others, for generously recommending their patients for this study.
Footnotes
Dr. Bathon has received consulting fees, speaking fees, and/or honoraria from Abbott, Amgen, and Centocor (less than $10,000 each) and research support from Amgen, Biogen Idec, and Bristol-Myers Squibb.
AUTHOR CONTRIBUTIONS
Dr. Giles had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Giles, Bathon.
Acquisition of data. Giles, Bathon.
Analysis and interpretation of data. Giles, Thompson, Bathon.
Manuscript preparation. Giles, Bartlett, Andersen, Fontaine, Bathon.
Statistical analysis. Giles, Thompson.
References
- 1.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36:729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 2.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 3.Keeling SO, Landewe R, van der Heijde D, Bathon J, Boers M, Garnero P, et al. Testing of the preliminary OMERACT validation criteria for a biomarker to be regarded as reflecting structural damage endpoints in rheumatoid arthritis clinical trials: the example of C-reactive protein. J Rheumatol. 2007;34:623–33. [PubMed] [Google Scholar]
- 4.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 5.Molenaar ET, Voskuyl AE, Familian A, van Mierlo GJ, Dijkmans BA, Hack CE. Complement activation in patients with rheumatoid arthritis mediated in part by C-reactive protein. Arthritis Rheum. 2001;44:997–1002. doi: 10.1002/1529-0131(200105)44:5<997::AID-ANR178>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Greenfield JR, Samaras K, Jenkins AB, Kelly PJ, Spector TD, Gallimore JR, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109:3022–8. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 7.Piche ME, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-α, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–7. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–9. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 10.Navarro-Cano G, del Rincon I, Pogosian S, Roldan JF, Escalante A. Association of mortality with disease severity in rheumatoid arthritis, independent of comorbidity. Arthritis Rheum. 2003;48:2425–33. doi: 10.1002/art.11127. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe F, Kleinheksel SM, Cathey MA, Hawley DJ, Spitz PW, Fries JF. The clinical value of the Stanford Health Assessment Questionnaire Functional Disability Index in patients with rheumatoid arthritis. J Rheumatol. 1988;15:1480–8. [PubMed] [Google Scholar]
- 12.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. Physical activity and health: a report of the Surgeon General. Atlanta: US Department of Health and Human Services; 1996. [Google Scholar]
- 14.Dessein PH, Norton GR, Woodiwiss AJ, Joffe BI, Solomon A. Independent role of conventional cardiovascular risk factors as predictors of C-reactive protein concentrations in rheumatoid arthritis. J Rheumatol. 2007;34:681–8. [PubMed] [Google Scholar]
- 15.Hyrich KL, Watson KD, Silman AJ, Symmons DP, the BSR Biologics Register Predictors of response to anti-TNF-α therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45:1558–65. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 16.Forslind K, Hafstrom I, Ahlmen M, Svensson B for the BARFOT Study Group. Sex: a major predictor of remission in early rheumatoid arthritis? Ann Rheum Dis. 2007;66:46–52. doi: 10.1136/ard.2006.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 18.Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86:2453–5. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, et al. Metabolic and vascular effects of tumor necrosis factor-α blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–25. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166:902–8. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Juanatey C, Testa A, Garcia-Castelo A, Garcia-Porrua C, Llorca J, Gonzalez-Gay MA. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti–tumor necrosis factor α antibody. Arthritis Rheum. 2004;51:447–50. doi: 10.1002/art.20407. [DOI] [PubMed] [Google Scholar]
- 22.Putz DM, Goldner WS, Bar RS, Haynes WG, Sivitz WI. Adiponectin and C-reactive protein in obesity, type 2 diabetes, and monodrug therapy. Metabolism. 2004;53:1454–61. doi: 10.1016/j.metabol.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379–86. doi: 10.1172/JCI117244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsmith J, Abad L, Kehayias J, Roubenoff R. Tumor necrosis factor-α production is associated with less body cell mass in women with rheumatoid arthritis. J Rheumatol. 2004;31:23–9. [PubMed] [Google Scholar]
- 25.Munro R, Capell H. Prevalence of low body mass in rheumatoid arthritis: association with the acute phase response. Ann Rheum Dis. 1997;56:326–9. doi: 10.1136/ard.56.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28:1018–25. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]