Abstract
Purpose
The goal of the present study was to determine whether the release of exosomes containing MYOC from trabecular meshwork (TM) cells is constitutive or regulated.
Methods
Conditioned media from TM cells were analyzed for MYOC-associated exosomes after treatment with IFN-γ, porcine aqueous humor, dexamethasone, or a calcium ionophore in cells pretreated with dexamethasone. Aqueous humor was tested whole or fractionated by size exclusion filters. Exosomes from conditioned media were purified by differential centrifugation. Proteins in whole, exosome, and soluble fractions were separated by SDS-PAGE and analyzed for MYOC content by Western blot and densitometry.
Results
Although treatment of TM cells with IFN-γ increased the appearance of extracellular MYOC-associated exosomes, results were not significantly different from those of control (P = 0.13). In contrast, treatment with dexamethasone increased the appearance of MYOC in the exosome fraction by 376% (P < 0.01). The increase in MYOC-associated exosomes caused by dexamethasone was enhanced by an additional 379% after short-term exposure to ionomycin (P < 0.05). When cultured in media containing aqueous humor, MYOC-associated exosomes increased 514% over control (P < 0.01). Such an increase was diminished in cells treated with aqueous humor that was first passed through a 3-kDa or a 30-kDa, but not a 100-kDa, size exclusion filter.
Conclusions
The appearance of MYOC-associated exosomes in conditioned media from human TM cells is regulated by a corticosteroid, a calcium ionophore, and a component of aqueous humor, suggesting that TM cells respond to environmental cues by releasing MYOC-associated exosomes.
Glaucoma is the second leading cause of irreversible blindness in the United States.1,2 Mutations causing ocular hypertension and open-angle glaucoma in some are located to a glaucoma gene on a chromosome 1 locus, GLC1A, which codes for a protein called myocilin (MYOC).3 Unfortunately, despite more than 10 years of research, the function of MYOC remains unknown. Contributing to the slow progress in understanding the role of MYOC in the control of intraocular pressure and its focal pathology (development of ocular hypertension in glaucoma) is its near ubiquitous distribution in the human body,4–7 its cell-specific induction characteristics,8–11 and its ambiguous cellular distribution and associations.4,6,7,10,12–25
MYOC is expressed by different cell types in several eye tissues, including the trabecular meshwork (TM), the likely site of pathology for ocular hypertension in primary open-angle glaucoma.4–7,10,13,26,27 Compared with other cell types that express MYOC, the TM appears unique with respect to expression level plus induction of expression in response to corticosteroids and to mechanical and oxidative stress.8–11 On a subcellular level, native MYOC localizes to the cytosolic and membrane compartments of TM cells16,17 and is found extracellularly in conditioned medium from TM cells in culture, perfused human anterior segments in organ culture, and aqueous humor.7,28–30
To explain such disparate localizations, we tested whether MYOC associates with a class of intracellular vesicles, called exosomes, that display properties coincident with the unique characteristics of MYOC. Exosomes are small vesicles that are formed by inward budding of the limiting membrane of the multivesicular body and are released from cells on fusion of the multivesicular body with the plasma membrane.31,32 When tested specifically, we observed that MYOC is not secreted in a traditional manner; rather, it exits trabecular meshwork cells and enters the extracellular compartment associated with exosomes.16 Moreover, we observed that MYOC-associated exosomes are present in human ocular samples and thus appear to serve a physiological function.33
We reasoned that if exosomes were involved in the dynamic control of conventional outflow facility, then exosome release must be subject to regulation. To better understand the role of MYOC in exosome release, we explored ways to modulate the appearance of MYOC-associated exosomes in conditioned media of TM cell monolayers in culture. We hypothesized that agents known to increase MYOC expression in TM cells in vitro and that affect outflow in vivo or compounds known to enhance exosome release/protein content in other cell types will increase MYOC-associated exosome release from TM cells. Our results show that a corticosteroid, a calcium ionophore, and a soluble component of aqueous humor measuring 30 kDa to 100 kDa significantly increased the appearance of MYOC-associated exosomes from cultured human TM cells.
Materials and Methods
Human Trabecular Meshwork Cells
Six different human TM cell strains were isolated and characterized by our laboratory, as previously described,34,35 and were used in experiments in the present study (Table 1). Cells were plated and maintained at confluence in low-glucose Dulbecco modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with a penicillin (100 U/mL), streptomycin (100 mg/mL), and glutamine (0.29 mg/mL) solution (Invitrogen) plus fetal bovine serum (FBS; Gemini Bio-Products, Irvine, CA) for at least 14 days before treatment. Media were supplemented with 10% FBS for the first week cells were at confluence, and then cells were maintained in media supplemented with 1% FBS until the start of treatment.
Table 1.
Drug Treatments and Cell Strains
| TM Strain | Donor Age | Ionomycin | Dex | IFN-γ | pAH |
|---|---|---|---|---|---|
| TM26 | 15 years | X | X | X | — |
| TM84 | 68 years | X | X | — | — |
| TM86 | 3 months | — | X | X | X |
| TM87 | 71 years | X | X | X | — |
| TM89 | 64 years | — | — | — | X |
| TM90 | 4 months | — | X | — | — |
Dex, dexamethasone; pAH, porcine aqueous humor.
All treatments were diluted in DMEM containing 0.1% exosome-free FBS. Endogenous exosomes were removed from FBS by centrifugation at 100,000g for 1 hour. All treatment regimens were different and were based on published data from our laboratory10,16 or from preliminary experiments that determined the optimal time course and dose for maximal MYOC appearance in conditioned media (data not shown). For all treatment regimens, only conditioned media from the final 72 hours of treatment were collected for analyses. Two of the treatments, IFN-γ (500 U/mL) and aqueous humor (50% vol/vol), lasted 72 hours. In contrast, cells were treated with dexamethasone (Sigma, St. Louis, MO) at a 100 nM concentration for 5 days, and conditioned media were collected from the final 72 hours of treatment. The only combination drug treatments occurred for cells that were treated with ionomycin (Calbiochem, San Diego, CA) at a concentration of 1 μM for 3 hours after cells had been pretreated with 5 days of 100 nM dexamethasone. Conditioned media were collected only for the final 72 hours, including the 3-hour ionomycin treatment. For each experimental condition, care was taken to ensure that equivalent amounts of medium-containing drug were added to cells, and equivalent amounts of conditioned media were collected from cells for analyses. To ensure equal loading between samples, proteins in conditioned media samples (after cell debris had been removed) from all experimental groups were fractionated by SDS-PAGE and stained with Coomassie Brilliant Blue (Sigma). For comparisons, paired samples (treated vs. controls) were run into the same gel, and protein banding patterns for entire lanes were digitized and analyzed by densitometry.
Porcine Aqueous Humor Treatment and Fractionation
Aqueous humor was collected from enucleated porcine eyes (porcine aqueous humor [pAH]; Hatfield Meat Products, Hatfield, PA) by inserting a 26-gauge needle through the cornea and positioning its tip into the center of the anterior chamber. Aqueous was removed by slow aspiration with care taken not to contact any internal structures. Aqueous humor (approximately 200 μL/eye) was pooled from 50 eyes and centrifuged at 10,000g for 1 hour at 4°C to pellet any pigment or cellular debris. Next, aqueous humor supernatant was transferred to clean tubes and centrifuged at 100,000g at 4°C to pellet endogenous exosomes. Supernatant was removed and tested on cells or fractionated further using centrifuge filters (Millipore, Billerica, MA). To fractionate, 2 mL aqueous humor was added atop filters with a 3-kDa, 30-kDa, or 100-kDa molecular weight cutoff, and filters were subjected to centrifugation at 3500g until 1 mL aqueous humor passed through the filters. The 1 mL aqueous humor that passed through the filters was tested for stimulation of exosome appearance in conditioned medium. In all cases, human TM cells were treated with a 50% (vol/vol) mixture of aqueous humor (unfractionated or filtrate) in DMEM supplemented with 0.1% exosome-free FBS plus antibiotics for 72 hours, after which conditioned media were collected and analyzed.
Exosome Isolation
Exosomes were isolated exactly as described previously.16 Briefly, conditioned media were collected from human TM cells with or without treatment and were centrifuged for 1 hour at 10,000g at 4°C to remove cell debris. Samples were taken from conditioned media before (whole) and after (cell debris) centrifugation, solubilized in 2× Laemmli buffer, and stored at −20°C for subsequent analyses. Exosomes were then isolated from precleared supernatant by centrifuging at 100,000g (SW41 rotor) for 1 hour at 4°C. Supernatant (after cell debris) was collected, and samples were placed in 2× Laemmli buffer and stored at −20°C. Pellets were resuspended in cold PBS, centrifuged at 100,000g again in a clean tube for 1 hour to ensure purity, and solubilized in 2× Laemmli buffer. Volumes of each fraction were kept identical between experimental conditions to allow for direct comparisons.
Western Blot Analyses
Proteins solubilized in Laemmli buffer were heated at 100°C for 10 minutes, loaded onto gel slabs containing 10% polyacrylamide, and separated by SDS-PAGE. Fractionated proteins were transferred electrophoretically onto nitrocellulose membranes incubated in Tris/HCl-buffered saline (100 mM Tris, 137 mM NaCl, 2.7 mM KCl, pH 7.4) containing 2% Tween-20 (TBS-T) and 5% nonfat dry milk (blocking buffer). Affinity-purified polyclonal IgG (300 ng/mL) raised in rabbits against myocilin was added to the blocking buffer, and membranes were incubated for 16 hours at 4°C.10,16,17,33 Membranes were washed in TBS-T four times for 15 minutes each, incubated in blocking buffer for 30 minutes, and transferred to blocking buffer containing horse-radish peroxidase-conjugated goat anti-rabbit IgG (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA) and incubated at 25°C with rocking for 1 hour. Membranes were washed in TBS-T four times for 15 minutes each, and protein antibody complexes were visualized by exposing membranes to reagent (Hyglo; Metuchen, Denville, NJ) followed immediately by timed exposures to x-ray film. Bands on x-ray film were digitized, and densitometry was performed (Labworks 4 software; (UVP, Upland, CA).
Statistical Analyses
Only data in which digitized bands corresponding to MYOC protein that fell in the linear range of the x-ray film were used for analyses. Values obtained from experimental and control samples were analyzed by two-tailed, paired Student’s t-test assuming unequal variance. Differences were considered significant at P < 0.05.
Results
To examine whether MYOC-associated exosomes are subject to regulation, we first explored whether a cytokine known to increase major histocompatibility complex class II antigen content on exosomes in several cell types36,37 (including human TM cells16) affected MYOC content on exosomes in conditioned media from human TM cells. After treating human TM cells for 72 hours with 500 U/mL IFN-γ, MYOC-associated exosomes in conditioned media were analyzed. Volume equivalents of whole conditioned media (and fractions) from control and treated cells were examined in parallel using SDS-PAGE, followed by Western blotting with anti-MYOC IgG (Fig. 1) or Coomassie Blue stain to control for total protein content between samples (data not shown). Figure 1 shows Western blots of whole conditioned media and its soluble and membrane component fractions that were probed for MYOC in the presence (+) or absence (−) of IFN-γ. All fractions were cleared of cell debris by centrifugation before analyses. The exosome fraction is the pellet of extracellular membranes isolated after two sequential high-speed (100,000g) centrifugation steps of precleared whole conditioned media. The supernatant atop the exosome fraction contained all soluble proteins in conditioned media. Results show that after 72 hours of treatment, MYOC content increased in all fractions but that it increased disproportionately in the exosome fraction by 256% ± 185%. A summary of results from five independent experiments is shown in Figure 1B and indicates that variability in results after IFN-γ treatment limited data from reaching significance (P = 0.13).
Figure 1.
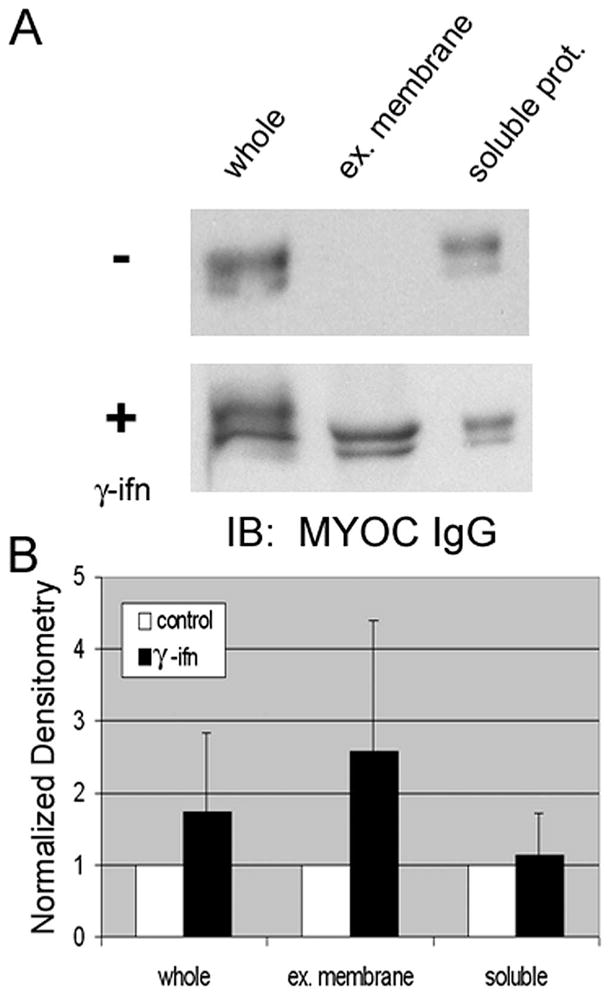
Effect of IFN-γ on myocilin content in conditioned media fractions from human trabecular meshwork cells. (A) Western blot analysis for myocilin in whole conditioned media or fractions generated by differential centrifugation before (−) and after (+) treatment with IFN-γ (500 U/mL). Conditioned media (72 hours) were first centrifuged to pellet cell debris. From this precleared conditioned media, exosomes (extracellular [ex.] membranes) were isolated by ultracentrifugation. The supernatant remaining after ultracentrifugation contained soluble proteins (prot.) from conditioned media. (B) Cumulative data (mean ± SD) from densitometric analyses of whole media and its fractions from five independent experiments.
Next, we examined whether dexamethasone, a corticosteroid that dramatically enhances the expression and release of MYOC in TM cells, affected the appearance of MYOC-associated exosomes in conditioned media. After 5 days of treatment with dexamethasone, we observed a significant increase in MYOC content in all fractions (Fig. 2). As in previous studies, we observed an increase in MYOC in whole conditioned media. When looking at fractions of conditioned media, the exosome fraction increased by 376% ± 111% compared with untreated controls (P < 0.01). Longer exposures of immunoblots (approximately 5 minutes) demonstrated the presence of MYOC in the exosome fraction of untreated controls (data not shown).
Figure 2.
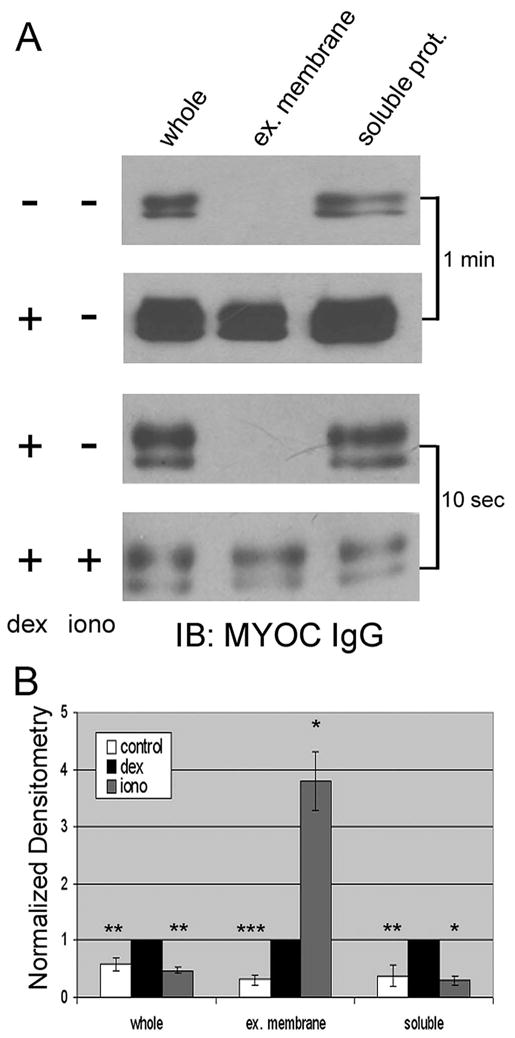
Effect of dexamethasone and a calcium ionophore (ionomycin) on myocilin content in conditioned media fractions from human trabecular meshwork cells. (A) Western blot analysis for myocilin in whole conditioned media or fractions generated by differential centrifugation before (−) and after (+) 5 days of treatment with 100 nM dexamethasone in the presence (+) or absence (−) of ionomycin (1 μM) for 3 hours. Conditioned media (72 hours) were first centrifuged to pellet cell debris. The cell debris fraction was then subject to ultracentrifugation to pellet extracellular (ex.) membranes, leaving soluble proteins (prot.) in the supernatant. Shown are 1-minute and 10-second exposures of film from untreated cells or from cells treated as indicated. (B) Cumulative data (mean ± SD) from densitometric analyses of whole conditioned media and its fractions from five independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Given that transient elevations in intracellular calcium have been shown to trigger the release of exosomes from other cell types,38–40 we tested whether the treatment of human TM cells with a calcium ionophore, ionomycin (1 μM), has an impact on the appearance of MYOC-associated exosomes in conditioned media. When ionomycin was tested alone, we were unable to detect a change in the extracellular appearance of MYOC (data not shown). However, in cells first treated with dexamethasone and then 3 hours with the calcium ionophore (iono), Figure 2 shows that MYOC appearance in the exosome fraction significantly increased 379% ± 52% over dexamethasone-alone control (P < 0.05). On treatment, the relative distribution of MYOC became more prominent in exosome than in soluble fractions after only 3 hours of treatment with ionomycin; in fact, the amount of MYOC in the soluble fraction decreased significantly in the treatment group (30% ± 8% of dexamethasone alone; P < 0.05). Similarly, MYOC in whole conditioned media samples receiving ionomycin treatment were decreased compared with dexamethasone alone.
Next, we examined the effect of aqueous humor on MYOC-associated exosome appearance in conditioned media. Similar to recent findings that extracellular MYOC increases when cultured in the presence of aqueous humor,41 we observed that exposure of human TM cells to media containing 50% pAH significantly increased MYOC in whole conditioned media by 66% ± 38% (Fig. 3; P < 0.01). When the exosome fraction was isolated from conditioned media, we found a dramatic and disproportionate increase in MYOC compared with other fractions (514% ± 178%; P < 0.01).
Figure 3.
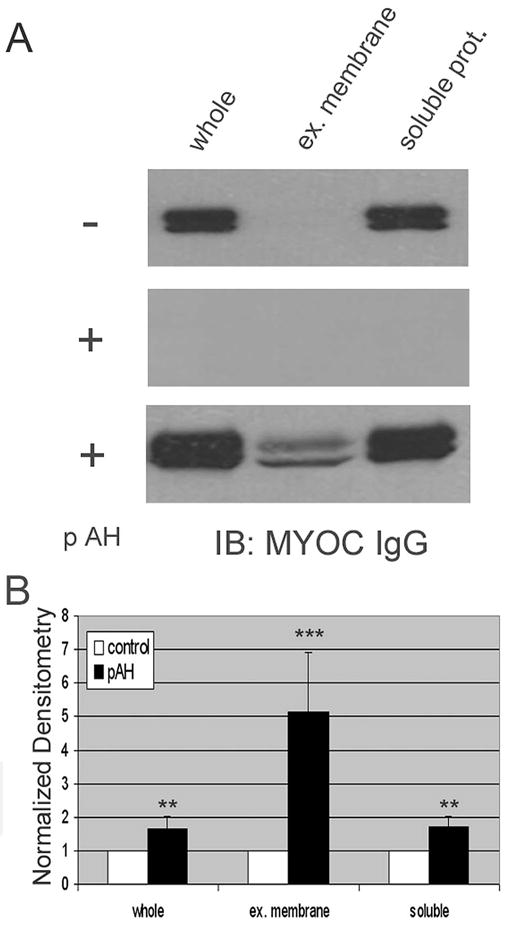
Effect of porcine aqueous humor (pAH) on myocilin content in conditioned media fractions from human TM cells. (A) Western blot analysis for myocilin in whole conditioned media or fractions generated by differential centrifugation before (−) and after (+) treatment with 50% (vol/vol) aqueous humor. Conditioned media (72 hours) were first centrifuged to pellet cell debris. Precleared conditioned media were then subject to ultracentrifugation to pellet extracellular (ex.) membranes, leaving soluble proteins (prot.) in the supernatant. (A, middle) Results when pAH itself was fractionated by differential centrifugation in a manner identical to that of conditioned media; proteins in each fraction were separated by SDS-PAGE and Western blotted for MYOC. (B) Cumulative data (mean ± SD) from densitometric analyses of whole conditioned media and its fractions from six independent experiments. **P < 0.01; ***P < 0.001.
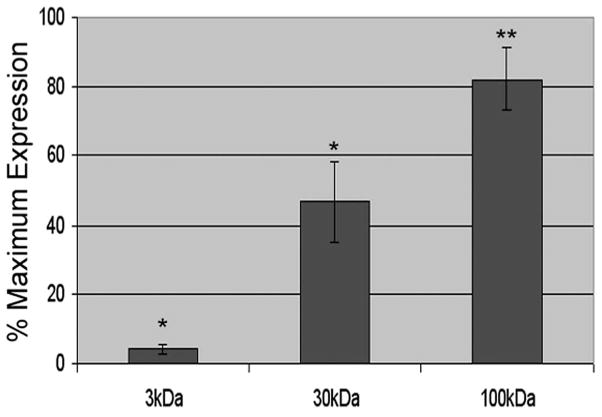
To better understand the identity of the components of aqueous humor that mediate the increased extracellular appearance of MYOC, we subjected fresh aqueous humor to size exclusion filtration (3 kDa, 30 kDa, 100 kDa) and tested the filtrate on human TM cells. Results in Figure 4 indicate that 3-kDa filters intercepted most of the component(s) in aqueous humor responsible for increasing MYOC-associated exosomes in conditioned media. Interestingly, though it had only approximately 15% of maximum stimulation of whole aqueous humor, the 3 K filtrate was significantly different from control. In contrast, the 30-kDa filters intercepted approximately half (53% ± 12%) of the active components in aqueous humor and was significantly different from untreated control (P < 0.05). Lastly, the 100-kDa filter failed to stop most aqueous humor components from increasing MYOC exosomes. In fact, as with whole aqueous humor (82% ± 9% of whole), the 100-kDa filtrate significantly increased MYOC-associated exosomes in conditioned media from human TM cells (P < 0.01).
Figure 4.
Effect of porcine aqueous humor filtrate on appearance of MYOC-associated exosomes in conditioned media from human trabecular meshwork cells. Cells were treated with porcine aqueous humor subjected to size-exclusion filtration (3 kDa, 30 kDa, 100 kDa), DMEM (control), or unfiltered porcine aqueous humor. Whole aqueous humor and filtrates were diluted in DMEM (50% vol/vol). MYOC-associated exosome content in conditioned media was assessed using Western blot analysis and anti-MYOC IgG and was quantified by densitometry. Data represent the combined results (mean ± SD) of three experiments. Values shown were calculated by setting the DMEM control equal to 0% and the mean effect of whole aqueous humor equal to 100% and interpolating the filtrate values by linear regression. *P < 0.05; **P < 0.01.
Discussion
Whether in stable human TM cell monolayers or in effluent from perfused anterior segments, we observed previously that MYOC-associated exosomes are constitutively released into the extracellular compartment over time.16,33 The primary finding of the present study was that steady state release of exosomes by stable monolayers of human TM cells was increased on long-term treatment with dexamethasone, medium-term treatment with aqueous humor, and short-term treatment with a calcium ionophore.
We chose to test the effect of a calcium ionophore on exosome release in our system because of two recent reports showing the involvement of intracellular calcium transients in the release of exosomes in other cells. Thus, treatment of K562 cells (reticulocytes) with monesin or A32187 for 7 hours resulted in elevated intracellular calcium transients corresponding to an increased release of exosomes.39 Similarly, calcium was observed to be required for exosome release by mast cells in response to stimulation with dinitrophenol for 1 hour.42 Our results here show a significant and dramatic increase in MYOC appearance in the exosome fraction after 3 hours of ionomycin treatment in dexamethasone-pretreated cells. Taken together, the regulation of exosome release as a result of increased intracellular calcium appears to share a common general mechanism for the fusion or release of many classes of vesicles.
Recent studies have demonstrated that exposure of cultured human TM cells or trabecular tissues in organ culture to aqueous humor has an impact on their morphologic appearance and expression profile.41 Relevant to the present study, Fautsch et al.41 found that culturing human TM cells in the presence of 50% aqueous humor (vol/vol in DMEM) increased the appearance of MYOC in conditioned media. The earliest point analyzed in this previous report was 5 days after the start of treatment. In the present study, we observed an increase in the appearance of MYOC in conditioned media 72 hours after aqueous humor treatment in whole conditioned media and exosome fraction. On closer examination, we found that the major component of aqueous humor responsible for MYOC-associated exosome stimulation measured between 30 kDa and 100 kDa. A minor active ingredient also appeared in aqueous between 3 and 30 K because significant stimulation above control was detected in the filtrate of both these filters. After reviewing known components of aqueous humor that lie within this range and that stimulate intracellular calcium, we noticed transferrin.43 A protein with a molecular mass of 33 kDa, transferrin (approximately 75 kDa with carbohydrate side chains) has been shown to stimulate intercellular calcium transients and exosome release from K562 cells in culture.39 In response to such tempting information, we conducted experiments to test the effects of transferrin on exosome release from human TM cells. Unfortunately, we were unable to detect a significant effect of transferrin on MYOC-associated exosomes using the conditions of concentration (20 μg/mL) and time (16 hours) reported previously (P = 0.57; data not shown), a concentration similar to that found in aqueous humor.44 Perhaps optimization of conditions in future studies may reveal a role for transferrin and transferrin receptor in exosome regulation in human TM cells.
IFN-γ was used previously by our group and others to induce the expression of major histocompatibility complex class II antigens in cultured cells (HLA-DR in human TM cells16) to characterize exosomes isolated from conditioned media.36,37 In a previous study, we treated cultured human TM cells for 5 days with IFN-γ to induce HLA-HR expression in exosome fractions containing MYOC but did not observe a change in MYOC content compared with control.16 In pilot experiments for this previous study, we noticed at earlier time points that IFN-γ treatment increased MYOC protein in the exosome fraction but not HLA-DR. Thus, in the present study, we characterized the extracellular appearance of MYOC after 3 days of IFN-γ treatment. Although we observed an increase in MYOC protein in the exosome fraction, statistical analysis of data did not distinguish a significant difference from control. We were unable to determine the origin of this variability.
Three of the four treatments tested significantly increased the release of exosomes from TM cells. Two of these substances, dexamethasone and aqueous humor, are known to increase the appearance of extracellular MYOC; thus, it was not surprising that exosomes were a significant contributor to such an increase. Interestingly, we could not detect a change in exosome release when cells were treated with the calcium ionophore alone. Only in cells that had basal levels of MYOC, increased by 5-day treatment with dexamethasone, were we able to detect the effects of the calcium ionophore, perhaps because in culture, baseline levels of MYOC are significantly decreased.11 Such an idea is supported by our finding that MYOC-associated exosomes are at higher concentrations in effluent collected from perfused human anterior segments and in aqueous humor compared with human TM cells in culture.33
We observed that after only 3 hours of ionomycin treatment, MYOC content in the exosome fraction increased whereas MYOC content in soluble fraction decreased. These data suggest that MYOC is associated with exosome vesicles on release from human TM cells and that it dissociates into media over time. Such an idea is consistent with our recent report showing a greater percentage of MYOC in exosome fraction in fresh aqueous humor collected from patients undergoing excisional surgery compared with aqueous humor collected from human donor eyes, in which MYOC is found predominantly in soluble fraction.33 These data also suggest that a reservoir of exosomes is awaiting release. After only 3 hours of ionomycin treatment, we observed changes in the association of MYOC with exosomes in conditioned media almost four times greater than at 72 hours of dexamethasone treatment alone.
The findings of the present study demonstrate that MYOC-associated exosome release is regulated, but they do not provide information about the role of exosomes in the control of outflow facility. We hypothesize that MYOC is a component of exosomes that function in the process of exosome release and that a second unknown protein resident of exosomes serves as a ligand that participates in signaling between outflow cells to regulate facility. With this in mind, the regulation of outflow facility is tied to functional MYOC and the presence of the ligand on exosomes. Such an idea is consistent with data showing that the expression of mutant MYOC inhibits the extracellular appearance of MYOC, resulting in a decreased outflow facility in those with glaucoma.28,45,46 The fact that overexpression of wild-type MYOC protein does not affect outflow facility47 indicates, in the context of our hypothesis, that the expression of MYOC and the signaling ligand on exosomes are not linked. Consistent with this idea are data showing that outflow facility is normal in MYOC knockout mice,48 a finding that implicates built-in redundancy in the vesicle targeting/release of exosomes from cells. However, long-term MYOC haploinsufficiency in humans, as is possible with those having the Q368STOP mutation, may tax backup systems over time and eventually have an impact on outflow function.
Acknowledgments
Supported in part by National Eye Institute Grant EY12797 and by the Research to Prevent Blindness Foundation.
Footnotes
Disclosure: E.A. Hoffman, None; K.M. Perkumas, None; L.M. Highstrom, None; W.D. Stamer, None
References
- 1.Quigley H. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leske M. The epidemiology of open-angle glaucoma: a review. Am J Epidemiol. 1983;118:166–191. doi: 10.1093/oxfordjournals.aje.a113626. [DOI] [PubMed] [Google Scholar]
- 3.Stone EM, Fingert JH, Alward WLM, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 4.Fingert J, Ying L, Swiderski R, et al. Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res. 1998;8:377–384. doi: 10.1101/gr.8.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam M, Belmouden A, Binisti P, et al. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet. 1997;12:2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- 6.Kubota R, Noda S, Wang Y, et al. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997;41:360–369. doi: 10.1006/geno.1997.4682. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen T, Chen P, Huang W, Chen H, Johnson D, Polansky J. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- 8.Shepard A, Jacobson N, Fingert J, Stone E, Sheffield V, Clark A. Delayed secondary glucocorticoid responsiveness of MYOC in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2001;42:33173–3181. [PubMed] [Google Scholar]
- 9.Polansky J, Fauss D, Zimmerman C. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye. 2000;14:503–514. doi: 10.1038/eye.2000.137. [DOI] [PubMed] [Google Scholar]
- 10.Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture and characterization of endothelial cells from Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:1804–1812. [PubMed] [Google Scholar]
- 11.Tamm E, Russell P, Epstein D, Johnson D, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- 12.Nagano T, Nakamura A, Mori Y, et al. Differentially expressed olfactomedin-related glycoproteins (pancortins) in the brain. Mol Brain Res. 1998;53:13–23. doi: 10.1016/s0169-328x(97)00271-4. [DOI] [PubMed] [Google Scholar]
- 13.Ortego J, Escribano J, Coca-Prados M. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett. 1997;413:349–353. doi: 10.1016/s0014-5793(97)00934-4. [DOI] [PubMed] [Google Scholar]
- 14.Wentz-Hunter K, Ueda J, Shimizu N, Yue BY. Myocilin is associated with mitochondria in human trabecular meshwork cells. J Cell Physiol. 2002;190:46–53. doi: 10.1002/jcp.10032. [DOI] [PubMed] [Google Scholar]
- 15.Shepard AR, Jacobson N, Millar JC, et al. Glaucoma-causing myocilin mutants require the peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16:609–617. doi: 10.1093/hmg/ddm001. [DOI] [PubMed] [Google Scholar]
- 16.Hardy K, Hoffman E, McKay B, Gonzalez P, Stamer W. Extracellular trafficking of myocilin in human trabecular meshwork cells. J Biol Chem. 2005;280:28917–28926. doi: 10.1074/jbc.M504803200. [DOI] [PubMed] [Google Scholar]
- 17.Stamer W, Perkumas K, Hoffman E, Roberts B, Epstein D, McKay B. Coiled-coil targeting of myocilin to intracellular membranes. Exp Eye Res. 2006;83:1386–1395. doi: 10.1016/j.exer.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Joe MK, Sohn S, Choi YR, Park H, Kee C. Identification of flotillin-1 as a protein interacting with myocilin: implications for the pathogenesis of primary open-angle glaucoma. BiochemBiophys Res Commun. 2005;336:1201–1206. doi: 10.1016/j.bbrc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Fautsch M, Johnson D. Characterization of myocilin-myocilin interactions. Invest Ophthalmol Vis Sci. 2001;42:2324–2331. [PubMed] [Google Scholar]
- 20.Sakai H, Shen X, Koga T, et al. Mitochondrial association of myocilin, product of a glaucoma gene, in human trabecular meshwork cells. J Cell Physiol. 2007;213:775–784. doi: 10.1002/jcp.21147. [DOI] [PubMed] [Google Scholar]
- 21.Park BC, Tibudan M, Samaraweera M, Shen X, Yue BY. Interaction between two glaucoma genes, optineurin and myocilin. Genes Cells. 2007;12:969–979. doi: 10.1111/j.1365-2443.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 22.Surgucheva I, Park BC, Yue BY, Tomarev S, Surguchov A. Interaction of myocilin with gamma-synuclein affects its secretion and aggregation. Cell Mol Neurobiol. 2005;25:1009–1033. doi: 10.1007/s10571-005-8471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wentz-Hunter K, Ueda J, Yue B. Protein interactions with myocilin. Invest Ophthalmol Vis Sci. 2002;43:176–182. [PubMed] [Google Scholar]
- 24.Peters DM, Herbert K, Biddick B, Peterson JA. Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp Cell Res. 2005;303:218–228. doi: 10.1016/j.yexcr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Fautsch MP, Vrabel AM, Johnson DH. The identification of myocilin-associated proteins in the human trabecular meshwork. Exp Eye Res. 2006;82:1046–1052. doi: 10.1016/j.exer.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Karali A, Russell P, Stefani F, Tamm E. Localization of myocilin/trabecular meshwork-inducible glucocorticoid response protein in the human eye. Invest Ophthalmol Vis Sci. 2000;41:729–740. [PubMed] [Google Scholar]
- 27.Abderrahim H, Jaramillo-Babb V, Zhou Z, Vollrath D. Characterization of the murine TIGR/myocilin gene. Mammal Gen. 1998;9:673–675. doi: 10.1007/s003359900844. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson N, Andrews M, Shepard A, et al. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- 29.Rao P, Allingham R, Epstein D. TIGR/Myocilin in human aqueous humor. Exp Eye Res. 2000;71:637–641. doi: 10.1006/exer.2000.0920. [DOI] [PubMed] [Google Scholar]
- 30.Russell P, Tamm E, Grehn F, Richt G, Johnson M. The presence and properties of myocilin in the aqueous humor. Invest Ophthalmol Vis Sci. 2001;42:983–986. [PubMed] [Google Scholar]
- 31.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 32.van Niel G, Raposo G, Candalh C, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 33.Perkumas K, Hoffman E, McKay BS, Stamer W. Myocilin-associated exosomes in human samples. Exp Eye Res. 2006;84:209–212. doi: 10.1016/j.exer.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamer W, Roberts B, Epstein D, Allingham R. Isolation of trabecular meshwork cells from primary open-angle glaucomatous tissue. Curr Eye Res. 2000;20:347–350. [PubMed] [Google Scholar]
- 35.Stamer W, Seftor R, Williams S, Samaha H, Snyder R. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- 36.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Niel G, Mallegol J, Bevilacqua C, et al. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52:1690–1697. doi: 10.1136/gut.52.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savina A, Fader C, Damiani M, Colombo M. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 39.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. JBiol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 40.Savina A, Videl M, Colombo M. The exosome pathway in K562 cells is regulated by rab11. J Cell Sci. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 41.Fautsch MP, Howell KG, Vrabel AM, Charlesworth MC, Muddiman DC, Johnson DH. Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Invest Ophthalmol Vis Sci. 2005;46:2848–2856. doi: 10.1167/iovs.05-0101. [DOI] [PubMed] [Google Scholar]
- 42.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, et al. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–22. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 43.Berman E. Biochemistry of the Eye. New York, NY: Plenum Press; 1991. pp. 151–200. [Google Scholar]
- 44.Inada K, Baba H, Okamura R. Quantitative determination of human aqueous proteins by crossed immunoelectrophoresis. Jpn J Ophthalmol. 1984;28:1–8. [PubMed] [Google Scholar]
- 45.Gould DB, Reedy M, Wilson LA, Smith RS, Johnson RL, John SW. Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma. Mol Cell Biol. 2006;26:8427–8436. doi: 10.1128/MCB.01127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson C, van der Straaten D, Craig J, et al. Tonography demonstrates reduced facility of outflow of aqueous humor in myocilin mutation carriers. J Glaucoma. 2003;12:237–242. doi: 10.1097/00061198-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Zillig M, Wurm A, Grehn F, Russell P, Tamm E. Overexpression and properties of wild-type and Tyr437His mutated myocilin in the eyes of transgenic mice. Invest Ophthalmol Vis Sci. 2005;46:223–234. doi: 10.1167/iovs.04-0988. [DOI] [PubMed] [Google Scholar]
- 48.Kim BS, Savinova OV, Reedy MV, et al. Targeted disruption of the myocilin gene (MYOC) suggests that human glaucoma-causing mutations are gain of function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]