Abstract
OBJECTIVE
The purpose of this study was to characterize differences in the acute insulin response to glucose (AIRg) relative to insulin sensitivity (SI) in black and white premenopausal normoglycemic South African women matched for body fatness.
RESEARCH DESIGN AND METHODS
Cross-sectional analysis including 57 black and white South African women matched for BMI, SI, AIRg, and the disposition index (AIRg × SI) were performed using a frequently sampled intravenous glucose tolerance test with minimal model analysis, and similar measures were analyzed using an oral glucose tolerance test (OGTT). Body composition was assessed by dual-energy X-ray absorptiometry and computed tomography.
RESULTS
SI was significantly lower (4.4 ± 0.8 vs. 9.4 ± 0.8 and 2.9 ± 0.8 vs. 6.0 ± 0. 8 × 10−5 min−1/[pmol/l], P < 0.001) and AIRg was significantly higher (1,028 ± 255 vs. 352 ± 246 and 1,968 ± 229 vs. 469 ± 246 pmol/l, P < 0.001), despite similar body fatness (30.9 ± 1.4 vs. 29.7 ± 1.3 and 46.8 ± 1.2 vs. 44.4 ± 1.3%) in the normal-weight and obese black women compared with their white counterparts, respectively. Disposition index, a marker of β-cell function, was not different between ethnic groups (3,811 ± 538 vs. 2,966 ± 518 and 3,646 ± 485 vs. 2,353 ± 518 × 10−5 min, P = 0.10). Similar results were obtained for the OGTT-derived measures.
CONCLUSIONS
Black South African women are more insulin resistant than their white counterparts but compensate by increasing their insulin response to maintain normal glucose levels, suggesting an appropriate β-cell response for the level of insulin sensitivity.
Type 2 diabetes is a significant contributor to morbidity and mortality worldwide and was ranked as the seventh leading cause of death among individuals of all ages in South Africa in 2000 (1). The relatively high prevalence of diabetes has been accompanied by a high prevalence of obesity in South-African women (2), as 87% of all type 2 diabetes in South Africa has been attributed to excess body weight (3).
For reasons that have not yet been explained, both black South African and African American women are more insulin resistant than their adiposity-matched Caucasian counterparts (4–8). Insulin resistance in African Americans has been associated with hyperinsulinemia (6–8), due in part to reduced hepatic insulin extraction (7,8). In contrast, insulin resistance in black South African women has been associated with insulinopenia (5,9,10). However, the women in the previous studies were either not matched for glucose tolerance (5,10) or glucose tolerance and insulin sensitivity were not measured (9), making interpretation of β-cell function difficult. Thus, it is an open question whether β-cell function, assessed using state-of-the-art methods, differs in normoglycemic South African women of different ethnicities who are otherwise well matched.
It has become clear that insulin sensitivity is an important modulator of the β-cell response (11). The relationship between insulin sensitivity and insulin response is hyperbolic in nature, such that as insulin sensitivity decreases, normal β-cells will increase their insulin response to maintain normoglycemia. This hyperbolic relationship allows for the product of insulin sensitivity and insulin response to be calculated, with the resultant parameter being termed the disposition index. The disposition index is highly heritable and correlates strongly with glucose tolerance such that individuals with the lowest disposition index are at increased risk for or already have type 2 diabetes (12).
By estimating the disposition index, we aimed to better define β-cell function in black and white South African women matched for body fatness. Further, we aimed to compare these measures with estimates of glucose metabolism obtained using an oral glucose tolerance test (OGTT).
RESEARCH DESIGN AND METHODS
The study sample consisted of 13 normal-weight (BMI 18–25 kg/m2) black, 14 normal-weight white, 16 obese (BMI >30 kg/m2) black, and 14 obese white South African women, who were recruited by advertisement in local news articles and from local church groups, community centers, and universities. Additional inclusion criteria were 1) age 18–45 years, 2) no known diseases and not taking medication for diabetes, hypertension, HIV infection/AIDS, or any other metabolic disease, 3) not currently pregnant, lactating, or postmenopausal, and 4) of South African ancestry. The study was undertaken in accordance with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of the Faculty of Health Sciences of the University of Cape Town. Before participating in the study, procedures and risks were explained to the subjects, all of whom gave written informed consent to participate in the study.
Testing procedures
Body composition assessment.
Basic anthropometric measurements, including weight, height, and waist (at the level of the umbilicus) and hip (largest gluteal area) circumferences were taken. Body fat was measured using dual-energy X-ray absorptiometry (Discovery-W, software version 4.40; Hologic, Bedford, MA). Body composition of subjects that exceeded the scanning region (n = 15 [26%]) was calculated using the arm-replaced method (13). The coefficients of variation for measurements of fat-free tissue mass and fat mass were 0.7 and 1.7%, respectively. Abdominal visceral and subcutaneous adipose tissue areas were measured using computed tomography (Xpress helical scanner; Toshiba, Tokyo, Japan) at the level of L4–L5 lumbar vertebrae.
Blood sampling and measurement of glucose tolerance, insulin sensitivity, and insulin release.
Subjects completed an insulin-modified frequently sampled intravenous glucose tolerance test (FSIGT). After an overnight fast, cannulae were inserted into the antecubital vein of each arm. One arm was used for intravenous glucose and insulin infusions and the contralateral arm was used for blood sampling. The arm used for blood sampling was heated to arterialize venous blood. Baseline samples were taken at −15, −5, and −1 min before a bolus of glucose (50% dextrose; 11.4 g/m2 body surface area) was infused intravenously over 60 s beginning at time 0 min. At 20 min, human insulin (0.02 unit/kg, Actrapid; Novo Nordisk) was infused over 5 min at a constant rate. Samples for determination of plasma glucose and serum insulin concentrations were drawn at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, 180, 200, 220, and 240 min. This sampling schedule allows for the identification of parameters using the minimal model of glucose kinetics (14) and the acute insulin response to glucose (AIRg). The samples were centrifuged at 3,000 rpm for 10 min at 4°C, the plasma was stored at −20°C for subsequent analysis of glucose concentrations, and the serum was stored at −80°C for the subsequent analysis of insulin concentrations.
On a separate day, a fasting venous blood sample was drawn for the determination of plasma glucose and serum insulin levels. Subjects then underwent a 75-g OGTT, during which blood was drawn at 30-min intervals for 2 h for the measurement of plasma glucose and serum insulin concentrations.
Biochemical analysis
Plasma glucose concentrations were determined using the glucose oxidase method (YSI 2300 STAT Plus; YSI, Yellow Springs, OH). Serum insulin concentrations were determined by immunochemiluminometric assays using the ADVIA Centaur (Bayer Diagnostics). The intra-assay and interassay coefficients of variation for plasma glucose and serum insulin concentrations were 0.6 and 2.5 and 4.5 and 12.2%, respectively.
Calculations
Glucose and insulin data from the FSIGT were used to calculate indexes of insulin sensitivity (SI) and glucose effectiveness (Sg) (the ability of glucose to promote its own uptake at basal insulin levels) using Bergman's minimal model of glucose kinetics (14). AIRg was calculated as the mean incremental insulin response above basal between 2 and 10 min after the intravenous glucose bolus was started. β-Cell function was determined as the disposition index, which was calculated as the product of SI and AIRg (11). The glucose disappearance constant (Kg), a measure of intravenous glucose tolerance, was calculated as the slope of the natural log of glucose from 10 to 19 min, expressed as percent change per minute.
The early insulin response during the OGTT, also known as the insulinogenic index, was calculated as the ratio of the change in insulin to the change in glucose from 0 to 30 min (Δinsulin0–30/Δglucose0–30). The oral disposition index (DIo), a measure of β-cell function derived from the OGTT, was calculated as Δinsulin0–30/Δglucose0–30 × 1/fasting insulin (15). Three subjects (one normal-weight and two obese black women) had a negative Δinsulin(0–30)/Δglucose(0–30) measurement and were excluded from analyses including this parameter.
Statistics
Results are presented as means ± SEM. Two-way ANCOVA, adjusting for age, was used to compare anthropometric and metabolic measures between normal-weight and obese women of different ethnicity, with Bonferroni post hoc analyses. Repeated-measures ANOVA with Tukey post hoc analyses was used to explore differences in the glucose and insulin responses during the OGTT over time. Data were normalized by log transformation for statistical analyses when required. Data were analyzed using STATISTICA (version 7; StatSoft, Tulsa, OK). P < 0.05 was considered significant.
RESULTS
Subject characteristics
On average, the obese women were significantly older than the normal-weight women (P = 0.002) (Table 1). Consequently, all subsequent analyses were covaried for age. By design, all measures of body composition and regional fat deposition were significantly greater in the obese than in the normal-weight women (Table 1). There was a difference in stature between the black and white women (Table 1), with the black women being on average 10 cm shorter than the white women (P < 0.001) and weighing significantly less (P = 0.039). However, the black and white groups were well matched for BMI and body fat. Although there were no ethnic differences in waist circumference and waist-to-hip ratio, obese black women had less visceral adipose tissue (P = 0.006) but more subcutaneous adipose tissue (P = 0.013) than obese white women.
Table 1.
Subject characteristics
| Normal-weight black | Normal-weight white | Obese black | Obese white | |
|---|---|---|---|---|
| n | 13 | 14 | 16 | 14 |
| Age (years) | 24 ± 2 | 26 ± 2 | 28 ± 1 | 31 ± 2 |
| Height (cm) | 158.8 ± 1.8* | 169.6 ± 1.8* | 157.1 ± 1.6† | 167.2 ± 1.8† |
| Weight (kg) | 59.2 ± 3.1* | 64.8 ± 3.0† | 94.1 ± 2.9* | 98.8 ± 2.8† |
| BMI (kg/m2) | 23.4 ± 1.0* | 22.6 ± 0.9† | 38.0 ± 0.9* | 35.2 ± 0.9† |
| Body fat (%) | 30.9 ± 1.4* | 29.7 ± 1.3† | 46.8 ± 1.2* | 44.4 ± 1.3† |
| Waist circumference (cm) | 76.7 ± 2.8* | 80.1 ± 2.8† | 113.6 ± 2.6* | 105.7 ± 2.8† |
| WHR | 0.77 ± 0.02* | 0.78 ± 0.02† | 0.90 ± 0.02* | 0.85 ± 0.02† |
| VAT (cm2) | 60.4 ± 11.5* | 59.9 ± 10.7† | 101.5 ± 11.1*‡ | 143.8 ± 11.1†‡ |
| Total SAT (cm2) | 176.7 ± 22.3* | 175.5 ± 20.7† | 591.3 ± 21.4*‡ | 492.2 ± 21.4†‡ |
Values are unadjusted means ± SEM. All P values were adjusted for age.
*,†,‡Matching superscript symbols represent groups that are significantly different from each other, P < 0.01. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; WHR, waist-to-hip ratio.
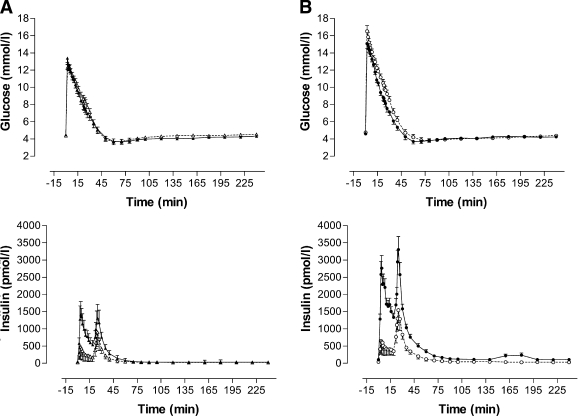
Glucose tolerance, insulin sensitivity, and insulin response to glucose
The glucose profile during the FSIGT was similar in black and white women, whereas the insulin profile after glucose injection was greater in black women (Fig. 1). SI was significantly lower and AIRg was significantly higher in black women than in white women (Table 2) and in obese women than in normal-weight women in both ethnic groups (P < 0.001). Ethnic differences in SI and AIRg persisted after adjustment for differences in visceral adipose tissue and subcutaneous adipose tissue areas. Disposition index (SI × AIRg) was not significantly different between ethnic and BMI groups, as was also the case for Sg. However, obese black women had better Kg than obese white women (P = 0.044).
Figure 1.
Plasma glucose and serum insulin responses during an FSIGT in normal-weight (A) and obese (B) black (●) and white (○) women.
Table 2.
Glucose tolerance, insulin sensitivity, and release
| Normal-weight black | Normal-weight white | Obese black | Obese white | |
|---|---|---|---|---|
| n | 13 | 14 | 16 | 14 |
| FSIGT | ||||
| SI (× 10−5 min−1/[pmol/l]) | 4.4 ± 0.8* | 9.4 ± 0.8* | 2.9 ± 0.8§ | 6.0 ± 0.8§ |
| AIRg (pmol/l) | 171.3 ± 42.5** | 58.6 ± 41.0** | 328.0 ± 38.2§ | 78.2 ± 41.0§ |
| Disposition index (× 10−5 min−1) | 3,811 ± 538 | 2,966 ± 518 | 3,646 ± 485 | 2,353 ± 518 |
| Sg (× 10−1 min) | 0.25 ± 0.03 | 0.24 ± 0.02 | 0.28 ± 0.02 | 0.24 ± 0.02 |
| Kg (%/min) | 2.81 ± 0.35 | 2.44 ± 0.34 | 3.46 ± 0.31* | 2.22 ± 0.34* |
| OGTT | ||||
| Fasting glucose (mmol/l) | 4.5 ± 0.1 | 4.4 ± 0.1 | 4.6 ± 0.1 | 4.7 ± 0.1 |
| Fasting insulin (pmol/l) | 59.0 ± 8.0* | 28.5 ± 7.7*§ | 90.6 ± 7.2‡ | 52.5 ± 7.7§‡ |
| Δinsulin(0–30)/Δglucose(0–30) | 437 ± 70* | 184 ± 65* | 507 ± 65† | 248 ± 65† |
| DIo (mmol/l) | 7.7 ± 1.3 | 7.6 ± 1.2 | 5.1 ± 1.2 | 5.0 ± 1.2 |
Values are unadjusted means ± SEM. All P values were adjusted for age.
*,†,‡,§Matching superscript symbols represent groups that are significantly different from each other, P < 0.05;
**P < 0.01.
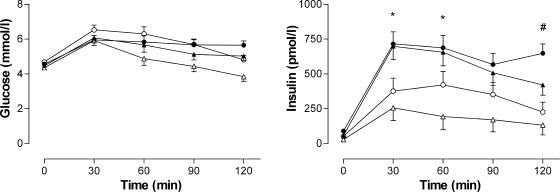
Glucose and insulin levels in response to the oral glucose challenge are presented in Table 2 and Fig. 2. All subjects had normal glucose tolerance by American Diabetes Association criteria. Two-hour plasma glucose levels were significantly higher in normal-weight black than in normal-weight white women (5.0 ± 0.3 vs. 3.8 ± 0.3 mmol/l, P = 0.016), with no differences between the obese black and white women (5.7 ± 0.2 vs. 4.8 ± 2.3 mmol/l, P = 0.132). Fasting serum insulin levels were greater in black women than in white women (P < 0.001) and in obese women than in normal-weight women (P < 0.001). The insulinogenic index [Δinsulin(0–30)/Δglucose(0–30)] was significantly higher in both normal-weight and obese black women than in their white counterparts (Table 2). With adjustment for the effect of insulin sensitivity to modulate the insulin response, DIo [Δinsulin(0–30)/Δglucose(0–30) × 1/fasting insulin] was not different between the BMI and ethnic groups.
Figure 2.
Plasma glucose and serum insulin responses to a 75-g oral glucose load in normal-weight (▲, ▵) and obese (●, ○) black (▲, ●) and white (▵, ○) women. *P < 0.05 for black versus white normal weight; #P < 0.05 for black versus white obese.
CONCLUSIONS
In the present study, we assessed for the first time in a sample of normoglycemic black and white South African women matched for BMI the relationship between insulin sensitivity and insulin response, which describes the ability of the β-cells to compensate for insulin resistance (11). We found that insulin resistance in the black women was associated with an appropriately greater insulin response to maintain normoglycemia compared with that in their white counterparts. Thus, the disposition index, which measures β-cell function, did not differ in black and white South African women.
The findings of this study, therefore, do not support the concept that insulin resistance in nondiabetic black South-African women is associated with insulinopenia, previously attributed to a reduction in β-cell mass (16). Rather, the results of this study correspond to those reported in African Americans, in whom insulin resistance is associated with hyperinsulinemia (6–8). The higher AIRg in African Americans has been attributed to both greater insulin secretion and reduced hepatic insulin extraction (8). In fact, the greater peak insulin level in the black women after insulin injection at 20 min during the FSIGT, occurring despite the fact that they weighed less and therefore received less insulin than the white women (Fig. 1), is also in keeping with black South African women having reduced insulin clearance.
The previous studies in which insulin resistance in black South African women was associated with insulinopenia measured insulin and C-peptide levels in the fasted state and in response to an OGTT and found them to be lower in black than in white women (4,5,10). However, the women in these studies were not matched for glucose tolerance. For example, in two separately published studies, 2-h post-OGTT glucose levels were ∼4.5 mmol/l in white women but as high as ∼7.5 mmol/l in the age- and BMI-matched black women (5,10). Thus, the relative degree of insulinopenia in the black women may be related to reduced glucose tolerance, which is known to be associated with impaired β-cell function (17). Other reports supporting the premise of insulinopenia in the black South African population either did not measure glucose tolerance or insulin sensitivity (9), made no direct comparisons with white subjects (16,18), or compared individuals with frank diabetes (19). More recent studies have shown no differences in the insulin response to an oral glucose load between well-matched black and white women (20,21). In fact, a greater 30-min insulin response to a mixed meal (22) and higher fasting C-peptide levels (23) have recently been reported in South African black compared with white women matched for BMI and glucose tolerance. The findings of our study showing a greater insulinogenic index in black compared with white women are thus compatible with these more recent reports.
Disposition index has been shown to decrease with increasing fasting glucose levels, even in subjects considered to be normoglycemic (17), and can be used to identify individuals at increased risk for type 2 diabetes (12). However, intravenous glucose tolerance testing is expensive and time consuming, reducing its use in resource-limited countries such as South Africa. Alternatively, the DIo can be calculated from the hyperbolic relationship between the early insulin response and insulin sensitivity during a standard OGTT (15). Although less precise than FSIGT-derived measures, DIo has been shown to be predictive of the development of diabetes over 10 years, even beyond that of fasting or 2-h post-OGTT glucose levels (15). The data from the current study, in which the insulinogenic index was higher and insulin sensitivity was lower in black than in white women, suggest that the degree of β-cell compensation in our sample of black women was appropriate, as DIo was not different based on ethnicity.
Based on the finding of similar disposition index values in the black and white women in this study, one would assume a similar risk for type 2 diabetes in these two population groups. However, the prevalence of type 2 diabetes in South Africa is higher in the black population than in the white population (24). A similar scenario is presented in African Americans, who have a higher prevalence of type 2 diabetes but display a similar or higher disposition index than their Caucasian counterparts (6,7). The mechanisms underlying the increased risk of type 2 diabetes in black populations from South Africa and the U.S. is not known. Joffe et al. (16) proposed a scheme for the pathogenesis of type 2 diabetes in the black population of Southern Africa, in which a reduction of β-cell mass (due to childhood malnutrition and/or genetic factors), followed by an accelerated phase of β-cell decompensation induced by a variable period of peripheral insulin resistance, would ultimately lead to insulinopenic type 2 diabetes. However, to our knowledge, there is no evidence of a reduction in β-cell mass in the general black population. In fact, exaggerated glucose-stimulated insulin secretion appears to be a characteristic of individuals with normal glucose tolerance who are at risk for type 2 diabetes (Pima Indians and African Americans) (25).
A limitation of our study is that the subjects were not randomly drawn from the population but comprised volunteers who had responded to a recruitment advertisement. Thus, there is the possibility that they may not be representative of the population at large. However, our finding of insulin resistance in black women is in keeping with previous observations reported from South Africa (4,5). The reasons for the differences in insulin sensitivity and insulin responses and whether they apply to the general population are unclear. Aside from genetic differences, environmental and lifestyle differences exist between the black and white South African populations, and it is quite possible that these may have an impact on measures associated with glucose metabolism. For example, we found that black South African women had lower socioeconomic status, consumed more dietary fat, and had more subcutaneous and less visceral fat than their white counterparts and that these factors explained in part some of the differences in insulin sensitivity (J. H. Goedecke, unpublished observations). However, even after adjustment for differences in fat distribution, the ethnic differences in SI and AIRg were not obviated. Identification of other factors and the mechanisms by which they may be operating to affect glucose metabolism is clearly needed. In addition, longitudinal studies are required to determine whether the β-cell will continue to compensate in the black women with increasing age or whether it will fail over time, leading to disturbances in glucose tolerance.
In summary, we have demonstrated that black South African women are more insulin resistant than their white counterparts but compensate by increasing their insulin response to maintain normal glucose levels. These findings refute previous suggestions that insulin resistance in black South African women is associated with relative insulinopenia but rather suggest an appropriate β-cell response for the level of insulin sensitivity. Future studies are required to explore the mechanisms underlying the high degree of insulin resistance in apparently healthy black populations and to establish whether their hyperinsulinemic response to insulin resistance is in fact associated with increased or decreased risk of diabetes with increasing age.
Acknowledgments
This study was funded by the Medical Research Council of South Africa (Career Development Award for J.H.G.), the International Atomic Energy Agency, the National Research Foundation of South Africa and Royal Society SA-U.K. Science Networks Programme, the University of Cape Town, the British Heart Foundation, the Wellcome Trust, and the U.S. Department of Veterans Affairs.
No potential conflicts of interest relevant to this article were reported.
We thank the research volunteers for their participation in this study and fieldworker Nandipha Sinyanya for subject recruitment and translation. We are extremely grateful to Hendriena Victor for coordinating the study, and together with Judy Belonje, for technical expertise. Jack Bergman and Naomi Fenton of Symington Radiology are thanked for performing the computed tomography scans and Linda Bewerunge is thanked for performing the dual-energy X-ray absorptiometry scans.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Bradshaw D, Norman R, Pieterse D, Levitt NS: Estimating the burden of disease attributable to diabetes in South Africa in 2000. S Afr Med J 2007; 97: 700– 706 [PubMed] [Google Scholar]
- 2. Puoane T, Steyn K, Bradshaw D, Laubscher R, Fourie J, Lambert V, Mbananga N: Obesity in South Africa: The South African Demographic and Health Survey. Obes Res 2002; 10: 1038– 1048 [DOI] [PubMed] [Google Scholar]
- 3. Joubert J, Norman R, Bradshaw D, Goedecke JH, Steyn NP, Puoane T: Estimating the burden of disease attributable to excess body weight in South Africa in 2000. S Afr Med J 2007; 97: 683– 690 [PubMed] [Google Scholar]
- 4. van der Merwe MT, Crowther NJ, Schlaphoff GP, Gray IP, Joffe BI, Lönnroth PN: Evidence for insulin resistance in black women from South Africa. Int J Obes 2000; 24: 1340– 1346 [DOI] [PubMed] [Google Scholar]
- 5. van der Merwe MT, Crowther NJ, Schlaphoff GP, Boyd IH, Gray IP, Joffe BI, Lonnroth PN: Lactate and glycerol release from the subcutaneous adipose tissue of obese urban women from South Africa; important metabolic implications. J Clin Endocrinol Metab 1998; 83: 4084– 4091 [DOI] [PubMed] [Google Scholar]
- 6. Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE: Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Diabetes 1996; 45: 742– 748 [DOI] [PubMed] [Google Scholar]
- 7. Rasouli N, Spencer HJ, Rashidi AA, Elbein SC: Impact of family history of diabetes and ethnicity on β-cell function in obese, glucose-tolerant individuals. J Clin Endocrinol Metab 2007; 92: 4656– 4663 [DOI] [PubMed] [Google Scholar]
- 8. Osei K, Schuster DP: Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med 1994; 11: 755– 762 [DOI] [PubMed] [Google Scholar]
- 9. Shires R, Joffe BI, Seftel HC: Maximal pancreatic β-cell stimulation and the counter-regulatory hormonal responses in South African black and white obese subjects. S Afr Med J 1985; 67: 845– 847 [PubMed] [Google Scholar]
- 10. van der Merwe MT, Wing JR, Celgow LH, Gray IP, Joffe BI, Lonnroth PN: Metabolic indices in relation to body composition changes during weight loss on dexfenfluramine in obese women from two South African ethnic groups. Int J Obes 1996; 20: 768– 776 [PubMed] [Google Scholar]
- 11. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D, Jr: Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes 1993; 42: 1663– 1672 [DOI] [PubMed] [Google Scholar]
- 12. Elbein SC, Hasstedt SJ, Wegner K, Kahn SE: Heritability of pancreatic β-cell function among nondiabetic members of Caucasian familial type 2 diabetic kindreds. J Clin Endocrinol Metab 1999; 84: 1398– 1403 [DOI] [PubMed] [Google Scholar]
- 13. Micklesfield LK, Reid S, Bewerunge L, Rush EC, Goedecke JH: A proposed method to measure body composition in obese individuals using dual-energy X-ray absorptiometry. Int J Body Comp Res 2007; 5: 147– 151 [Google Scholar]
- 14. Bergman RN, Ider YZ, Bowden CR, Cobelli C: Quantitative estimation of insulin sensitivity. Am J Physiol Endocrinol Metab 1979; 236: E667– E677 [DOI] [PubMed] [Google Scholar]
- 15. Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE: An oral disposition index predicts the development of future diabetes above and beyond fasting and 2-hour glucose levels. Diabetes Care 2009; 32: 335– 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joffe BI, Panz VR, Wing JR, Raal FJ, Seftel HC: Pathogenesis of non-insulin-dependent diabetes mellitus in the black population of Southern Africa. Lancet 1992; 340: 460– 462 [DOI] [PubMed] [Google Scholar]
- 17. Utzschneider KM, Prigeon RL, Carr DB, Hull RL, Tong J, Shofer JB, Retzlaff BM, Knopp RH, Kahn SE: Impact of differences in fasting glucose and glucose tolerance on the hyperbolic relationship between insulin sensitivity and insulin responses. Diabetes Care 2006; 29: 356– 362 [DOI] [PubMed] [Google Scholar]
- 18. Shires R, Joffe BI, Seftel HC: Hormonal and metabolic responses to an oral glucose load in obese black diabetics. S Afr Med J 1978; 53: 446– 448 [PubMed] [Google Scholar]
- 19. Wicks ACB, Jones JJ: Insulinopenic diabetes in Africa. Br Med J 1973; 1: 773– 776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Punyadeera C, van der Merwe MT, Crowther NJ, Toman M, Schlaphoff GP, Gray IP: Ethnic differences in lipid metabolism in two groups of obese South African women. J Lipid Res 2001; 42: 760– 767 [PubMed] [Google Scholar]
- 21. Punyadeera C, van der Merwe MT, Crowther NJ, Toman M, Immelman AR, Schlaphoff GP, Gray IP: Weight-related differences in glucose metabolism and free fatty acid production in two South African population groups. Int J Obes Relat Metab Disord 2001; 25: 1196-1205. [DOI] [PubMed] [Google Scholar]
- 22. Punyadeera C, Crowther NJ, van der Merwe MT, Toman M, Immelman AR, Schlaphoff GP, Gray IP: Metabolic response to a mixed meal in obese and lean women from two South African populations. Obes Res 2002; 10: 1207– 1216 [DOI] [PubMed] [Google Scholar]
- 23. Reimann M, Schutte AE, Huisman HW, Schutte R, Van Rooyen JM, Malan L, Malan NT, Schwarz PE: Ethnic differences in C-peptide secretion but not in non-esterified fatty acid metabolism in pre-menopausal women with and without abdominal obesity. Diabetes Res Clin Pract 2007; 77: 62– 69 [DOI] [PubMed] [Google Scholar]
- 24. Levitt NS, Katzenellenbogen JM, Bradshaw D, Hoffman MN, Bonnici F: The prevalence and identification of risk factors for NIDDM in urban Africans in Cape Town, South Africa. Diabetes Care 1993; 16: 601– 607 [DOI] [PubMed] [Google Scholar]
- 25. Stefan N, Stumvoll M, Weyer C, Bogardus C, Tataranni PA, Pratley RE: Exaggerated insulin secretion in Pima Indians and African-Americans but higher insulin resistance in Pima Indians compared with African-Americans and Caucasians. Diabet Med 2004; 21: 1090– 1095 [DOI] [PubMed] [Google Scholar]