Abstract
Genes of the Eya family and of the Six1/2 subfamily are expressed throughout development of vertebrate cranial placodes and are required for their differentiation into ganglia and sense organs. How they regulate placodal neurogenesis, however, remains unclear. Through loss of function studies in Xenopus we show that Eya1 and Six1 are required for neuronal differentiation in all neurogenic placodes. The effects of overexpression of Eya1 or Six1 are dose dependent. At higher levels, Eya1 and Six1 expand the expression of SoxB1 genes (Sox2, Sox3), maintain cells in a proliferative state and block expression of neuronal determination and differentiation genes. At lower levels Eya1 and Six1 promote neuronal differentiation, acting downstream of and/or parallel to Ngnr1. Our findings suggest that Eya1 and Six1 are required for both the regulation of placodal neuronal progenitor proliferation, through their effects on SoxB1 expression, and subsequent neuronal differentiation.
Keywords: Xenopus, NeuroD, Neurogenin, otic placode, epibranchial placodes, trigeminal placode, olfactory placode, lateral line placodes, p27Xic1, cell cycle
Introduction
The cranial placodes, which contribute to the cranial ganglia and sense organs of the vertebrate head, originate from the pre-placodal ectoderm that surrounds the anterior neural plate (reviewed in Baker and Bronner-Fraser, 2001; Bailey and Streit, 2006; Schlosser, 2006). Genes of the Eya family and the Six1/2 and Six4/5 subfamilies are specifically expressed in this pre-placodal ectoderm and continue to be expressed later in various placodes (Pandur and Moody, 2000; Ghanbari et al., 2001; David et al., 2001). Six proteins bind directly to DNA to regulate gene transcription; interactions with various cofactors including Eya modulate their activity (Pignoni et al., 1997; Ohto et al., 1999; Ikeda et al., 2002). Mutants or morphants of Six1 and Eya1 in mice, humans and zebrafish display similar developmental deficits affecting multiple placodal derivatives (Xu et al., 1999, Zheng et al., 2003; Li et al., 2003; Laclef et al., 2003; Zou et al., 2004; Ozaki et al., 2004; Friedman et al., 2005; Kozlowski et al., 2005; Bricaud and Collazo, 2006; Ikeda et al., 2007). In Xenopus, Six1 is required for the formation of the pre-placodal ectoderm (Brugmann et al., 2004). While these mutant phenotypes suggest that Six1 and Eya1 play a central role in the regulation of placodal neurogenesis the underlying mechanisms remain poorly understood.
Regulation of neurogenesis is particularly well studied in the Xenopus neural plate (reviewed in Bertrand et al., 2002). Neuronal differentiation is initiated by neuronal determination (proneural) genes coding for basic helix loop helix (bHLH) transcription factors, such as the neurogenin related gene Ngnr1 (Ma et al., 1996). These are transiently expressed in proliferating neural progenitors, and promote cell cycle exit and the expression of bHLH neuronal differentiation genes such as NeuroD (Ma et al., 1996; Farah et al., 2000). Proneural proteins also induce expression of ligands of the Notch pathway such as Delta1, which prevent neighboring cells from adopting a neuronal fate (lateral inhibition; Chitnis et al., 1995; Ma et al., 1996). Cell cycle exit is required for the progression of neuronal differentiation and is regulated by inhibitors of cyclin dependent kinases including p27Xic1 (Hardcastle and Papalopulu, 2000; Carruthers et al 2003). p27Xic1 also promotes neuronal differentiation more directly, possibly by stabilizing Ngnr1 protein (Vernon et al., 2003; Nguyen et al., 2006). Neuronal differentiation genes then activate batteries of neuron-specific genes including N-Tubulin (Lee et al., 1995). However, neuronal differentiation occurs only in a small subpopulation of neural plate cells; most cells are kept in a proliferative progenitor state where differentiation is blocked by various transcription factors including Sox1, Sox2 and Sox3, members of the SoxB1 subfamily of HMG box containing transcription factors (Bylund et al., 2003; Graham et al., 2003). In addition, SoxB1 genes bias lineage choices of progenitor cells towards a neural or neuronal fate (Mizuseki et al., 1998; Pevny et al., 1998; Kishi et al., 2000; Zhao et al., 2004; Kan et al., 2004, 2007; Wang et al., 2006).
Neurogenic placodes express many of the same genes that regulate neurogenesis in the neural plate (Schlosser and Northcutt, 2000; Abu-Elmagd et al., 2001; Vernon et al., 2003; Schlosser and Ahrens, 2004). Ngn1, Ngn2 and NeuroD act as neuronal determination and differentiation genes, respectively, in some placodes (Ma et al., 1998; Fode et al., 1998; Liu et al., 2000; Kim et al., 2001). The phenotypes of mutants or morphants of Eya1 and Six1 suggest that these genes are required both for the proliferation and survival of placodal neuronal progenitors (Zheng et al., 2003; Li et al., 2003; Bricaud and Collazo, 2006) as well as for the proper expression of neuronal determination and differentiation genes (Zou et al., 2004; Friedman et al. 2005; Bricaud and Collazo, 2006; Ikeda et al., 2007). However, it is unclear which target genes mediate the various effects of Eya1 and Six1. Here we use gain- and loss-of-function approaches in Xenopus to address this question. We show that high levels of expression of Eya1 and/or Six1 promote proliferating neuronal progenitors via activation of SoxB1 genes, while at lower levels they permit cell cycle exit and promote neuronal differentiation downstream of and/or parallel to Ngnr1. Our findings indicate that Eya1 and Six1 are required during multiple steps of placodal neurogenesis.
Materials and Methods
Expression constructs
Six1, Eya1, Sox3 and GR-Sox3 mRNAs were made from pCS2+-Eyalα(Ahrens and Schlosser, 2005), pDH105-Six1 (Pandur and Moody, 2000), pCS2-Sox3-V5His (Zhang et al., 2003) and pCS2-GR-Sox3-GFP (Zhang and Klymkowsky, 2007) plasmids. Myc-tagged Eya1 mRNA (myc-Eya) was made from pCS2+-myc-Eya1α generated by releasing the insert from pT-Adv-Eya1α (David et al., 2001) with EcoRI and subcloning it downstream of the myc-tag of pCS2+-myc. The construct was verified in immunoblot analyses following in vitro transcription and translation (TNT-coupled reticulocyte lysate kit, Promega) using a mouse anti-c-myc antibody (9E10, Developmental Studies Hybridoma Bank). Myc-tagged or untagged versions of Eya1 mRNA had identical effects on all markers analyzed.
To generate a hormone-inducible Eya1 expression vector (pCS2+-GR-myc-Eyalα ligand-binding domain of the human glucocorticoid receptor (GR) plus the myc-tag were released by digesting pCS2+-GR-myc (Hutcheson and Vetter, 2001) with BamHI and NcoI. This fragment was inserted into the BamHI/NcoI site upstream of Xenopus Eya1 replacing the myc-tag in the pCS2+-myc-Eya1α plasmid. To generate a hormone-inducible Six1 expression vector (pCS2+-GR-myc-Six1), the full open-reading frame of Xenopus Six1 was generated by PCR with primers containing XhoI sites and the pDH105-Six1 plasmid (Brugmann et al., 2004). The Six1 fragment was inserted into the XhoI site downstream of GR-myc in pCS2+-GR-myc. Both plasmids were confirmed by sequencing and used as templates to generate mRNAs (GR-Six1 and GR-Eya1).
To ensure that GR-fusion constructs function comparable to wild-type protein, injected embryos were cultured in dexamethasone (DEX) (10 μM) immediately after mRNA injection. For each construct these embryos displayed nearly the identical phenotype as those injected with the wild-type mRNAs (cf. Supplemental Table S1 to Table 2 and Brugmann et al., 2004). Embryos injected with GR-constructs and raised in the absence of DEX showed minimal effects (Supplemental Table S1) in accord with published accounts of this method (Hollenberg et al., 1993; Mattioni et al., 1994, Kolm and Sive, 1995; de Graaf et al., 1998). DEX treatment alone does not significantly alter gene expression (Supplemental Table S1).
Morpholinos
Morpholino antisense oligonucleotides (MO) against two different potential translational start sites in Six1 (Six1MO1: 5′-GGAAGGCAGCATAGACATGGCTCAG-3′; Six1MO2: 5′-CGCACACGCAAACACATACACGGG-3′; both lissamine-tagged) were previously described and analyzed for specificity (Brugmann et al., 2004). Two different MOs against Eya1 were generated (GeneTools): Eya1MO1 (5′-TACTATGTGGACTGGTTAGATCCTG-3′) targeted base pairs 10 to 34 of the Eya1 coding region, whereas Eya1MO2 (5′-ATATTTGTTCTGTCAGTGGCAAGTC-3′) was directed against base pairs -7 to-31 in the 5′ UTR of Eya1 (EST BJ066588, Genbank). The efficacy of Eya1MO1 was verified in western blots following in vitro transcription and translation (TNT-coupled reticulocyte lysate kit, Promega) of pCS2+-Eya1α (1 μg /25 μl reaction) with and without MO (1 μg of MO/25 μl reaction) using guinea pig anti-Xenopus-Eya1 antibody as previously described (Ahrens and Schlosser, 2005). MOs directed against the Sox2 (5′-CTCCATCATGCTGTACATGCAGGCG-3′) and Sox3 (5′-AACATGCTATACATTTGGAGCTTCA-3′) UTR/coding regions (Supplemental Fig. S1) were synthesized (GeneTools). To evaluate MO efficacy and specificity, epitope-tagged 5′ UTR-Sox2 and Sox3 constructs (pCS2-utr-Sox3-V5 and pCS2-utr-Sox2-V5) that include the sequences targeted by the Sox2MO and Sox3MO, respectively, were generated by PCR. A plasmid encoding ΔNXTCF3 (Molenaar et al., 1996) was supplied by Oliver Destree. mRNAs synthesized from pCS2-utr-Sox3-V5 and pCS2-utr-Sox2-V5 were injected into fertilized eggs (650 pg) together with Sox2 or Sox3 MOs (20 ng) and proteins were analyzed in western blots using anti-V5 and anti-XTCF3c antibodies as previously described (Zhang et al., 2003). A standard control MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′) obtained from GeneTools was used in control injections.
Microinjections
Embryos of Xenopus laevis were staged according to Nieuwkoop and Faber (1967) and injected according to standard procedures (Sive et al., 2000). Capped mRNAs were synthesized with Message Machine Kit (Ambion) and injected into single blastomeres at the 2-to 16-cell stage that give rise to the dorsal ectoderm. Unless otherwise noted, the following amounts of mRNAs were injected: Eya1 or myc-Eya1: 500-1000 pg; Six1: 250-500 pg; GR-Eya1: 400-500 pg; GR-Six1: 400-500 pg; Sox3: 500 pg; GR-Sox3: 500 pg. Morpholinos (see above) were injected singly or as a cocktail (20 ng each) into single blastomeres at the 2-16 cell stage. For rescue experiments, Eya1MO2 alone or in combination with Eya1MO1 (20 ng each) were co-injected with myc-Eya1 (250-500 pg). GR-Eya1+GR-Six1 (500 pg each) were co-injected with a combination of Sox2MO and Sox3MO (12-24 ng each). In injections of non-tagged mRNA or morpholinos, co-injection of myc-GFP (125-250 pg) (pCMTEGFP; kindly provided by Doris Wedlich) or lacZ (250 pg) identified the injected side. For activation of hormone-inducible constructs, embryos were incubated in DEX (10 μM; Sigma) from stages 16-18 onwards. To block protein synthesis, cycloheximide-treatment (50 μg/ml; Sigma) at stage 16-18 was followed after 30′ by incubation in cycloheximide supplemented with DEX (10 μM) for 3-4 hours at room temperature.
Tissue grafts
Grafts were performed as previously described (Ahrens and Schlosser, 2005). The lateral placodal region of neural plate stage albino embryos injected unilaterally with Eya1 (1000 pg) and myc-GFP (250 pg) at the 2-cell stage, was grafted orthotopically into pigmented host embryos of the same stage. Uninjected donor embryos were used in control experiments.
In situ hybridization and immunohistochemistry
Embryos injected with myc-GFP or with tagged morpholinos were sorted under a fluorescent stereomicroscope and then fixed according to standard procedures (Sive et al., 2000). LacZ injected embryos were fixed and then stained to reveal lacZ. Wholemount in situ hybridization was carried out as previously described (Schlosser and Ahrens, 2004) using digoxigenin-labeled antisense probes for Eya1 (David et al., 2001), Six1 (Pandur and Moody, 2000), Sox3 (Penzel et al., 1997), Sox2 (de Robertis et al., 1997), N-Tubulin (Oschwald et al., 1991), NeuroD (Lee et al., 1995), Ngnr1 (Ma et al., 1996), Delta1 (Chitnis et al., 1996), p27Xic1 (Ohnuma et al., 1999), and CyclinA1 (Carter et al., 2006).
After in situ hybridization, myc-tagged proteins were revealed immunohistochemically using mouse anti-c-myc antibody (9E10, Developmental Studies Hybridoma Bank) as previously described (Ahrens and Schlosser, 2005). Phosphohistone H3 (PH3) was identified with a rabbit anti-PH3 antibody (06-570, Upstate; 1:100) and revealed with either anti-rabbit-TRITC (T-6778, Sigma, 1:100) or anti-rabbit-IgG-HRP (111-035-003, Jackson ImmunoResearch, 1:200) followed by reaction in diaminobenzidine (Dent et al., 1989). PH3-immunopositive cells were counted in areas of defined size in the lateral placodal ectoderm of injected embryos. Uninjected sides of these embryos or lacZ injected embryos served as control.
Vibratome sections (30 μm) were cut after whole mount in situ hybridization. Immunohistochemistry on sections for myc-tagged proteins or Sox3 was performed as previously described (Schlosser and Ahrens, 2004). Sox3 was revealed using a polyclonal rabbit anti-Sox3 antibody (Zhang et al., 2003; 1:1000) and a TRITC-conjugated anti-rabbit-IgG antibody (T-6778, Sigma, 1:100). PCNA immunohistochemistry followed published protocols (Wullimann et al., 2005) except that anti-mouse-IgG2a-TXRD (1080-07, Southern Biotechnol., 1: 100) was used as secondary antibody. Secondary antibodies were sometimes coincubated with DAPI (100 ng/μl). Nonspecific binding of secondary antibodies was not observed when the primary antibody was omitted in control reactions.
Results
Placodal neurons differentiate adjacent to Eya1 and Six1 expression domains
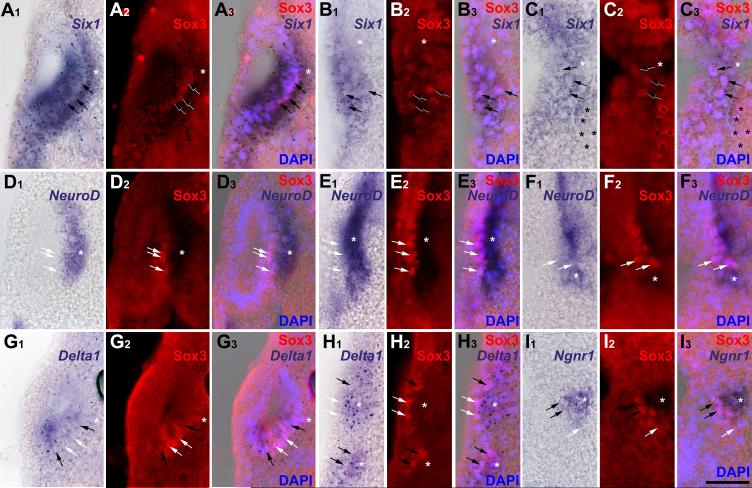
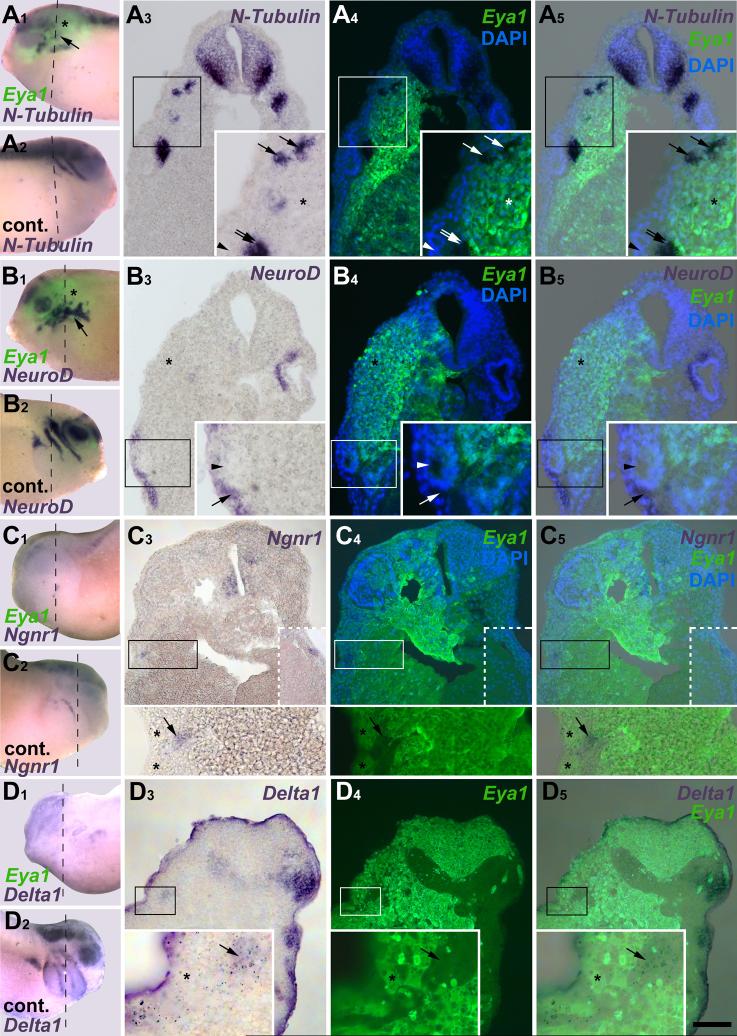
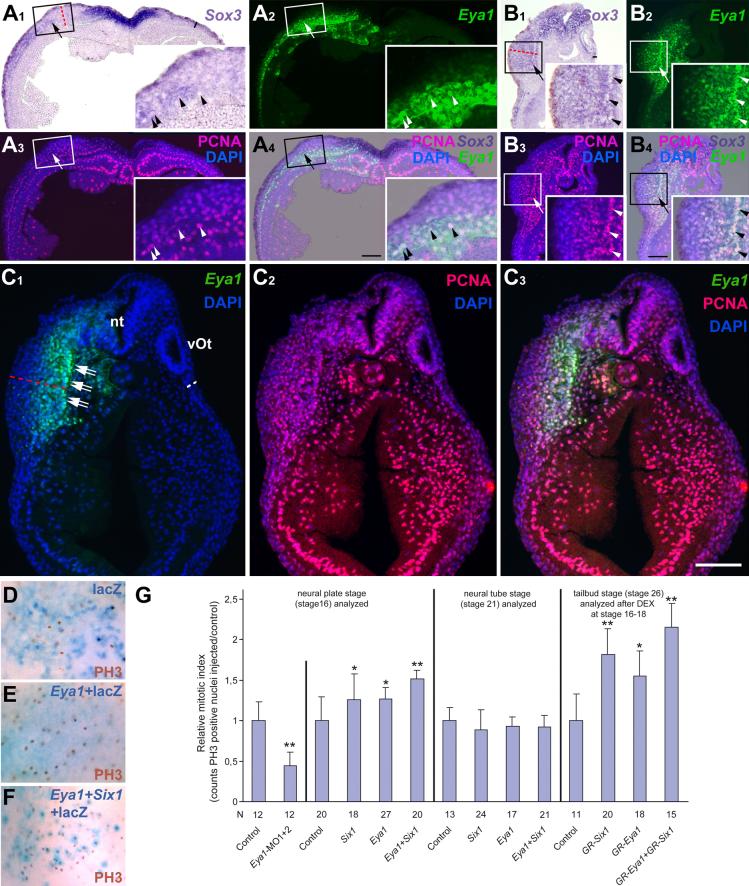
As the pre-placodal ectoderm begins to separate into discrete placodes, it maintains expression of Six1 and Eya1, while several genes regulating neurogenesis are expressed in delaminating cells (Pandur and Moody, 2000; Schlosser and Northcutt, 2000; David et al., 2001). To determine the progression of gene expression in placodal cells, we combined Sox3-immunostaining with in situ hybridization for Eya1, Six1, NeuroD, Ngnr1 or Delta1 (Fig. 1). Sox3-immunopositive nuclei were present in all neurogenic placodes except the profundal and trigeminal placodes and were always located fully within the expression domains of Six1 (Fig. 1 A-C) and Eya1 (not shown). However, Sox3+ cells always constituted a subpopulation in these domains. Expression of neurogenesis markers was analyzed in otic, lateral line and epibranchial placodes. Each of these expresses NeuroD and Delta1, whereas Ngnr1 is confined to epibranchial placodes. Delta1 and Ngnr1 were expressed in both the placodes and in incipient ganglion cells migrating away from the placodes. Delta1 and Ngnr1 expressing cells were both adjacent to Sox3+ cells and overlapping with them (Fig.1 G-H), suggesting that: 1) Sox3 expression is upstream of Delta1 and Ngnr1; and 2) cells that are down-regulating Sox3 expression initiate the expression of neuronal determination genes. In contrast, NeuroD expression was observed in incipient ganglion cells immediately adjacent to, but not overlapping with, Sox3+ nuclei (Fig. 1 D-F). These results indicate that Sox3+ neuronal progenitors arise from the Eya1 and Six1-positive pool, then initiate neuronal determination programs via Ngnr1 and Delta1, but complete neuronal differentiation only after Eya1, Six1 and Sox3 expression declines.
Fig. 1.
Placodal distribution of Sox3-immunopositive cells in relation to Six1, NeuroD, Delta1 and Ngnr1 expression in transverse sections through stage 26 embryos. Each section is shown in bright field (A1-I1: gene expression), red fluorescence (A2-I2: Sox3 protein), and a superposition of these two with blue DAPI fluorescence (A3-I3). White asterisks indicate placodally derived cranial ganglia (main body of ganglia out of level of section in B, C and G). Black asterisks mark endodermal Sox3 nuclei. A-C: Six1 expression domains in otic vesicle (A), anterodorsal lateral line (B) and facial epibranchial (C) placodes encompass Sox3 immunopositive nuclei (arrows). D-F: NeuroD is expressed in ganglia derived from otic vesicle (D), anterodorsal lateral line (E) and facial epibranchial placodes (F) immediately abutting Sox3-positive placodal cells (arrows). G-I: Delta1 expression in the otic vesicle (G), and the anterodorsal lateral line and glossopharyngeal epibranchial placodes and ganglia (H upper and lower part, respectively) and Ngnr1 expression in the facial epibranchial placode (I) overlap with some (black arrows) but not all (white arrows) Sox3-cells. Bar in I: 50 μm (for all panels).
Eya1 and Six1 are required for placodal neurogenesis
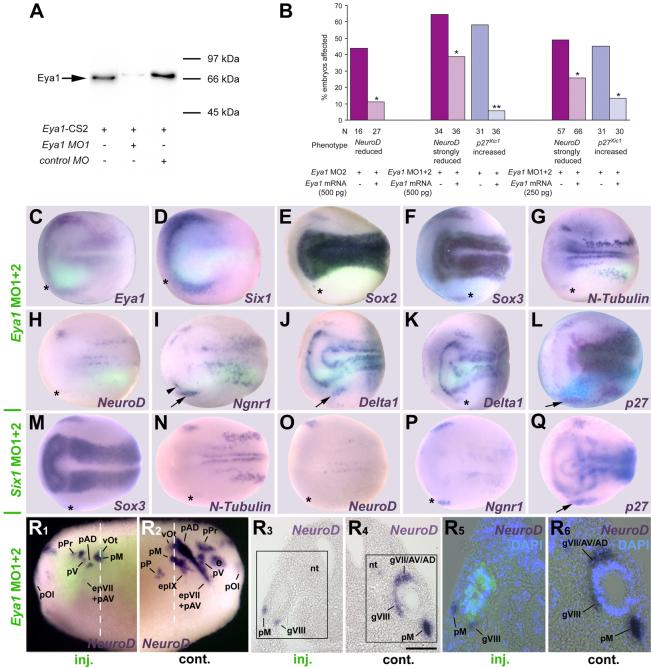
To test whether Eya1 and/or Six1 are required for placodal neurogenesis (Fig. 2, Tables 1,2) we generated morpholino antisense oligonucleotides (MOs) that specifically block translation of Six1 or Eya1 mRNAs. The efficacy and specificity of the two Six1 MOs were previously reported (Brugmann et al., 2004). The efficacy of Eya1MO1 was verified in vitro, where it strongly inhibited Eya1 protein synthesis (Fig. 2 A). Both Eya1MO1 and Eya1MO2, when injected individually, affected the expression of a wide range of markers in a similar fashion; co-injection of both MOs produced a higher frequency of stronger phenotypes (Tables 1, 2). Co-injection of a myc-tagged Eya1 mRNA rescued the effects of Eya1MO2 or Eya1MO1+2 injection on NeuroD and p27Xic1 expression (Fig. 2 B), demonstrating that these MOs specifically block Eya1 synthesis. Unless otherwise noted, control MOs had no or significantly less frequent effects on the markers analyzed than injections of Eya1MO1+2 or Six1MO1+2 (Table 2).
Fig. 2.
Effects of Eya1 and Six1 knockdown on markers of neurogenesis and placodal ectoderm. A: Immunoblot showing that Eya1MO1 but not control MO blocks synthesis of Eya1 protein. B: Co-injecting Eya1MO2 or Eya1MO1+2 with myc-tagged Eya1 mRNA significantly restores NeuroD and prevents ectopic p27Xic1 expression (X2 test; *: p< 0.05, **: p< 0.001) in three independent rescue experiments. C-Q: Neural plate stage embryos after unilateral injection (lower half) of Eya1MO1+2 (C-L) or Six1MO1+2 (M-Q). In all cases, where myc-GFP was coinjected as lineage tracer (C-K), embryos are shown superimposed with green fluorescent channel. Asterisks indicate reductions, arrows and arrowheads indicate increased marker gene expression. For Delta1 after Eya1MO1+2 injections two different phenotypes are depicted (J, K). R: Tail bud stage embryo after unilateral injection (R1, R3, R5: injected side; R2, R4, R6: control side) of Eya1MO1+2 reveals reduction of NeuroD expression in all neurogenic placodes or derivative ganglia. R3 and R4 depict a section at the level indicated (white line) with boxed areas shown magnified in R5 and R6, respectively, superimposed with green (myc-GFP co-injected with Eya1MO1+2) and blue (DAPI) fluorescence. Residual NeuroD expression is confined to cells receiving little or no MO. Bar in R4: 100 μm (for R3, R4). Abbreviations: e: eye; epVII+AV: facial epibranchial and anteroventral lateral line placode; epIX: glossopharyngeal epibranchial placode; gVII/AV/AD: ganglion of the facial, anteroventral and anterodorsal lateral line nerve; gVIII: vestibulocochlear ganglion; nt: neural tube; pAD: anterodorsal lateral line placode; pM: middle lateral line placode; pOl: olfactory placode; pP: posterior lateral line placode; pPr: profundal placode; pV: trigeminal placode; vOt: otic vesicle.
Table 1.
Changes in marker gene expression in the placodal and non-neural ectoderm after injection of Eya1 morpholinos
| Injection |
Eya1 MO11 % (n) |
Eya1 MO21 % (n) |
|---|---|---|
| Phenotype | ||
| Eya1 | ||
| Reduced | 40** (15) |
75** (8) |
| Six1 | ||
| Reduced | 41* (17) |
52** (31) |
| Sox3 | ||
| Reduced | 8 (26) |
29* (45) |
| Increased/ectopic | 15* (26) |
11* (45) |
| N-Tubulin | ||
| Reduced | 58** (40) |
73** (41) |
| Increased/ectopic | 0 (40) |
3 (41) |
| NeuroD | ||
| Reduced | 71** (38) |
68** (79) |
| Ngnr1 | ||
| Reduced | 18 (40) |
36* (50) |
| Increased/ectopic | 38** (40) |
20* (50) |
| Delta1 | ||
| Reduced | 42* (19) |
50** (32) |
| Increased/ectopic | 16* (19) |
3 (32) |
n: number of embryos analyzed at neural plate (stage 14-16) and tail bud (stage 21-26) stages
significant differences (X2 test; *: p< 0.05, **: p< 0.001) to control MO injections (see Table 2) are indicated
Table 2.
Changes in marker gene expression in the placodal and non-neural ectoderm after injection of various constructs
| Injection | Control MO % (n) |
Eya1 MO1+21 % (n) |
Six1 MO1+21 % (n) |
Sox2MO+ Sox3MO1 % (n) |
Eya1 | Six1 | Eya1+Six1 |
Sox3 % (n) |
GR- Eya12 % (n, tb) |
GR- Six12 % (n, tb) |
GREya1+ GR-Six12 % (n, tb) |
GR- Sox32 % (n, tb) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | % (n, np) |
% (n, tb) |
% (n, np) |
% (n, tb) |
% (n, np) |
% (n, tb) |
|||||||||
| Eya1 | |||||||||||||||
| Reduced | 4 (45) |
54** (39) |
nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Increased/ectopic | 0 (45) |
15* (39) |
nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Six1 | |||||||||||||||
| Reduced | 8 (38) |
45** (81) |
nd | nd | 0 (62) |
0 (77) |
nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Increased/ectopic | 0 (38) |
0 (81) |
nd | nd | 55 (62)3 |
44 (77)3 |
nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Sox3 | |||||||||||||||
| Reduced | 12 (60) |
30* (156) |
62** (153) |
nd | 29 (52) |
53 (117) |
52 (46) |
59 (69) |
85 (40) |
90 (48) |
nd | 32 (34) |
70 (125) |
56 (135) |
nd |
| Increased/ectopic | 0 (60) |
12* (156) |
0 (153) |
nd | 29 (52) |
22 (117) |
4 (46) |
0 (69) |
35 (40) |
38 (48) |
nd | 32 (34) |
39 (137) |
63 (135) |
nd |
| Sox2 | |||||||||||||||
| Reduced | 6 (32) |
58** (51) |
54** (26) |
nd | 30 (63) |
50 (38) |
86 (28) |
71 (68) |
42 (26) |
36 (25) |
68 (34) |
35 (40) |
58 (106) |
46 (52) |
nd |
| Increased/ectopic | 0 (32) |
26* (31) |
0 (26) |
nd | 38 (63) |
37 (38) |
0 (28) |
6 (33) |
46 (26) |
59 (32) |
9 (34) |
68 (40) |
28 (114) |
69 (52) |
nd |
| N-Tubulin | |||||||||||||||
| Reduced | 9 (32) |
90** (206) |
66** (85) |
80** (40) |
60 (52) |
48 (42) |
88 (57) |
86 (14) |
79 (19) |
88 (17) |
91 (46) |
41 (41) |
41 (32) |
43 (82) |
42 (26) |
| Increased/ectopic | 0 (32) |
0 (206) |
0 (85) |
0 (40) |
4 (52) |
33 (42) |
0 (57) |
0 (14) |
11 (19) |
25 (20) |
0 (46) |
30 (41) |
22 (32) |
42 (82) |
0 (26) |
| NeuroD | |||||||||||||||
| Reduced | 24 (89) |
79** (238) |
70** (182) |
75** (60) |
64 (63) |
17 (201) |
83 (78) |
74 (87) |
72 (39) |
87 (37) |
81 (67) |
12 (42) |
62 (141) |
16 (195) |
49 (78) |
| Increased/ectopic | 0 (89) |
1 (146) |
0 (182) |
0 (60) |
3 (63) |
45 (201) |
0 (78) |
0 (87) |
5 (39) |
87 (37) |
0 (67) |
52 (42) |
29 (141) |
71 (195) |
0 (53) |
| Ngnr1 | |||||||||||||||
| Reduced | 10 (41) |
21 (116) |
45** (75) |
38* (37) |
44 (50) |
50 (117) |
75 (68) |
57 (62) |
76 (45) |
86 (21) |
45 (51) |
27 (33) |
57 (136) |
61 (79) |
67 (30) |
| Increased/ectopic | 0 (41) |
34** (116) |
36** (75) |
38** (37) |
14 (50) |
9 (117) |
4 (68) |
14 (52) |
4 (45) |
0 (21) |
18 (51) |
24 (33) |
5 (168) |
18 (79) |
0 (30) |
| Delta1 | |||||||||||||||
| Reduced | 7 (30) |
33* (75) |
58** (79) |
69** (36) |
49 (33) |
48 (58) |
68 (40) |
68 (66) |
93 (27) |
80 (25) |
52 (60) |
44 (27) |
72 (127) |
54 (41) |
64 (36) |
| Increased/ectopic | 0 (30) |
22* (75) |
18* (79) |
0 (36) |
27 (33) |
4 (58) |
48 (44) |
0 (66) |
0 (27) |
0 (25) |
32 (60) |
0 (27) |
2 (136) |
10 (41) |
0 (36) |
| p27Xic1 | |||||||||||||||
| Reduced | 17 (35) |
22 (72) |
29 (59) |
42* (33) |
43 (115) |
76 (72) |
75 (60) |
82 (71) |
80 (10) |
nd | 78 (55) |
35 (161) |
84 (83) |
39 (209) |
24 (46) |
| Increased/ectopic | 0 (35) |
51** (72) |
34** (59) |
49** (33) |
48 (115) |
20 (110) |
15 (40) |
0 (71) |
0 (10) |
nd | 38 (55) |
29 (161) |
8 (49) |
50 (209) |
17 (46) |
| CyclinA1 | |||||||||||||||
| Reduced | nd | nd | nd | nd | nd | 2 (46) |
0 (27) |
0 (38) |
nd | nd | nd | 0 (112) |
0 (37) |
0 (91) |
nd |
| Increased/ectopic | nd | nd | nd | nd | nd | 0 (46) |
0 (27) |
0 (38) |
nd | nd | nd | 0 (112) |
0 (37) |
1 (91) |
nd |
n: number of embryos analyzed at both neural plate (stage 14-16) and tail bud (stage 21-26) stage;
n, np: number of embryos analyzed at neural plate (stage 14-16) stage;
n, tb: number of embryos analyzed at tail bud (stage 21-26) stage;
nd: not determined;
Significant differences (X2 test; *: p< 0.05, **: p< 0.001) to control MO injections are indicated
Dexamethasone treatment from stages 16-18 on
Expression broader but weaker
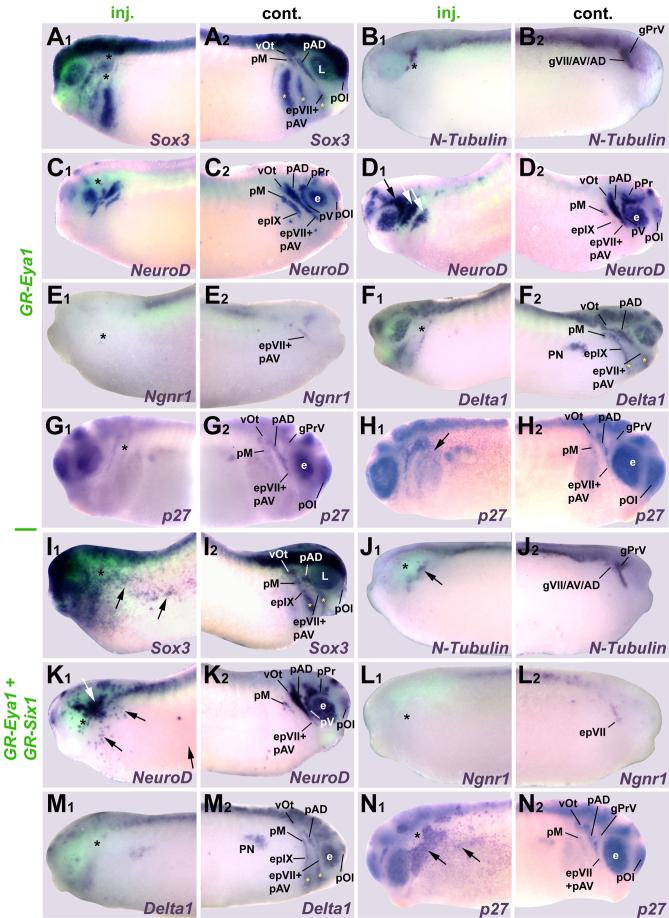
Similar to findings after Six1 loss of function (Brugmann et al., 2004), injection of Eya1MOs reduced expression of Eya1 and Six1 (Fig. 2 C, D; Table 2), demonstrating its requirement for establishing the pre-placodal ectoderm. In addition, injection of either Eya1MOs or Six1MOs resulted in significant reduction of placodal neurons as evidenced by reduced N-Tubulin and NeuroD expression in all neurogenic placodes (Fig. 2 G, H, N, O, R), corroborating results in mutant mice that Eya1 and Six1 are required for neuronal differentiation in placodes (Zou et al., 2004, Ikeda et al., 2007). We further demonstrate that this requirement is cell autonomous, since NeuroD is reduced in cells containing Eya1MOs (Fig. 2 R). We then investigated which neurogenesis genes are involved in the reduction of placodal neurons. Both Eya1MOs and Six1MOs generally reduced placodal expression of Sox3 (Fig. 2 F, M) and Sox2 (Fig. 2 E), although occasionally Eya1MOs led to an expansion of the Sox3 and Sox2 expression domain (Table 2). This was manifested as a broader, but more faintly stained expression domain or scattered ectopic spots in the head. Loss of Eya1 or Six1 function often reduced Ngnr1 (Fig. 2 P) and Delta1 (Fig. 2 K) expression domains, likely because Eya1 and Six1 are required to establish the placodal ectoderm. However, in about a third of the embryos Ngnr1 and Delta1 domains were broadened (Fig. 2 I, J), indicating that neuronal determination proceeds more effectively when Eya1 or Six1 levels are reduced. Eya1 loss of function significantly reduced the number of mitotic (PH3-positive) cells (Fig. 9 G), while reduction of either Eya1 or Six1 led to the expansion of the p27Xic1 expression domain (Fig. 2 L, Q), suggesting a role in blocking the cell cycle exit that is necessary for neuronal differentiation. These results demonstrate that loss of Eya1 and Six1 function interferes with proliferation, SoxB1 expression and neuronal differentiation but allows neuronal determination to proceed. This suggests a dual requirement for Eya1 and Six1 during placodal neurogenesis upstream of SoxB1 genes as well as downstream of and/or parallel to Ngnr1 and p27Xic1.
Eya1 and Six1 delay the onset of neuronal differentiation
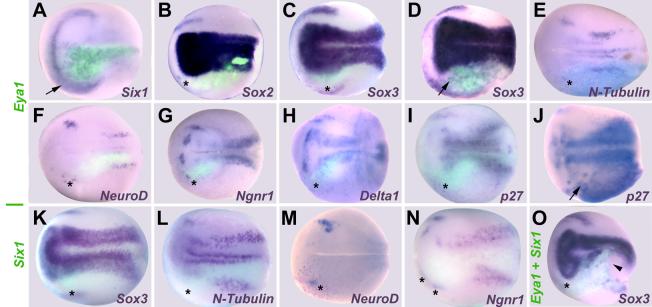
To complement our loss of function studies, we overexpressed myc-tagged and untagged forms of Eya1 and Six1 either individually or in combination by mRNA injection (Figs. 3-5, Table 2). Injection of Six1 and Eya1+Six1 mRNAs expanded the pre-placodal ectoderm at the expense of neural crest and epidermal genes (Brugmann et al., 2004). Likewise, injection of Eya1 mRNA led to broader and more diffuse expression of Six1 in cranial ectoderm (Fig. 3 A). However, injection of Eya1, Six1 or Eya1+Six1 mRNAs at levels that expand placodal ectoderm tended to repress the expression of genes required for neurogenesis at neural plate stages (Fig. 3, Table 2). Placodal Sox2 and Sox3 expression were frequently reduced, in particular after Six1 mRNA injection (Figs. 3 B, C, K, O) while Eya1 mRNA injection also caused ectopic expression of low levels of Sox2 and/or Sox3 in both head and trunk ectoderm (Fig. 3 D). Eya1 and/or Six1 overexpression reduced placodal expression of Ngnr1, Delta1 and p27Xic1 (Figs. 3 G, H, I, N). For Eya1-injected embryos, Ngnr1 and Delta1 occasionally displayed a broader domain of expression, and ectopic p27Xic1 expression was observed in head and trunk ectoderm in nearly half of the embryos (Fig. 3 J). For Six1-injected embryos, Ngnr1 and p27Xic1 were rarely expanded, whereas this phenotype occurred frequently for Delta1. Finally, both N-Tubulin (Fig. 3 E, L) and NeuroD (Figs. 3 F, M) were strongly reduced by Eya1, Six1 or Eya1+Six1.
Fig. 3.
Effects of Eya1 and Six1 overexpression on markers of neurogenesis and placodal ectoderm at neural plate stages. Embryos after unilateral injection (lower half) of Eya1 (A-J), Six1 (K-N) or Eya1+Six1 (O) mRNA. In all cases, where myc-GFP was coinjected as lineage tracer (A-D, F-I, K, L, N, O), embryos are shown superimposed with green fluorescent channel. Black asterisks indicate reductions in the placodal or non-neural ectoderm. Arrows mark increased or ectopic marker gene expression in the placodal or non-neural ectoderm; black arrowhead identifies deformed blastopore due to gastrulation defects (O). For Sox3 (C,D) and p27Xic1 (I,J) after Eya1 injections two different phenotypes are depicted.
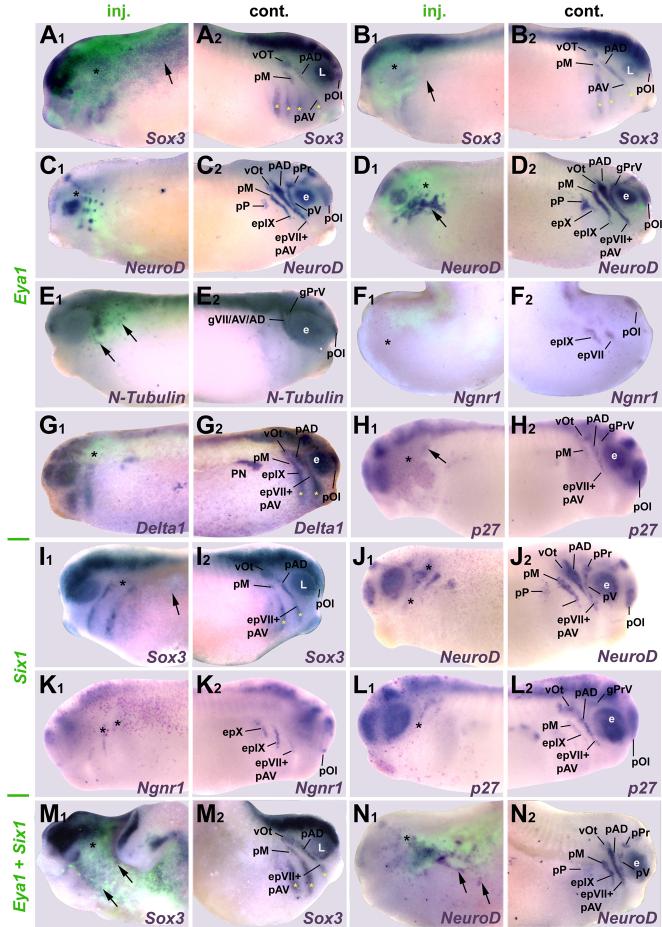
To determine whether the effects of Six1 or Eya1 overexpression were transient, similarly injected embryos were analyzed at tail bud stages (Fig. 4, Table 2) when most placodes have separated and initiated neuronal differentiation. The effects of Eya1 and/or Six1 mRNA injection on Sox3, Sox2, and Ngnr1 were similar to effects at neural plate stages (Fig. 4 A, B, F, I, K, M). The frequencies at which Delta1 and p27Xic1 were expanded decreased significantly (Fig. 4 G, H, L). While NeuroD and N-Tubulin continued to be frequently reduced, increased or ectopic expression was now also observed after injection of Eya1 mRNA (Fig. 4 C-E, J) in the vicinity of placodes and in spots throughout head ectoderm. After coinjection of Eya1 and Six1 mRNAs, ectopic spots of NeuroD expression in the cranial ectoderm occurred at high frequency (Figs. 4 N, 10 B). Often placodal expression of these genes was reduced throughout the region containing the exogenous Eya1 or Six1 mRNAs, with ectopic expression occurring at its border (Fig. 4 D). These data suggest that increased levels of Eya1 and Six1 delay the expression of neuronal determination and differentiation genes, which ultimately results in larger numbers of placodal neurons. Moreover, Eya1 and Six1 affect neuronal differentiation synergistically and Eya1 rather than Six1 is limiting in vivo because ectopic neurons form only after Eya1 but not after Six1 overexpression.
Fig. 4.
Effects of Eya1, Six1 and Eya1+Six1 overexpression on various neurogenesis markers at tail bud stages (stage 26). Injected (A1-N1) and control (A2-N2) sides of tail bud stage embryos after unilateral injection of Eya1 (A-H), Six1 (I-L) or Eya1+Six1 (M, N) mRNAs. In all cases, where myc-GFP was coinjected as lineage tracer (A-G, M-N), embryos are shown superimposed with green fluorescent channel. Black asterisks indicate reductions, while black arrows mark increased or ectopic marker gene expression in the placodal or non-neural ectoderm. Yellow asterisks identify expression in pharyngeal pouches. For Sox3 (A, B) and NeuroD (C, D) after Eya1 injections two different phenotypes are depicted. Abbreviations as in Fig. 2. gPrV: profundal-trigeminal ganglionic complex; L: lens placode; PN: pronephros.
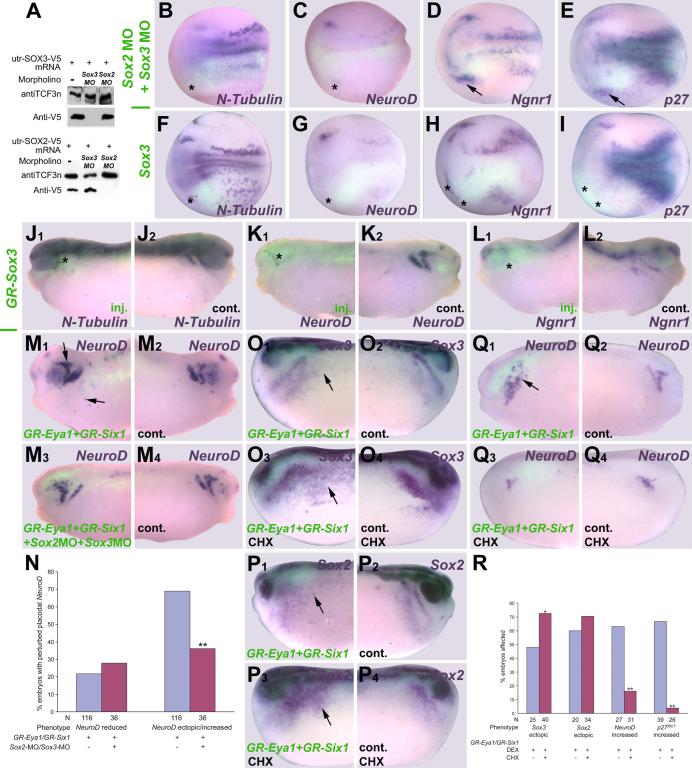
To ensure that these phenotypes are not due to effects in the early embryo, prior to the induction of the pre-placodal ectoderm, we injected mRNAs encoding GR-fusion constructs of Eya1 and Six1 (GR-Eya1, GR-Six1; see Supplemental Table S1 for validation). These proteins were activated by adding DEX at neural fold stages (stage16-18), when pre-placodal ectoderm has become committed (Ahrens and Schlosser, 2005). Embryos were then analyzed at early tail bud stages. The phenotypes observed were very much as described for wild-type constructs analyzed at tail bud stages (Fig. 5; Table 2). However, injection of GR-Eya1 or GR-Six1 led to higher frequencies of ectopic expression of Sox3 (Fig. 5 I) and Sox2 in head and trunk ectoderm; this was particularly extensive after co-injection of both constructs. Ectopic expression of Sox2 and Sox3 were seen only with GR-Six1 but not wild-type Six1. Similarly, ectopic expression of N-Tubulin and NeuroD was only seen after GR-Six1 but not after wild-type Six1 injection.
Fig. 5.
Effects of overexpression of GR-Eya1 or GR-Eya1 + GR-Six1 on various neurogenesis markers at tail bud stages (stage 26). Injected (A1-N1) and control (A2-N2) sides of tail bud stage embryos after unilateral injection of GR-Eya1 (A-H) or GR-Eya1+GR-Six1 (I-N) and DEX activation at stage 16-18. In all cases, where myc-GFP was coinjected as lineage tracer (A-F, I-M), embryos are shown superimposed with green fluorescent channel. Black asterisks indicate reductions, while black or white arrows mark increased or ectopic marker gene expression in the placodal or non-neural ectoderm.Yellow asterisks identify expression in pharyngeal pouches. For NeuroD (C, D) and p27Xic1 (G, H) after GR-Eya1 injection two different phenotypes are depicted. Abbreviations as in Figs. 2 and 4.
High levels of Eya1 and Six1 inhibit neuronal differentiation but expand the pool of proliferative SoxB1-positive neuronal progenitors
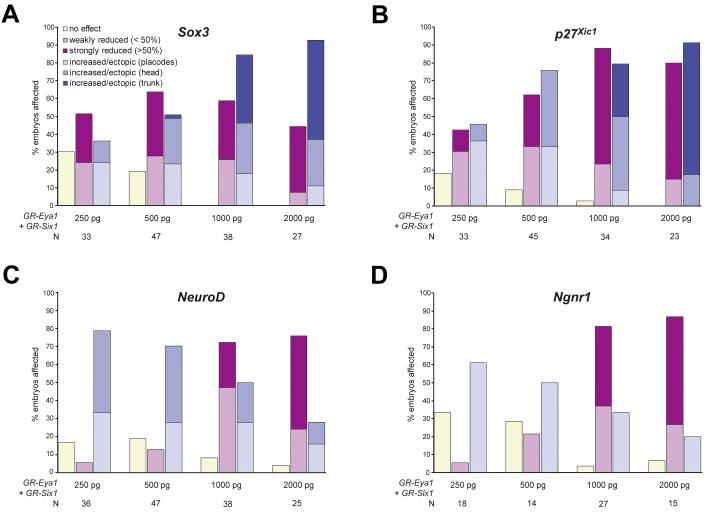
One explanation for the diverse phenotypes observed involves a threshold effect; at protein levels above the threshold level these proteins act to maintain cells in a SoxB1-positive proliferative neuronal progenitor state, whereas below this level ectopic neurons can differentiate from the expanded pool of progenitors. This model was supported by injecting increasing doses of GR-Eya1+GR-Six1 followed by DEX activation at stage 16-18 (Fig. 6). The frequency of embryos with ectopic Sox3 expression as well as the extent of ectopic Sox3 expression increased with increasing doses of injected Eya1 and Six1 mRNA (although Sox3 expression in the placodes was often reduced) (Fig. 6 A). In contrast, the frequency and extent of increased and/or ectopic expression of Ngnr1 or NeuroD declined and the frequency and extent of reductions in p27Xic1, Ngnr1 or NeuroD expression increased (Fig. 6 B-D). This suggests that increasing concentrations of Eya1 and Six1 increasingly delay cell cycle exit. In contrast to Ngnr1 and NeuroD, frequency and extent of increased and/or ectopic expression of p27Xic1 also increased with increasing doses of injected Eya1 and Six1 mRNA (althoughp27Xic1 expression in the placodes was often reduced in the same embryos) (Fig. 6 B), suggesting that high levels of Eya1 and Six1 prevent onset of neuronal differentiation even in cells that have left the cell cycle.
Fig. 6.
Dose-dependent effects of Eya1 on neurogenesis markers. Changes in placodal expression of Sox3 (A), p27Xic1 (B), NeuroD (C), and Ngnr1 (D) at stages 21-26 after injecting one blastomere at the two cell stage with various doses of GR-Eya1+GR-Six1 mRNA followed by DEX activation at stage 16-18. For all markers analyzed, reductions of marker gene expression was categorized as weak (less than 50 % reduced) or strong (more than 50 % reduced), while increased or ectopic marker gene expression was categorized as being confined to the vicinity of placodes (placodes), extending to other parts of head ectoderm (head) or extending into trunk ectoderm (trunk). Note that reductions in placodal marker gene expression were sometimes associated with increased and/or ectopic marker gene expression elsewhere in the same embryo.
In addition, analysis of the expression domains of neurogenesis genes in relation to injected Eya1 or Six1 in tissue sections through the placodal region of tail bud embryos showed that in ectoderm containing exogenous Eya1, identified by myc-immunostaining, N-Tubulin, NeuroD, Ngnr1, p27Xic1 and Delta1 were repressed, whereas residual and ectopic expression were located in adjacent areas with weak Eya1 expression (Fig. 7). Similar results were found with GR-Eya1 and co-expression with Six1 or GR-Six1.
Fig. 7.
Dose-dependent effects of Eya1 on neurogenesis markers as revealed in tissue sections. Distribution of N-Tubulin (A), NeuroD (B), Ngnr1 (C) and Delta1 (D) in stage 26 embryos after injection of myc-Eya1 or co-injection of Eya1 with myc-GFP mRNA. Embryos are shown as wholemounts (injected side: A1-D1; control side: A2-D2) and in transverse sections (at level indicated by black line) shown in bright field (A3-D3), superimposition of green (myc-immunostaining) and blue (DAPI) fluorescence (A4-D4, no DAPI image available in D), and as a merged image (A5-D5). Boxed areas are magnified in inserts. The DAPI channel is not shown in the inserts of C to make Ngnr1 staining more clearly visible. Hatched boxed area in C3-C5 shows Ngnr1 positive epibranchial placode on control side in an adjacent section. Asterisks indicate reductions, while arrows mark ectopic marker gene expression in the placodal or non-neural ectoderm. Double arrows identify areas of residual expression. Arrowheads mark displaced otic vesicle on injected side. Bar in D5: 100 μm (for A3-5-D3-5).
To test whether this distribution of neuronal differentiation is due to non-cell autonomous action of Eya1, we orthotopically grafted placodal ectoderm from Eya1 mRNA injected embryos or uninjected control embryos into uninjected hosts (Fig. 8). While some reductions of placodal NeuroD expression were observed in grafts from both control (2/5) and Eya1 injected (10/19) embryos, an ectopic increase of NeuroD expression was only observed in grafts from Eya1 injected donors (5/19) and was confined to the graft. This indicates that Eya1 promotes neuronal differentiation in a cell-autonomous fashion.
Fig. 8.
Cell-autonomous effects of Eya1 on placodal neurogenesis. Panels show distribution of NeuroD in grafts of placodal ectoderm from embryos injected with Eya1 and myc-GFP mRNA to uninjected hosts. Boxed area is magnified in insert of A. Insert of A and C are shown superimposed with green fluorescent channel to reveal myc-immunostaining. While NeuroD expression in profundal (pPr) and trigeminal (pV) placodes is reduced in the graft (asterisk, A, C) compared to control side (B), NeuroD is expressed ectopically (arrow) at graft border but not in host ectoderm (A, C).
High levels of Eya1 appear to be incompatible with high levels of Sox3 expression since native Sox3 expression domains in the placodes are repressed in areas of intense exogenous Eya expression. At the same time, there is ectopic low level expression of Sox3 throughout these regions, and the ectoderm is dramatically thickened and contains multiple layers of cells compared to the thin bilayered ectoderm on the control side (Fig. 9 A-C). Sox3-expressing cells within these ectodermal thickenings are immunopositive for proliferating cell nuclear antigen (PCNA), indicating that the thickenings result from increased proliferation (Fig. 9 A, B).
Fig. 9.
Eya1 and Six1 promote proliferative SoxB1-positive neuronal progenitors. A-C: Transverse sections through neural plate (A) or tail bud stage (B, C) embryos that received unilateral myc-Eya1 mRNA injection at the two cell stage. A, B: Panels show Sox3 expression in bright field (A1-B1), distribution of injected myc-Eya1 protein (A2-B2), PCNA together with DAPI staining (A3-B3) and a merged image (A4-B4). C: Panels show distribution of injected myc-Eya1 protein (C1), PCNA (C2) and a merged image (C3) together with DAPI staining. Ectoderm that received high levels of Eya1 expresses Sox3 (A1-B1, arrows), is drastically thickened (A1-C1, red lines) compared to control side (black or white lines) and consists of multiple layers of PCNA positive cells (A3, B3, C2). The otic vesicle has failed to form on the injected side of embryos in B and C. Arrowheads identify cells coexpressing myc-Eya1 and PCNA. Double arrows in C identify the basal lamina of the ectoderm on the injected side. Abbreviations: nt: neural tube. vOt: otic vesicle. All bars: 100 μm. D-G: Effects of Eya1 and Six1 gain and loss of function on phosphohistone H3 (PH3). D-F: PH3 immunopositive nuclei in placodal ectoderm of neural plate stage embryos after injection of lacZ (D) Eya1 + lacZ (E) or Eya1 + Six1 + lacZ mRNAs (F). G: Relative mitotic index after injections of Six1 or Eya1 mRNAs or morpholinos. Standard deviations and significant increases are indicated (t-Test; *: p< 0.05, **: p< 0.001).
To quantify the effects on cell proliferation we counted nuclei immunopositive for PH3, a marker for cells in mitosis (Saka and Smith, 2001), in the placodal region after injection of mRNAs encoding wild-type Eya1 and/or Six1. Each significantly increased the proportion of PH3-positive cells at neural plate stages and co-injection augmented this effect (Fig. 9 D-G). By neural tube stages the mitotic index returned to control levels indicating that this is a transient response. After injections of mRNA encoding GR-Eya1 and/or GR-Six1 and DEX-activation at neural fold stages, a similar significant increase of the mitotic index was observed (Fig. 9 G). These results are compatible with the observation that both wild-type and inducible forms of Eya1 and Six1 reduced placodal expression of p27Xic1 (Figs. 3 I, 4 H, L, 5 G, N), indicating that they delay cell cycle exit. However, neither Eya1 nor Six1 affected CyclinA1 expression in the developing placodal ectoderm (Table 2), indicating that neuronal progenitor pool expansion is mediated by other target genes of Eya1 and Six1.
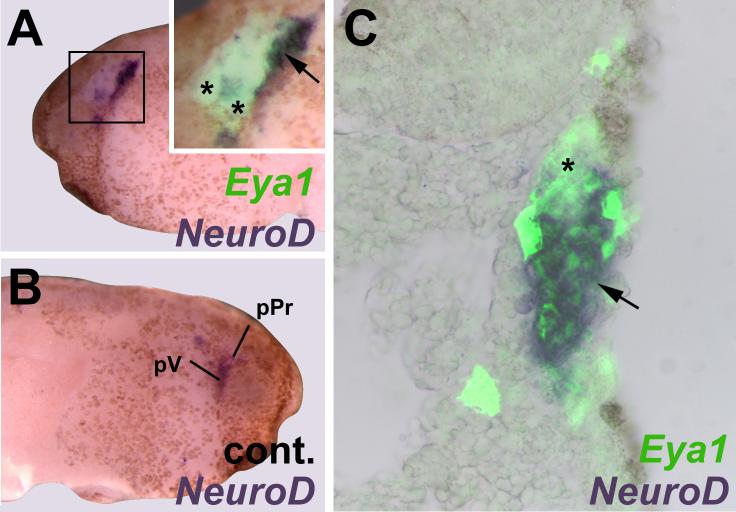
Taken together these findings substantiate the proposal that high levels of Eya1 and Six1 synergistically delay cell cycle exit and prevent neuronal differentiation but promote the proliferation of SoxB1-positive neuronal progenitor cells. To confirm that the ectopic neurons observed in areas of low exogenous Eya1 and Six1 expression originate from Sox3+ neuronal progenitors, we examined NeuroD expression in relation to Sox3-immunostaining (Fig. 10). While Sox3-immunostaining and NeuroD expression are mutually exclusive, ectopic NeuroD expressing cells are found adjacent to Sox3+ cells and some of the latter are localized within ectoderm expressing high levels of exogenous Eya1 (Fig. 10 A) or Eya1 + Six1 (Fig. 10 B). This spatial relationship suggests that Sox3+ cells differentiate as neurons once Eya1 and Six1 levels decline below a certain threshold.
Fig. 10.
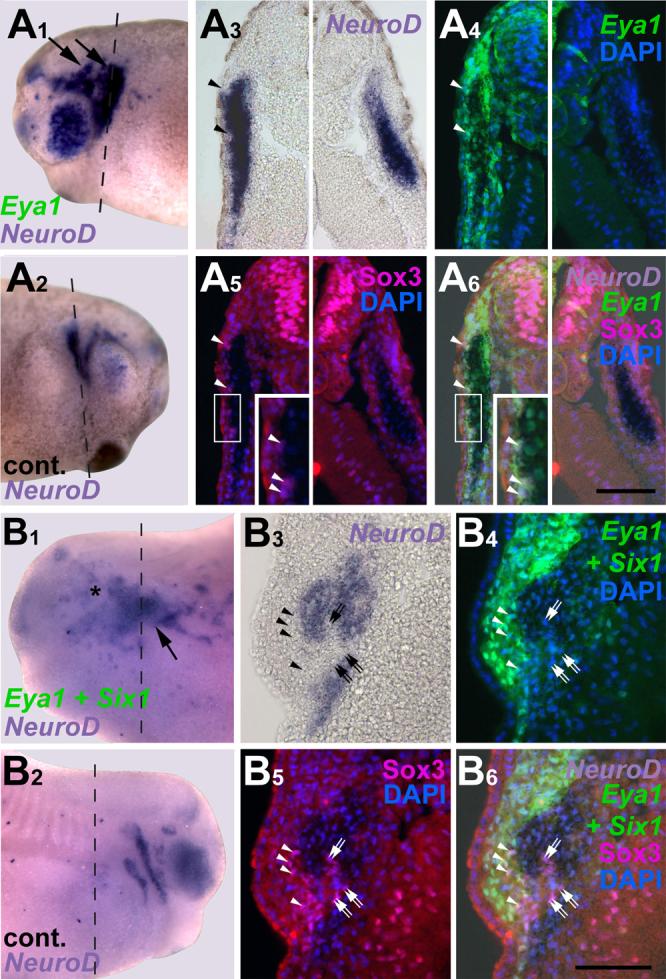
Ectopic neurons in embryos overexpressing Eya1 or Eya1+Six1 originate from Sox3 immunopositive neuronal progenitors. Panels show distribution of NeuroD expressing cells in relation to Sox3-immunopositive cells in tail bud stage embryos after injection of myc-Eya1 mRNA alone (A) or together with Six1 mRNA (B). Embryos are shown as wholemounts (injected side: A1-B1; control side: A2-B2) and in transverse sections (at level indicated by black line). Panels show bright field images (A3-B3), myc-immunostaining together with DAPI (A4-B4), Sox3-immunostaining together with DAPI (A5-B5), and merged images (A6-B6). Asterisks indicate reductions, while arrows mark ectopic NeuroD expression in the non-neural ectoderm. Ectopic NeuroD expression is found next to Sox3 immunopositive cells on the fringes of ectodermal regions that received high levels of myc-Eya1. Sox3 cells reside in ectoderm that received high levels of myc-Eya1 (arrowheads) as well as in areas of lower myc-Eya1 levels immediately abutting NeuroD expressing cells (double arrows). Boxed areas are shown at higher magnifiction in inserts. All bars: 100 μm
Eya1 and Six1 control placodal neurogenesis in a SoxB1-dependent fashion
Our model implies that Eya1 and Six1 modulate placodal neurogenesis in a SoxB1-dependent fashion. To test this hypothesis, we injected either SoxB1 morpholinos or mRNAs into one blastomere at the two cell stage and analyzed neurogenesis marker expression at neural plate and tail bud stages (Fig. 11, Table 2). To test efficacy and specificity of MOs designed to block the translation of Sox2 and Sox3 mRNAs (Supplemental Fig. S1), we co-injected them together with utr-Sox2 and utr-Sox3 mRNA into fertilized eggs. The Sox2MO inhibited protein synthesis from the utr-Sox2 mRNA but not from the utr-Sox3 mRNA and vice versa (Fig. 11 A). Neither MO affected TCF3 synthesis, an internal control.
Fig. 11.
Role of SoxB1 genes in placodal neurogenesis. A: Immunoblot showing that Sox2MO and Sox3MO specifically and efficiently block protein synthesis from co-injected utr-Sox2 and utr-Sox3 mRNA, respectively. B-E, F-I: Effects of unilateral injection (lower half) of Sox2/Sox3MO (B-E), or Sox3 mRNA (F-I) on various neurogenic markers at neural plate stage. Embryos are shown superimposed with green fluorescent channel to reveal distribution of myc-GFP coinjected as lineage tracer. Asterisks indicate reductions, while arrows mark increased or ectopic marker gene expression in the placodal or non-neural ectoderm. J-L: Effects of unilateral injection of GR-Sox3 and DEX-activation at stage 16-18 on neurogenic markers at tail bud stages (J1-L1: injected side; J2-L2: control side). Asterisks indicate reductions in the placodal or non-neural ectoderm. M-N: Ectopic neuronal differentiation (arrows) after injection of GR-Eya1+GR-Six1 mRNAs (M1: inj. side; M2: control side) and DEX-activation at stage 16-18 is significantly reduced after co-injection of Sox2/Sox3 MO (M3: inj. side; M4: control side; N: quantitation, X2 test; **: p< 0.001). O-R: Ectopic expression (arrows) of Sox3 (O1, O3: inj. side; O2, O4: control side) and Sox2 (P1, P3: inj. side; P2, P4: control side) but not of NeuroD (Q1, Q3: inj. side; Q2, Q4: control side) and p27xicl after injection of GR-Eya1 + GR-Six1 mRNAs and DEX activation at stage 16-18 persists and is more extensive with CHX treatment (R: quantitation; X2 test; *: p< 0.05, **: p< 0.001).
In embryos co-injected with Sox2 and Sox3 morpholinos, N-Tubulin (Fig. 11 B) and NeuroD expression (Fig. 11 C) were strongly repressed in all neurogenic placodes including the profundal and trigeminal placodes. Delta1 expression was also strongly repressed (not shown) suggesting that reduced neuronal differentiation was not due to an increase in lateral inhibition. On the other hand, the expression of p27Xic1 and Ngnr1 was sometimes reduced but often enhanced in the placodal region (Fig. 11 D, E). Thus, Sox2 and Sox3 appear essential for maintaining cells in the cell cycle and to promote neuronal differentiation after cell cycle exit, since elevation of p27Xic1 and Ngnr1 after SoxB1 knockdown is not accompanied by increased neuronal differentiation.
Overexpression of Sox3, however, reveals that high levels of SoxB1 proteins are incompatible with neuronal differentiation. Injections of Sox3 or of GR-Sox3 followed by DEX-activation at stage 16-18 gave similar results. N-Tubulin (Fig. 11 F, J) and NeuroD (Fig. 11 G, K) were strongly repressed in all neurogenic placodes and the Sox3-overexpressing regions of the ectoderm were thickened (not shown). However, unlike the results with Eya1 or Six1, we never found ectopic expression of N-Tubulin or NeuroD. p27Xic1 (Fig. 11 I), Ngnr1 (Fig. 11 H, L) and Delta1 (not shown) were mostly reduced by increasing Sox3 or GR-Sox3 levels although increased or ectopic expression was sometimes observed, indicating that Sox3 overexpression delays cell cycle exit and onset of neuronal differentiation. Increasing Sox3 expression, therefore, mimics many of the effects of Eya1 and Six1 on placodal neurogenesis suggesting that these effects are SoxB1-mediated. However, in contrast to Eya1 or Six1 injection, Sox3 injection never led to ectopic neuronal differentiation indicating that Eya1 and Six1 probably promote neuronal differentiation by additional and Sox3-independent mechanisms.
To directly test whether Eya1 and Six1 promote placodal neurogenesis in a SoxB1 dependent fashion, we injected GR-Eya1 + GR-Six1 together with Sox2MO and Sox3MO followed by DEX activation at stage 16-18. Co-injection of SoxB1 MOs significantly compromised the ability of GR-Eya1 + GR-Six1 to generate ectopic NeuroD expression (Fig. 11 M, N). To determine whether SoxB1 genes are direct target genes of Eya1 and Six1 we treated GR-Eya1 + GR-Six1 injected embryos with the protein synthesis inhibitor cycloheximide (CHX) prior to and during DEX-activation at stage 16-18. Eya1 and Six1 retained the ability to ectopically induce Sox2 (Fig. 11 P, R) and Sox3 (Fig. 11 O, R) under these conditions, indicating that the latter are likely direct targets of transcriptional regulation by Eya1 and Six1. Ectopic induction of Sox2 and Sox3 was stronger and more frequent with CHX treatment suggesting that there also is an indirect inhibitory effect on Sox2 and Sox3 expression that is relieved when protein synthesis is blocked. Ectopic NeuroD and p27Xic1 expression were no longer observed after CHX treatment (Fig. 11 Q, R) indicating that these genes are likely to be indirect targets of regulation by Eya1 and Six1.
Discussion
Eya1 and Six1 regulate several steps in placode development
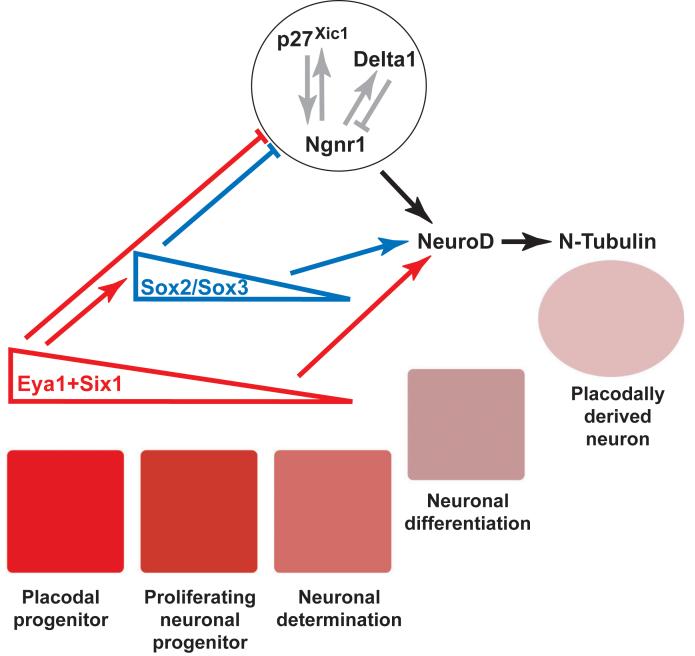
Eya1 and Six1 are required for establishing the pre-placodal ectoderm in Xenopus and distinguishing it from the adjacent neural crest and non-neural epidermis (Brugmann et al., 2004). In addition, members of the Eya and Six1/2 gene families have important roles in the proliferation and survival of neuronal and sensory progenitors (Zheng et al., 2003; Li et al., 2003; Bricaud and Collazo, 2006; Zou et al., 2006; Kriebel et al., 2007) as well as for the proper expression of neuronal determination and differentiation genes in the olfactory, otic and epibranchial placodes (Zou et al., 2004; Friedman et al., 2005; Bricaud and Collazo, 2006; Ikeda et al., 2007). We have extended these observations to demonstrate that both Eya1 and Six1 are required for neuronal differentiation in all neurogenic placodes, substantiating the suggestion that Eya1 and Six1 have pan-placodal functions (Schlosser, 2006). In addition, we show that they are important in multiple steps during placodal neurogenesis, that their specific roles are determined by their levels of expression, and many of their effects are mediated by SoxB1 genes (Fig. 12).
Fig. 12.
Model of regulatory interactions between Six1, Eya1 and SoxB1 genes in placodal ectoderm. Blue and red arrows and bars indicate positive and negative regulatory interactions, respectively, of Eya1 + Six1 (red) and SoxB1 (blue) proteins supported by present study. The circle encloses a network of proteins known to cross-regulate during early stages of neuronal determination. High levels of Eya1 and Six1 in the placodes (red squares) maintain proliferative neuronal progenitors. These levels decrease as cells leave the placodes (pink squares), permitting cell cycle exit and onset of neuronal determination and promoting neuronal differentiation. See text for details.
After establishing the pre-placodal ectoderm, Eya1 and Six1 continue to be expressed at high levels in the developing placodes (Pandur and Moody, 2000; Schlosser and Northcutt, 2000; David et al., 2001). We show that a subset of these cells express Sox2 and Sox3, and that Eya1 and Six1 are required for Sox expression. Our expression analysis suggests that incipient placodal neurons are mostly Eya1, Six1 and Sox3-negative but originate from Sox3+ cells. This extends observations in the zebrafish and chick, where neurons in the otic and epibranchial placodes were found to differentiate from Sox3+ cells (Abu-Elmagd et al., 2001; Neves et al., 2007; Sun et al., 2007; Nikaido et al., 2007). It appears that it is the reduction of Eya1 and Six1 protein levels that permits expression of neuronal determination and differentiation markers. Thus, levels of expression of Eya1 and Six1 regulate the progression from proliferating placodal progenitor to differentiated placodal neuron (Fig. 12).
High levels of Eya1 and/or Six1 maintain cells in a proliferative neuronal progenitor state
Our gain of function experiments suggest that at high levels of expression Eya1 and Six1 keep cells in a proliferative neuronal progenitor state and block onset of neuronal determination. Although Six1 is able to directly activate cell cycle regulators such as CyclinA1 and c-Myc (Li et al., 2003; Coletta et al., 2004), neither Eya1 nor Six1 affected CyclinA1 expression in our experiments. This suggests that other target genes of Eya1 and Six1 are involved in progenitor pool expansion. Our finding that Sox2 and Sox3 are likely direct targets of Eya1 and Six1 suggest that SoxB1 genes may be involved in this process.
SoxB1 genes are well known to be required for neuronal differentiation in the central nervous system and to bias lineage choice towards neural or neuronal fates (Pevny et al., 1998; Kishi et al., 2000; Zhao et al., 2004; Kan et al., 2004, 2007; Wang et al., 2006). However, overexpression of SoxB1 genes interferes with neuronal differentiation and often reduces Ngnr1 and p27Xic1 expression (Bylund et al., 2003; Graham et al., 2003; Hardcastle and Papalopulu, 2000), while loss of SoxB1 function increases their expression, indicating that SoxB1 proteins maintain a proliferative neural or neuronal progenitor cell. Our loss of function experiments show that neuronal differentiation in placodes is blocked even though p27Xic1 and Ngnr1 are frequently increased indicating that SoxB1 genes are also required for neuronal differentiation in placodes, acting either downstream of and/or parallel to p27Xic1 and Ngnr1. However, we also observed that increasing Sox3 expression in the placodal ectoderm reduced the expression of both neuronal determination and differentiation genes, while knockdown could expand Ngnr1 and p27Xic1. These findings suggest that SoxB1 genes play a dual role in the neurogenic placodes as they do in the CNS: they are (1) required for neuronal differentiation, but (2) maintain cells in a proliferative neuronal progenitor state.
Surprisingly, we find SoxB1 genes to be required for neuronal differentiation even in the profundal and trigeminal placodes, which do not express Sox2 or Sox3 from neural plate stages on (Schlosser and Ahrens, 2004). These are the earliest placodal neurons to be born and to differentiate (Lamborghini, 1980; Schlosser and Northcutt, 2000), and therefore may be influenced by the more widely expressed Sox2/Sox3 at gastrula stages (Penzel et al., 1997; Mizuseki et al., 1998).
Our experiments demonstrate that Sox2/Sox3 are regulated in the placodes by Eya1 and Six1. Loss- and gain-of function of either Eya1 or Six1 affect neuronal determination and differentiation genes in a similar manner to the loss- and gain-of function of Sox2/Sox3, suggesting that these two sets of transcription factors act in the same pathway. Furthermore, the endogenous expression of Sox2 and Sox3 in the placodes is confined to domains of Eya1 and Six1 expression, and requires both of these genes. These data suggest that the ability of Eya1 and Six1 to promote proliferative neuronal progenitors, from which ectopic neurons later differentiate, is mediated by their capacity to activate SoxB1 expression (Fig. 12). We confirmed this by showing that SoxB1 knockdown interferes with the ability of Eya1 and Six1 to induce ectopic neurons. Our gain-of-function experiments further suggest that when expressed at high levels Eya1 and Six1 promote a proliferative SoxB1+ neuronal progenitor state. As levels of expression of both sets of genes begin to decrease, the SoxB1+ cells transition to a state in which cell cycle exit is permitted, the neuronal fate is determined, and neurons differentiate from an expanded pool of neuronal progenitors.
Low levels of Eya1 and/or Six1 promote neuronal differentiation
Our observation that ectopic neuronal differentiation occurs only after Eya1 or Six1 but not after Sox3 overexpression, indicates that low levels of Eya1 and Six1 do not merely play a permissive role for the onset of neuronal determination but additionally promote neuronal differentiation downstream of and/or in parallel to Sox3 (Fig. 12). Furthermore, we do not observe ectopic neuronal differentiation in Eya1 or Six1 loss of function experiments even though Ngnr1 and p27Xic1 are often increased. This suggests that Eya1 and Six1 are both required for neuronal differentiation to proceed beyond expression of proneural genes.
Neurogenesis in different placodes is known to rely on different proneural genes with interspecific differences between vertebrates. In mammals, neuronal determination depends on Ngn1 in the trigeminal and otic placodes, on Ngn2 in the epibranchial placodes and on Ash1 in the olfactory placode (Fode et al., 1998, Ma et al., 1998; Cau et al., 2002), while in the zebrafish neuronal determination in all (except possibly the olfactory placodes) depends on ngn1 (Andermann et al., 2002). Although two different Ngn genes are also present in Xenopus (Furlong et al., 2005), only the expression of the Ngn2 homologue Ngnr1 has been described and it is confined to the olfactory, profundal/trigeminal and epibranchial placodes (Schlosser and Northcutt, 2000). This suggests that in Xenopus similar to amniotes, neuronal determination in different placodes relies on different neuronal determination genes and that possibly the Xenopus Ngn1 homologue may play this role in placodes that do not express Ngnr1 (otic and lateral line placodes). Because knockdown of Eya1 or Six1 blocks neuronal differentiation in placodes that express Ngnr1as well as in those that do not express it, we propose that these genes promote neuronal differentiation both downstream of and/or in parallel to Ngnr1 and via other pathways (possibly downstream of and/or in parallel to other neuronal determination genes such as Ngn1).
Increased levels of Eya1 or Six1 also reduce Delta1, suggesting that relief of Notch-mediated lateral inhibition (Chitnis et al., 1995) allows Eya1 and Six1 to promote ectopic neuronal differentiation. However, several observations indicate that this is unlikely to be a major contributor. First, repression of Delta1 is confined to regions of high levels of Eya1 and Six1, where cells maintain a proliferative progenitor state, whereas ectopic neuronal differentiation occurs in adjacent regions, which maintain Delta1 expression. Second, Ngnr1 as well as NeuroD is expected to be increased after relief of lateral inhibition (Ma et al., 1996), which we did not observe. Third, increases in Delta1 after Six1 or Eya1 loss-of-function are observed much too rarely to account for the frequent reductions in neuronal differentiation.
Are other factors interacting with Eya1 and/or Six1 in placodal neurogenesis?
In the experiments presented herein, Eya1 and Six1 have similar effects on the expression of most neurogenesis markers and the phenotypes were stronger after co-expression of both. These results support their known ability to directly interact to affect transcription (Ohto et al., 1999; Ikeda et al., 2002). However, other Eya genes and genes of the Six1/2 and Six4/5 subfamilies are also expressed in multiple placodes in different vertebrates with some interspecific differences in expression patterns (Brugmann and Moody, 2005; Schlosser, 2006). This suggests that some of these other genes may also be involved in the regulation of placodal neurogenesis perhaps with redundant functions. Indeed, in mice trigeminal neurogenesis is affected only in Six1/Six4 double knockouts but not in mutants of either gene alone (Konishi et al., 2006). In Xenopus, Six2 and Six4 are expressed in most placodes similar to Six1 (Ghanbari et al., 2001). Xenopus Eya3 is not strongly expressed in the pre-placodal ectoderm, in contrast to Eya1, but subsequently is expressed in the lens and otic placodes, while the expression of other Xenopus Eya genes has not yet been reported (Kriebel et al. 2007). Taken together, this suggests that Six2, Six4, Eya3 and possibly other uncharacterized family members (Six5, Eya2, Eya4) as well may cooperate with Six1 and Eya1 in regulating neurogenesis in at least some placodes in Xenopus.
From our experiments, we noted that Eya1 and Six1 effects were not always identical. Eya1MOs caused expansion of Sox2 and Sox3 whereas Six1MOs did not. Injection of Eya1 mRNA expanded Ngnr1 and p27Xic1 domains more frequently and reduced SoxB1 expression less frequently than did injection of Six1 mRNA. Finally, Eya1 knockdown had a much smaller effect on Sox3 compared to Six1 knockdown. These results suggest that both Eya1 and Six1 may synergize with some other factors during placodal neurogenesis.
In conclusion, we have identified multiple previously unrecognized roles of Eya1 and Six1 in the regulation of placodal neurogenesis. Based on the data presented herein, we propose a model of the gene cascade regulating this important developmental process (Fig. 12). High levels of Eya1 and Six1 expression in the neurogenic placodes promote a SoxB1-dependent proliferative neuronal progenitor state and block onset of neuronal determination. As Eya1 and Six1 are downregulated, SoxB1 expression subsides and cell cycle inhibitors (p27Xic1) and proneural genes (Ngnr1 or other unidentified genes) are de-repressed, thereby permitting neuronal determination and differentiation to proceed. Low Eya1, Six1 and SoxB1 expression levels then promote neuronal differentiation downstream of and/or in parallel to Ngnr1 and possibly other proneural genes. Because Eya1 and Six1 only promote neuronal differentiation in cranial ectoderm, additional regionalized and yet unidentified factors must contribute to the regulation of placodal neurogenesis. Our data demonstrate that Eya1 and Six1 play a central role in multiple steps of placodal neurogenesis: they regulate neuronal progenitor pool size, mediated via the SoxB1-genes, control the onset of neuronal determination and differentiation, and promote neuronal differentiation.
Supplementary Material
Acknowledgements
We thank Eddy de Robertis (Sox2), Horst Grunz (Sox3), Chris Kintner (Ngnr1, NeuroD, N-Tubulin, Delta-1), Naoko Koyano-Nakagawa (LacZ), Nancy Papalopulu (p27Xic1), Jill Sible (CyclinA1), and Doris Wedlich (myc-GFP) for constructs. The c-myc antibody (9E10) developed by J.M. Bishop was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Dept. Biol. Sci., Iowa City, IA 52242. We are grateful to Gerhard Roth, Barbara Reinhold-Hurek and Reimer Stick for providing lab space and to Dorothea Kittlaus, Himani Datta Majumdar, Annette Peter, Elke Rink, and Anna Schultz for technical assistance. This work was supported by grants SCHL 450/5-1, 5-3 and 5-4 from the German Science Foundation (G. Schlosser), and NIH grants GM54001 (M. Klymkowsky) and NS23158 (S. Moody).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Elmagd M, Ishii Y, Cheung M, Rex M, Le Rouedec D, Scotting PJ. cSox3 expression and neurogenesis in the epibranchial placodes. Dev. Biol. 2001;237:258–269. doi: 10.1006/dbio.2001.0378. [DOI] [PubMed] [Google Scholar]
- Ahrens K, Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev. Biol. 2005;288:40–59. doi: 10.1016/j.ydbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev. Biol. 2002;251:45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Streit A. Sensory organs: making and breaking the pre-placodal region. Curr. Top. Dev. Biol. 2006;72:167–204. doi: 10.1016/S0070-2153(05)72003-2. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. Vertebrate cranial placodes. I. Embryonic induction. Dev. Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J. Neurosci. 2006;26:10438–10451. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugmann SA, Moody SA. Induction and specification of the vertebrate ectodermal placodes: precursors of the cranial sensory organs. Biol. Cell. 2005;97:303–319. doi: 10.1042/BC20040515. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Carruthers S, Mason J, Papalopulu N. Depletion of the cell-cycle inhibitor p27(Xic1) impairs neuronal differentiation and increases the number of ElrC(+) progenitor cells in Xenopus tropicalis. Mech. Dev. 2003;120:607–616. doi: 10.1016/s0925-4773(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Carter AD, Wroble BN, Sible JC. Cyclin A1/Cdk2 is sufficient but not required for the induction of apoptosis in early Xenopus laevis embryos. Cell Cycle. 2006;5:2230–2236. doi: 10.4161/cc.5.19.3262. [DOI] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375:761–766. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- Coletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Muller-Tidow C, Golub TR, Kawakami K, Ford HL. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc. Natl. Acad. Sci. USA. 2004;101:6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Ahrens K, Wedlich D, Schlosser G. Xenopus Eya1 demarcates all neurogenic placodes as well as migrating hypaxial muscle precursors. Mech. Dev. 2001;103:189–192. doi: 10.1016/s0925-4773(01)00355-0. [DOI] [PubMed] [Google Scholar]
- de Graaf M, Zivkovic D, Joore J. Hormone-inducible expression of secreted factors in zebrafish embryos. Dev. Growth Differ. 1998;40:577–582. doi: 10.1046/j.1440-169x.1998.t01-3-00001.x. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kim S, Leyns L, Piccolo S, Bachiller D, Agius E, Belo JA, Yamamoto A, Hainski-Brousseau A, Brizuela B, Wessely O, Lu B, Bouwmeester T. Patterning by genes expressed in Spemann’s organizer. Cold Spring Harb. Symp. Quant. Biol. 1997;62:169–175. [PubMed] [Google Scholar]
- Dent JA, Polson AG, Klymkowsky MW. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development. 1989;105:61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, Lemeur M, Goridis C, Guillemot F. The bHLH protein neurogenin 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc. Natl. Acad. Sci. USA. 1998;95:12608–12613. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong RF, Graham A. Vertebrate neurogenin evolution: long-term maintenance of redundant duplicates. Dev. Genes Evol. 2005;215:639–644. doi: 10.1007/s00427-005-0023-x. [DOI] [PubMed] [Google Scholar]
- Friedman RA, Makmura L, Biesiada E, Wang X, Keithley EM. Eya1 acts upstream of Tbx1, Neurogenin 1, NeuroD and the neurotrophins BDNF and NT-3 during inner ear development. Mech. Dev. 2005;122:625–634. doi: 10.1016/j.mod.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Ghanbari H, Seo HC, Fjose A, Brändli AW. Molecular cloning and embryonic expression of Xenopus Six homeobox genes. Mech. Dev. 2001;101:271–277. doi: 10.1016/s0925-4773(00)00572-4. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Hardcastle Z, Papalopulu N. Distinct effects of XBF-1 in regulating the cell cycle inhibitor p27XlC1 and imparting a neural fate. Development. 2000;127:1303–1314. doi: 10.1242/dev.127.6.1303. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Cheng PF, Weintraub H. Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. Proc. Natl. Acad. Sci. USA. 1993;90:8028–8032. doi: 10.1073/pnas.90.17.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DA, Vetter ML. The bHLH factors Xath5 and XNeuroD can upregulate the expression of XBrn3d, a POU-homeodomain transcription factor. Dev. Biol. 2001;232:327–338. doi: 10.1006/dbio.2001.0178. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Watanabe Y, Ohto H, Kawakami K. Molecular interaction and synergistic activation of a promoter by Six, Eya, and Dach proteins mediated through CREB binding protein. Mol. Cell Biol. 2002;22:6759–6766. doi: 10.1128/MCB.22.19.6759-6766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ookawara S, Sato S, Ando Z, Kageyama R, Kawakami K. Six1 is essential for early neurogenesis in the development of olfactory epithelium. Dev. Biol. 2007;311:53–68. doi: 10.1016/j.ydbio.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Kan L, Jalali A, Zhao LR, Zhou X, McGuire T, Kazanis I, Episkopou V, Bassuk AG, Kessler JA. Dual function of Sox1 in telencephalic progenitor cells. Dev. Biol. 2007;310:85–98. doi: 10.1016/j.ydbio.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L, Israsena N, Zhang Z, Hu M, Zhao LR, Jalali A, Sahni V, Kessler JA. Sox1 acts through multiple independent pathways to promote neurogenesis. Dev. Biol. 2004;269:580–594. doi: 10.1016/j.ydbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Mizuseki K, Sasai N, Yamazaki H, Shiota K, Nakanishi S, Sasai Y. Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development. 2000;127:791–800. doi: 10.1242/dev.127.4.791. [DOI] [PubMed] [Google Scholar]
- Kolm PJ, Sive HL. Efficient hormone-inducible protein function in Xenopus laevis. Dev. Biol. 1995;171:267–272. doi: 10.1006/dbio.1995.1279. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Ikeda K, Iwakura Y, Kawakami K. Six1 and Six4 promote survival of sensory neurons during early trigeminal gangliogenesis. Brain Res. 2006;1116:93–102. doi: 10.1016/j.brainres.2006.07.103. [DOI] [PubMed] [Google Scholar]
- Kozlowski DJ, Whitfield TT, Hukriede NA, Lam WK, Weinberg ES. The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev. Biol. 2005;277:27–41. doi: 10.1016/j.ydbio.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Kriebel M, Müller F, Hollemann T. Xeya3 regulates survival and proliferation of neural progenitor cells within the anterior neural plate of Xenopus embryos. Dev. Dyn. 2007;236:1526–1534. doi: 10.1002/dvdy.21170. [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six1 deficient mice. Mech. Dev. 2003;120:669–679. doi: 10.1016/s0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Lamborghini JE. Rohon Beard cells and other large neurons in Xenopus originate during gastrulation. J. Comp. Neurol. 1980;189:323–333. doi: 10.1002/cne.901890208. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Gene Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QF, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Ma QF, Chen ZF, Barrantes ID, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Mattioni T, Louvion JF, Picard D. Regulation of protein activities by fusion to steroid binding domains. Methods Cell Biol. 1994;43(Pt A):335–352. doi: 10.1016/s0091-679x(08)60611-1. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus zic-related-1 and sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J. Comp. Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) North-Holland; Amsterdam: 1967. [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev. Dyn. 2007;236:564–571. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Philpott A, Wang K, Holt CE, Harris WA. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell. 1999;99:499–510. doi: 10.1016/s0092-8674(00)81538-x. [DOI] [PubMed] [Google Scholar]
- Ohto H, Kamada S, Tago K, Tominaga S, Ozaki H, Sato S, Kawakami K. Cooperation of Six and Eya in activation of their target genes through nuclear translocation of Eya. Mol. Cell Biol. 1999;19:6815–6824. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oschwald R, Richter K, Grunz H. Localization of a nervous system-specific class II beta-tubulin gene in Xenopus laevis embryos by whole-mount in situ hybridization. Int. J. Dev. Biol. 1991;35:399–405. [PubMed] [Google Scholar]
- Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131:551–562. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- Pandur PD, Moody SA. Xenopus Six1 gene is expressed in neurogenic cranial placodes and maintained in differentiating lateral lines. Mech. Dev. 2000;96:253–257. doi: 10.1016/s0925-4773(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Penzel R, Oschwald R, Chen Y, Tacke L, Grunz H. Characterization and early embryonic expression of a neural specific transcription factor xSOX3 in Xenopus laevis. Int. J. Dev. Biol. 1997;41:667–677. [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Saka Y, Smith JC. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev. Biol. 2001;229:307–318. doi: 10.1006/dbio.2000.0101. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev. Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev. Biol. 2004;271:439–466. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Northcutt RG. Development of neurogenic placodes in Xenopus laevis. J. Comp. Neurol. 2000;418:121–146. [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. [Google Scholar]
- Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev. Biol. 2007;303:675–686. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Vernon AE, Devine C, Philpott A. The cdk inhibitor p27Xic1 is required for differentiation of primary neurones in Xenopus. Development. 2003;130:85–92. doi: 10.1242/dev.00193. [DOI] [PubMed] [Google Scholar]
- Wang TW, Stromberg GP, Whitney JT, Brower NW, Klymkowsky MW, Parent JM. Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. J. Comp. Neurol. 2006;497:88–100. doi: 10.1002/cne.20984. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rink E, Vernier P, Schlosser G. Secondary neurogenesis in the brain of the African clawed frog, Xenopus laevis, as revealed by PCNA, Delta-1, Neurogenin-related-1, and NeuroD expression. J. Comp. Neurol. 2005;489:387–402. doi: 10.1002/cne.20634. [DOI] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nature Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Zhang C, Basta T, Hernandez-Lagunas L, Simpson P, Stemple DL, Artinger KB, Klymkowsky MW. Repression of nodal expression by maternal B1-type SOXs regulates germ layer formation in Xenopus and zebrafish. Dev. Biol. 2004;273:23–37. doi: 10.1016/j.ydbio.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Zhang C, Basta T, Jensen ED, Klymkowsky MW. The beta-catenin/VegT-regulated early zygotic gene Xnr5 is a direct target of SOX3 regulation. Development. 2003;130:5609–5624. doi: 10.1242/dev.00798. [DOI] [PubMed] [Google Scholar]
- Zhang C, Klymkowsky MW. The Sox axis, Nodal signaling, and germ layer specification. Differentiation. 2007;75:536–545. doi: 10.1111/j.1432-0436.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- Zhao S, Nichols J, Smith AG, Li M. SoxB transcription factors specify neuroectodermal lineage choice in ES cells. Mol. Cell Neurosci. 2004;27:332–342. doi: 10.1016/j.mcn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–5572. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Rodrigo-Blomqvist S, Enerback S, Xu PX. Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear. Dev. Biol. 2006;298:430–441. doi: 10.1016/j.ydbio.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.