Abstract
OBJECTIVE
Early puberty is associated with increased risk of subsequent cardiovascular disease. Low sex hormone–binding globulin (SHBG) levels are a feature of early puberty and of conditions associated with increased cardiovascular risk. The aim of the present study was to evaluate SHBG as a predictor of glucose metabolism and metabolic risk during puberty.
RESEARCH DESIGN AND METHODS
This was a cross-sectional study on 132 healthy Caucasian children and adolescents evaluated by an oral glucose tolerance test, a dual-energy X-ray absorptiometry scan, direct oxygen uptake measurement during cycle ergometry, and fasting blood samples.
RESULTS
SHBG levels declined with advancement of puberty in both boys (P < 0.001) and girls (P = 0.019). SHBG was significantly positively associated with insulin sensitivity in boys (P < 0.001) and girls (P < 0.001). In addition, SHBG was a strong predictor of insulin sensitivity (P = 0.001) and the only predictor of the disposition index (P = 0.031) after adjustment for puberty, fat mass, and aerobic fitness. SHBG was significantly negatively associated with metabolic risk (P = 0.032) and with hypersensitive C-reactive protein levels (P = 0.030) after adjustment for relevant confounders.
CONCLUSIONS
SHBG was a strong predictor of insulin sensitivity and metabolic risk during puberty. Thus, we hypothesize that SHBG integrates the marked changes in glucose metabolism and body composition that occur during the pubertal transition.
Early puberty has been associated with increased cardiovascular risk in adulthood (1–3), the underlying mechanism of which is unknown. However, a shared feature of both early puberty and cardiovascular risk is low serum levels of sex hormone–binding globulin (SHBG).
In adults, SHBG levels are negatively associated with a cluster of conditions, all of which have a strong association with obesity and insulin resistance. Thus, low levels of SHBG have consistently been associated with a wide array of cardiovascular risk factors including visceral and subcutaneous adiposity, hypertension, dyslipidemia, and insulin resistance (4,5). Consequently, SHBG levels are low in overt type 2 diabetes and have more recently been associated with the metabolic syndrome (6–8). In addition, SHBG levels have been found to be a determinant of cardiovascular risk independently of obesity and insulin resistance (9) and can be used to predict future type 2 diabetes and metabolic syndrome in adults (7,10).
Despite the increasing focus on SHBG as a marker of cardiovascular risk in adults, few studies have examined the relation of SHBG to glucose metabolism and metabolic risk during puberty. SHBG levels have been negatively associated with fasting insulin levels (11), body composition, and sex steroid levels (12) during puberty. We have recently shown that early pubertal timing in girls is associated with lower SHBG levels than predicted by pubertal stage and BMI (12), suggesting that differences in glucose metabolism could be at least partly responsible. However, no study to date has evaluated the predictive value of SHBG on glucose metabolism during puberty.
The aim of the present study was to investigate SHBG as a predictor of insulin sensitivity, insulin secretion, and metabolic risk after correction for other known influential confounders such as puberty, adiposity, and aerobic fitness.
RESEARCH DESIGN AND METHODS
All participants were recruited as a part of the Copenhagen Puberty Study from three primary schools in the Copenhagen community. A total of 132 healthy Caucasian children and adolescents (70 girls) aged 8.5–16.1 years volunteered. No prior or present medical history of confounding conditions was reported. Four of the adolescent girls were excluded from analysis of SHBG because of intake of contraceptive pills. No other intakes of medications were reported. All participants filled out a lifestyle questionnaire. None of the participants were smokers or reported consuming alcohol. The median time spent on physical activity was 5–6 h/week. Median hours spent per day in front of the television and computer were 1–2 and <1 h, respectively.
Pubertal development
Pubertal development was described according to the Tanner classification. The date of last menstrual bleeding was recorded in postmenarchal girls (n = 21).
Body composition
Whole-body dual-energy X-ray absorptiometry scanning was performed on all subjects using a Hologic CDR 1000/W densitometer (Hologic, Bedford, MA) with software version 6.2. Waist circumference was measured three times at the midaxillary line at the level midway between the anterior superior iliac spine and the 12th rib.
Aerobic fitness
Maximal oxygen uptake (Vo2max) was assessed during a cycle ergometry test using an electronically braked cycle ergometer (Ergomedic 839; Monark, Varberg, Sweden). Vo2max was measured directly using an online pulmonary gas analyzer system (Quark CPET; Cosmed, Rome, Italy). Initial and incremental workloads were 20 W for children weighing <30 kg and 25 W for children weighing ≥30 kg. For adolescents >15 years of age, initial workload was 70 W for girls and 100 W for boys, and incremental workload was 35 W. Workload was increased every third minute until exhaustion for children and adolescents <15 years of age, and every second minute until exhaustion for adolescents >15 years of age. Heart rate was recorded continuously throughout the test using a heart rate monitor (Polar Electro, Oulu, Finland). Criteria for a maximal effort were heart rate of ≥185 beats/min and a subjective judgment by the observer that the individual could no longer continue, even after encouragement. For a valid directly measured test, the oxygen consumption curve should show signs of leveling off. The cycle ergometer was electronically calibrated on every test day. Maximal oxygen consumption per kilogram body weight is an expression of aerobic fitness.
Two subjects could not participate in cycle ergometry because of a foot injury; 122 (92.4%) subjects fulfilled the criteria for a valid cycle ergometry test, of which only 113 (85.6%) had a valid directly measured Vcm;1o2max owing to technical problems with the online pulmonary gas analyzer system. To include all participants with a valid cycle ergometry test, the predicted Vo2max was estimated by linear regression analysis from the maximal power output from all participants with valid direct Vo2max.
 |
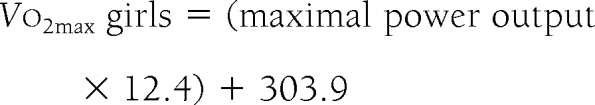 |
The estimated values were highly significantly correlated to the direct measurements in both boys (r = 0.938) and girls (r = 0.895).
Blood sampling
An intravenous cannula was inserted into an antecubital vein, from which fasting venous blood samples were drawn into standard vacuum tubes. Blood was centrifuged (3,000g at 10 min), and plasma samples were isolated and stored at −20°C until analysis.
Oral glucose tolerance test
Standard 2-h oral glucose tolerance tests (OGTTs) with an oral glucose load of 1.75 g glucose/kg body weight (maximum 75 g glucose) were performed for all subjects. Blood samples were drawn with 30-min intervals. Blood was centrifuged within 30 min, and plasma was immediately stored at −20°C until determination of insulin and glucose levels. The areas under the curve for plasma glucose and insulin were calculated by the trapezoidal rule. The whole-body insulin sensitivity index (WBISI) was calculated by the formula developed by Matsuda and DeFronzo (13) and subsequently converted to picomoles per liter insulin per millimoles per liter glucose. First-phase insulin release was calculated by the formula developed by Stumvoll et al. (14). β-Cell compensatory capacity was evaluated by the disposition index, as the product of WBISI and first-phase insulin release (1st PH), which has recently been validated from OGTTs in adults (15). Application of the same statistical model (see statistics) to the values from the present children confirmed the rectangular hyperbolic relationship with the following formula: 1347.8/WBISI + 516.9 = 1st PH (R2 = 0.512, P < 0.001).
Analyses
SHBG was determined by a time-resolved immunofluorescence assay (Delfia; Wallac Oy, Turku, Finland) with a detection limit of 0.23 nmol/l. Intra- and interassay coefficients of variation (CVs) were 5.8 and 6.4%, respectively. Insulin was determined by an electrochemiluminescence immunoassay (Elecsys insulin reagents kit; Roche Diagnostics, Mannheim, Germany) on an automated Roche Modular Analytics Module E170 (Roche Diagnostics). The detection limit was 2 pmol/l. The intra- and interassay CVs were 4.2 and 8.2%, respectively. Glucose, lipids (HDL cholesterol and triglycerides), and hypersensitive C-reactive protein (hsCRP) were all determined on a Roche Modular Analytics SWA Module P (Roche Diagnostics). Glucose was determined by enzymatic absorption photometry, and the intra- and interassay CVs were 1.1 and 1.7%, respectively. HDL cholesterol and triglycerides were determined by enzymatic colorimetric analyses (CFAS HDL plus, triglyceride GPO-PAP; Roche Diagnostics). For HDL, the intra- and interassay CVs were 0.9 and 1.85%, respectively. For triglycerides, the intra- and interassay CVs were 1.5 and 1.8%, respectively. hsCRP levels were determined by immunoturbidimetric analyses (Cobas; Roche Diagnostics), and the intra- and interassay CVs were 1.0 and 2.4%, respectively.
Blood pressure
Blood pressure was measured with a conventional sphygmomanometer (Heine Gamma G5; Heine Optotechnik, Herrsching am Ammersee, Germany) in the left arm after a 10-min rest in the supine position.
Metabolic syndrome score
Metabolic syndrome is defined as a cluster of dyslipidemia, hypertension, glucose intolerance, and obesity in adults. Until recently, no definition of the metabolic syndrome existed for children and adolescents, but the International Diabetes Federation (IDF) came out with a consensus in 2007 (16). No participant met the diagnostic criteria of metabolic syndrome based on the IDF criteria. To evaluate the metabolic parameters as a continuous variable, we generated a combined Z score based on the five variables included in the IDF consensus. Age- and sex-specific Z scores for waist circumference were obtained by using combined cross-sectional and longitudinal data from the Copenhagen Puberty Study, with 2,103 observations on 1,093 girls and 765 boys (K.S., L.A., A.J., unpublished data). The Z score for HDL cholesterol was inversed because of the negative association with metabolic syndrome. All five Z scores were added and subsequently divided by 5, generating a joint Z score with positive and negative scores indicating values higher and lower, respectively, than the mean risk profile for this specific population.
Statistics
No differences in outcome variables were found between postmenarchal girls tested during the luteal phase versus the follicular phase, and data were pooled before analyses. Descriptive statistics are shown as medians (10th percentile; 90th percentile). Mann-Whitney U tests were used for nonparametric comparison between groups. SHBG, fat mass, insulin sensitivity, insulin secretion, disposition index, and hsCRP were log-transformed to obtain approximate normal Gaussian distribution of the residuals and to obtain a residual variance that did not depend on the level. General linear models were used to assess the main determinants of insulin sensitivity, insulin secretion, disposition index, metabolic risk score, and hsCRP, respectively. No interactions between puberty and sex were observed in any of these models. Analyses with insulin sensitivity, insulin secretion, and disposition index as dependent variables, respectively, included the following covariates: sex, age, puberty, fat mass, SHBG, and aerobic fitness.
To confirm the inverse relationship between insulin secretion and insulin sensitivity, a regression analysis was done using the following model: 1st PH = constant × WBISIβ → log (1st PH) = constant + β × log(WBISI). The inverse relationship was confirmed with β = −0.583 and a 95% CI of β from −0.691 to −0.474. A linear regression analysis was done with direct measured Vo2max as the response and maximal power output as the explanatory variable to predict the Vo2max in all subjects with a valid cycle ergometry test, including those with invalid direct measurements of Vo2max. No other variables contributed significantly in this regression.
Ethics
The study was performed in accordance with the ethnical principles of the Declaration of Helsinki II. The study protocol was approved by the local ethics committee (reference nos. KF 01 282214 and KF 11 2006-2033). All children and parents gave their informed written consent.
RESULTS
Descriptive characteristics according to sex are shown in Table 1.
Table 1.
Descriptive characteristics
| Boys | Girls | P * | |
|---|---|---|---|
| n | 62 | 70 | |
| Pubertal stage 1–5 (%) | 34; 24; 11; 11; 19 | 14; 11; 19; 43; 13 | |
| Age (years) | 12.4 (9.2; 14.9) | 12.3 (9.1; 15.2) | NS |
| Fat mass (kg) | 7.5 (5.1; 15.8) | 9.6 (5.2; 15.0) | 0.042 |
| Lean mass (kg) | 34.1 (23.7; 52.8) | 32.5 (22.4; 44.2) | NS |
| Fasting insulin (pmol/l) | 43 (21; 72) | 49 (32; 91) | 0.010 |
| Fasting glucose (mmol/l) | 4.8 (4.1; 5.5) | 4.8 (4.2; 5.3) | NS |
| Mean AUCinsulin (pmol/l) | 242 (118; 379) | 248 (155; 458) | NS |
| Mean AUCglucose (mmol/l) | 5.8 (4.5; 7.3) | 5.5 (4.6; 6.7) | NS |
| WBISI‡ | 2.1 (1.3; 4.3) | 1.9 (1.0; 3.0) | NS |
| Insulin release (pmol)§ | 1,002 (635; 1,647) | 1,305 (876; 2,088) | 0.003 |
| Disposition index | 2,260 (1,377; 3,924) | 2,492 (1,524; 3,555) | NS |
| Vo2max (ml · kg−1 · min−1)‖ | 46.8 (35.6; 55.0) | 40.0 (33.4; 48.0) | <0.001 |
| Triglycerides (mmol/l) | 0.61 (0.43; 1.11) | 0.79 (0.53; 1.36) | 0.001 |
| HDL cholesterol (mmol/l) | 1.47 (1.15; 1.88) | 1.52 (1.04; 1.84) | NS |
| Mean ABP (mmHg) | 78.3 (70.0; 93.3) | 76.7 (71.7; 90.0) | NS |
| Waist circumference Z score | 0.22 (−0.99; 1.34) | 0.07 (−1.02; 1.32) | NS |
| Metabolic Z score¶ | −0.13 (−0.56; 0.78) | 0.04 (−0.53; 0.77) | NS |
| hsCRP (mg/l) | 0.28 (0.16; 1.40) | 0.28 (0.16; 2.68) | NS |
| SHBG (nmol/l) | 63.0 (23.0; 122.0) | 81.5 (34.5; 130.5) | NS |
Data are medians (10th percentile; 90th percentile).
*Comparisons between boys and girls by a Mann-Whitney U test.
‡Insulin sensitivity (ref. 13).
§Insulin release as first-phase (ref. 14).
‖Vo2max indicates predicted aerobic fitness.
¶Z score represents the SD score. ABP, arterial blood pressure; AUC, area under the curve. NS, nonsignificant (P > 0.05).
SHBG levels decreased with increasing stage of puberty in boys (P < 0.001) and girls (P = 0.019), respectively. However, SHBG levels in girls tended to plateau during midpuberty, after which a slight nonsignificant increase was noted. Girls had statistically significantly higher SHBG levels (P = 0.023) than boys after adjustment for age, puberty, fat mass, and insulin sensitivity.
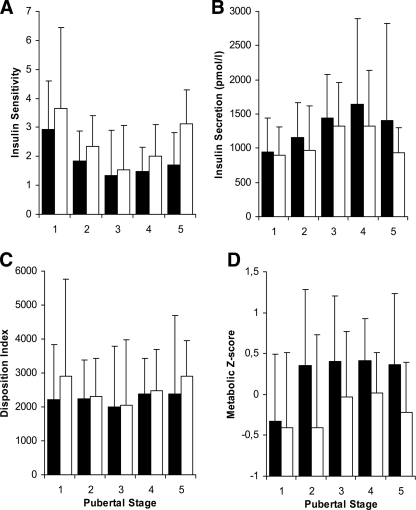
Insulin sensitivity during puberty in relation to SHBG levels above and below the median is illustrated in Fig. 1A. In univariate analyses, insulin sensitivity was significantly positively associated with SHBG and aerobic fitness and negatively associated with fat mass in boys (P < 0.001, P = 0.042, and P < 0.001, respectively) and in girls (all P < 0.001). SHBG remained a significant predictor of insulin sensitivity after adjustment for fat mass and aerobic fitness (Table 2).
Figure 1.
Insulin sensitivity (A), insulin secretion (B), disposition index (C), and metabolic Z score (D) during puberty based on OGTTs in healthy children and adolescents. For each pubertal stage, the effect of low versus high SHBG levels is illustrated by grouping all children according to the median SHBG level for pubertal stage and sex. ■, below median SHBG group; □, above median SHBG group. The whiskers represent the 90th percentiles. Insulin sensitivity (WBISI) (ref. 13) and insulin secretion (first-phase release) (ref. 14) were calculated using glucose in millimoles per liter and insulin in picomoles per liter concentrations. To convert WBISI to glucose in milligrams per deciliter and insulin in microunits per milliliter, multiply by a factor 3. To convert insulin secretion from picomoles per liter to microunits per milliliter, divide by a factor 6. Disposition index was calculated as the product of insulin sensitivity and insulin secretion. The metabolic Z score is generated by combining Z scores from fasting glucose, triglyceride levels, inverse HDL cholesterol levels, waist circumference, and mean blood pressure levels divided by 5. An increase in the combined Z score indicates an increase in metabolic risk.
Table 2.
Predictors of insulin sensitivity, disposition index, and metabolic risk in healthy nonobese children and adolescents
| Insulin sensitivity (WBISI) |
Disposition index |
Metabolic risk (metabolic Z score) |
||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| SHBG (nmol/l) | 21.4% (7.9 to 35.7) | 0.001 | 14.1% (1.4 to 28.3) | 0.031 | −0.15 (−0.29 to −0.01) | 0.032 |
| Fat mass (kg) | −6.7% (−24.2 to 14.9) | 0.49 | 0.7% (−19.9 to 23.1) | 0.94 | 0.27 (0.01 to 0.53) | 0.041 |
| Vo2max (ml · kg−1 · min−1) | 38.5% (−12.3 to 120.4) | 0.16 | −5.4% (41.4 to 51.6) | 0.81 | −0.35 (−0.91 to 0.21) | 0.23 |
| Puberty† | 0.004 | 0.18 | 0.88 | |||
| Tanner II vs. I | −13.9% (−30.9 to 7.3) | −6.8% (25.9 to 17.4) | 0.07 (−0.20 to 0.34) | |||
| Tanner III vs. I | −26.7% (−42.9 to −5.8) | −4.9% (−26.7 to 23.4) | 0.14 (−0.17 to 0.45) | |||
| Tanner IV vs. I | −11.3% (−32.3 to 17.4) | 9.4% (−18.1 to 44.8) | 0.05 (−0.28 to 0.39) | |||
| Tanner V vs. I | 15.0% (−18.9 to 64.9) | 29.7% (−9.5 to 87.8) | 0.05 (−0.39 to 0.49) | |||
| Adjusted R2 | 0.48 | 0.04 | 0.38 | |||
Data are parameter estimates (95% CIs) for the continuous covariates. All models were adjusted for sex and age, none of which contributed significantly to any of the models. CIs are presented as the changes in the response resulting from a 100% increase in the covariate, i.e., a doubling of SHBG results in a 21.4% increase in WBISI and 0.15 SD reduction in the metabolic risk score. To calculate the change (d) in the dependent variable of a given change (c) in covariates, the following formulas can be used: for WBISI and disposition index (log-transformed), the change is d = clog2(Est). For the metabolic Z score (untransformed), the change is d = [Est/ln(2)] × ln(c). As an example, an isolated (i.e., not changing other covariates) 50% increase in SHBG levels will lead to a 12% [1.5log2(1.21) = 1.50.28] increase in WBISI and a 0.09 [(−0.15/ln(2)) × ln(1.5) = −0.22 × ln(1.5)] decrease in metabolic Z score. Significant P values (P < 0.05) are in boldface.
†For pubertal stages, the changes in relation to Tanner stage I are presented as relative changes for WBISI and disposition index and as absolute changes for metabolic Z score. As an example, the SHBG level is 26.7% lower and metabolic Z score 0.14 SD higher in Tanner stage III than in Tanner stage I.
Insulin secretion during puberty in relation to SHBG levels above and below the median is illustrated in Fig. 1B. In univariate analyses, insulin secretion was significantly positively associated with SHBG and fat mass and negatively associated with aerobic fitness in boys (P = 0.009, P < 0.001, and P = 0.013, respectively) and in girls (all P < 0.001). However, SHBG was no longer a significant predictor of insulin secretion after adjustment for insulin sensitivity.
Disposition index during puberty in relation to SHBG levels above and below the median is shown in Fig. 1C. SHBG was significantly negatively associated with disposition index (P = 0.005). Neither fat mass nor aerobic fitness were significantly associated with disposition index in univariate analyses. SHBG and puberty contributed the most to the variance in disposition index (P = 0.004, R2 = 0.08). Predictors of disposition index are shown in Table 2.
Metabolic risk during puberty was evaluated by the combined Z score on risk factors derived from the recent IDF consensus on childhood metabolic syndrome. Metabolic risk during puberty in relation to SHBG levels above and below the median is shown in Fig. 1D. Metabolic risk was negatively associated with SHBG and aerobic fitness and positively associated with fat mass in boys (P < 0.001, P = 0.002, and P < 0.001, respectively) and in girls (P = 0.003, P < 0.001, and P < 0.001, respectively). Predictors of metabolic risk are shown in Table 2.
hsCRP levels were not associated with puberty per se. hsCRP was positively associated with metabolic risk and fat mass and negatively associated with SHBG and aerobic fitness in univariate analyses in both boys (P = 0.002, P < 0.001, P = 0.003, and P < 0.001, respectively) and girls (P = 0.006, P = 0.035, P = 0.018, and P = 0.006, respectively). After adjustment for fat mass and aerobic fitness, both SHBG (P = 0.030) and metabolic risk (P = 0.019) remained significant predictors of hsCRP during puberty.
CONCLUSIONS
In the present study of healthy children and adolescents, we found strong associations between SHBG levels and markers of glucose metabolism and metabolic risk. SHBG was a significant positive predictor of insulin sensitivity and disposition index during puberty independent of fat mass and aerobic fitness. In addition, SHBG negatively predicted childhood metabolic risk and low-grade inflammation.
Early puberty has been associated with an increased risk of insulin resistance, dyslipidemia, and adiposity in adulthood (1–3). The underlying mechanism responsible for this association is unknown, but a shared feature of both conditions is low SHBG levels.
In accordance with previous studies (11,12), SHBG levels declined during puberty in boys and girls, although levels in girls tended to plateau from around midpuberty. During puberty, SHBG levels have been associated with body composition, sex steroids (12), and fasting insulin levels (11). Interestingly, girls with precocious puberty have lower SHBG levels than predicted by pubertal stage and BMI (12), indicating that some of the residual variation in SHBG in such patients might be related to differences in insulin sensitivity and secretion.
Puberty is characterized by a marked physiological decline in insulin sensitivity, leading to a compensatory increase in insulin secretion, both of which recover by late puberty (17). In the present study, a similar curvilinear pattern with declining insulin sensitivity and increasing insulin responses until midpuberty was confirmed. Because of the inverse relationship between insulin sensitivity and insulin secretion, it has been speculated that risk assessment for future development of type 2 diabetes is most accurately measured during periods of β-cell challenge (18). Pregnancy may constitute such an example in that β-cells are challenged by low insulin sensitivity. Low SHBG levels during the first trimester of pregnancy predict gestational diabetes mellitus (19), which has been found to be strongly associated with subsequent development of overt type 2 diabetes (18). In analogy, puberty might constitute a comparable sensitive period during childhood and adolescence in which low SHBG might predict increased risk of type 2 diabetes and related metabolic changes.
Previous studies in adults have consistently shown strong correlations between SHBG and different indexes of insulin sensitivity (8,20). A similar positive correlation has been found in children and adolescents during puberty, although this has previously only been studied in relation to fasting insulin levels (11). In the present study, we found insulin sensitivity assessed by OGTT to be strongly associated with SHBG levels in both boys and girls during puberty. In addition, SHBG was a strong predictor of insulin sensitivity independently of fat mass and aerobic fitness. In accordance with previous studies (21), insulin sensitivity was strongly associated with both fat mass and aerobic fitness. However, because of the strong interrelation between aerobic fitness and fat mass, these covariates explain much of the same variability in insulin sensitivity and therefore are nonsignificant when both are included in the same variance analysis.
Insulin secretion was strongly positively associated with SHBG but not when adjusted for insulin sensitivity. To evaluate the predictive value of SHBG on β-cell function, we calculated the disposition index by a method that has recently been validated during OGTTs (15). The disposition index was lowest during midpuberty, which is in accordance with previous studies (17). As reported in adults (22), we found that SHBG was significantly associated with the disposition index. In addition, SHBG was the only significant predictor of the disposition index during puberty. However, it should be noted that SHBG and puberty only accounted for ∼8% of the variance in disposition index.
Obesity and insulin resistance are major risk factors for development of the metabolic syndrome in children and adolescents (16,23). In light of the strong associations between SHBG levels, adiposity, and insulin resistance (8,20), it is not surprising that SHBG levels have been shown to be strongly associated with the metabolic syndrome and overt type 2 diabetes in adults (6–8). In the present study, we found SHBG to be a significant predictor of the combined Z score on all variables derived from the IDF consensus on childhood metabolic syndrome, even after adjustment for fat mass and aerobic fitness. In accordance with previous studies (24), aerobic fitness did not independently predict metabolic risk in these children, indicating that aerobic fitness influences metabolic risk indirectly through effects on fat mass. However, the metabolic syndrome score is partially based on waist circumference, an abdominal fat mass estimate, and therefore is fitted to be strongly associated with fat mass per se. Thus, coexistence of adverse metabolic characteristics was independently associated with low SHBG levels, indicating that SHBG might be valuable in the assessment of cardiovascular risk during puberty. High metabolic risk was independently associated with high hsCRP levels in the present study. Elevated hsCRP levels have been associated with the metabolic syndrome in obese children and adolescents in previous studies (25), and the present finding extends this result to include nonobese children without overt metabolic syndrome. In addition, high hsCRP levels were associated with low SHBG independent of fat mass and aerobic fitness. Thus, SHBG might serve as an independent marker of low-grade systemic inflammation during puberty, which further strengthens its candidacy as a predictor of cardiovascular risk during childhood and adolescence.
In summary, strong associations were found between SHBG and markers of glucose metabolism in healthy nonobese children during puberty. Importantly, SHBG was a strong predictor of insulin sensitivity and a predictor of disposition index independently of total fat mass and aerobic fitness. In addition, the clustering of adverse metabolic characteristics and elevated hsCRP levels was independently associated with low SHBG levels. We hypothesize that SHBG integrates the marked changes in glucose metabolism and body composition that occur during the pubertal transition.
Acknowledgments
The Copenhagen Puberty Study received financial support from the Kirsten and Freddy Johansen's Foundation. J.W.H. received financial support from The 1991 Pharmacy Foundation.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Feng Y, Hong X, Wilker E, Li Z, Zhang W, Jin D, Liu X, Zang T, Xu X, Xu X: Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis 2008; 196: 590– 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM: Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab 2005; 90: 2718– 2724 [DOI] [PubMed] [Google Scholar]
- 3. Frontini MG, Srinivasan SR, Berenson GS: Longitudinal changes in risk variables underlying metabolic syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord 2003; 27: 1398– 1404 [DOI] [PubMed] [Google Scholar]
- 4. Tchernof A, Labrie F, Belanger A, Prud'homme D, Bouchard C, Tremblay A, Nadeau A, Despres JP: Relationships between endogenous steroid hormone, sex hormone-binding globulin and lipoprotein levels in men: contribution of visceral obesity, insulin levels and other metabolic variables. Atherosclerosis 1997; 133: 235– 244 [DOI] [PubMed] [Google Scholar]
- 5. Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, Haug E, Larsen R, Andersen M: Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab 2007; 92: 2696– 2705 [DOI] [PubMed] [Google Scholar]
- 6. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB: Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab 2006; 91: 843– 850 [DOI] [PubMed] [Google Scholar]
- 7. Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT: Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004; 27: 1036– 1041 [DOI] [PubMed] [Google Scholar]
- 8. Tchernof A, Toth MJ, Poehlman ET: Sex hormone-binding globulin levels in middle-aged premenopausal women: associations with visceral obesity and metabolic profile. Diabetes Care 1999; 22: 1875– 1881 [DOI] [PubMed] [Google Scholar]
- 9. Onat A, Hergenc G, Karabulut A, Albayrak S, Can G, Kaya Z: Serum sex hormone-binding globulin, a determinant of cardiometabolic disorders independent of abdominal obesity and insulin resistance in elderly men and women. Metabolism 2007; 56: 1356– 1362 [DOI] [PubMed] [Google Scholar]
- 10. Ding EL, Song Y, Malik VS, Liu S: Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295: 1288– 1299 [DOI] [PubMed] [Google Scholar]
- 11. Holly JM, Smith CP, Dunger DB, Howell RJ, Chard T, Perry LA, Savage MO, Cianfarani S, Rees LH, Wass JA: Relationship between the pubertal fall in sex hormone binding globulin and insulin-like growth factor binding protein-I: a synchronized approach to pubertal development? Clin Endocrinol (Oxf) 1989; 31: 277– 284 [DOI] [PubMed] [Google Scholar]
- 12. Sorensen K, Andersson AM, Skakkebaek NE, Juul A: Serum sex hormone-binding globulin levels in healthy children and girls with precocious puberty before and during gonadotropin-releasing hormone agonist treatment. J Clin Endocrinol Metab 2007; 92: 3189– 3196 [DOI] [PubMed] [Google Scholar]
- 13. Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462– 1470 [DOI] [PubMed] [Google Scholar]
- 14. Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van HT, Renn W, Gerich J: Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000; 23: 295– 301 [DOI] [PubMed] [Google Scholar]
- 15. Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B: Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008; 16: 1901– 1907 [DOI] [PubMed] [Google Scholar]
- 16. Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S: The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr Diabetes 2007; 8: 299– 306 [DOI] [PubMed] [Google Scholar]
- 17. Goran MI, Gower BA: Longitudinal study on pubertal insulin resistance. Diabetes 2001; 50: 2444– 2450 [DOI] [PubMed] [Google Scholar]
- 18. Kahn SE: The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003; 46: 3– 19 [DOI] [PubMed] [Google Scholar]
- 19. Spencer K, Yu CK, Rembouskos G, Bindra R, Nicolaides KH: First trimester sex hormone-binding globulin and subsequent development of preeclampsia or other adverse pregnancy outcomes. Hypertens Pregnancy 2005; 24: 303– 311 [DOI] [PubMed] [Google Scholar]
- 20. Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ: Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care 2004; 27: 861– 868 [DOI] [PubMed] [Google Scholar]
- 21. Lee S, Bacha F, Gungor N, Arslanian SA: Cardiorespiratory fitness in youth: relationship to insulin sensitivity and β-cell function. Obesity (Silver Spring) 2006; 14: 1579– 1585 [DOI] [PubMed] [Google Scholar]
- 22. Fernandez-Real JM, Grasa M, Casamitjana R, Pugeat M, Barret C, Ricart W: Plasma total and glycosylated corticosteroid-binding globulin levels are associated with insulin secretion. J Clin Endocrinol Metab 1999; 84: 3192– 3196 [DOI] [PubMed] [Google Scholar]
- 23. Bacha F, Saad R, Gungor N, Arslanian SA: Are obesity-related metabolic risk factors modulated by the degree of insulin resistance in adolescents? Diabetes Care 2006; 29: 1599– 1604 [DOI] [PubMed] [Google Scholar]
- 24. Eisenmann JC: Aerobic fitness, fatness and the metabolic syndrome in children and adolescents. Acta Paediatr 2007; 96: 1723– 1729 [DOI] [PubMed] [Google Scholar]
- 25. Ford ES, Ajani UA, Mokdad AH: The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005; 28: 878– 881 [DOI] [PubMed] [Google Scholar]