Abstract
OBJECTIVE
The purpose of this study was to examine the prevalence and correlates of elevated A1C in a large, nationally representative sample of adults without diabetes in the U.S.
RESEARCH DESIGN AND METHODS
We analyzed data from 15,934 participants aged ≥20 years without diagnosed diabetes who had A1C measurements in the 1999–2006 National Health and Nutrition Examination Survey, a cross-sectional and nationally representative sample of the U.S. population.
RESULTS
The overall prevalence of A1C >6% was 3.8%, corresponding to 7.1 million adults without diabetes in the U.S. population. Approximately 90% of these individuals had fasting glucose ≥100 mg/dl. Older age, male sex, non-Hispanic black race/ethnicity, hypercholesterolemia, higher BMI, and lower attained education were significantly associated with having a higher A1C level even among individuals with normal fasting glucose (<100 mg/dl) and after multivariable adjustment.
CONCLUSIONS
A single elevated A1C level (A1C >6%) is common in the general population of adults without a history of diabetes and is highly reliable for the detection of elevated fasting glucose. Nondiabetic adults with elevated A1C are likely to have impaired fasting glucose and an array of other risk factors for type 2 diabetes and cardiovascular disease.
A1C is an integrated measure of circulating glucose levels and tracks well in individuals over time. Epidemiological studies have shown that A1C values in nondiabetic adults predict incident diabetes (1–5), cardiovascular disease morbidity and mortality (6–10), and total mortality (7). In these studies, A1C values well within in the “normal” range (i.e., A1C <6%) were independently associated with clinical outcomes. There is currently renewed interest in using A1C for diagnosis and/or screening for diabetes (11); however, there have been few epidemiological investigations of A1C in nondiabetic adults. The objective of the present study was to examine the prevalence and correlates of elevated A1C in a large, nationally representative sample of U.S. adults without diagnosed diabetes who participated in the National Health and Nutrition Examination Survey (NHANES) (1999–2006). We hypothesized that 1) elevated A1C levels (e.g., A1C >6%) are common in the general population of nondiabetic adults in the U.S. and 2) A1C levels would be associated with risk factors for type 2 diabetes and its complications even in the absence of elevated glucose levels.
RESEARCH DESIGN AND METHODS
NHANES is an ongoing cross-sectional, multistage, stratified, clustered probability sample of the U.S. civilian noninstitutionalized population conducted by the National Center for Health Statistics (NCHS), a branch of the Centers for Disease Control and Prevention (12). Detailed in-person interviews, physical examinations, and blood samples were obtained from 18,986 participants aged ≥20 years in the 1999–2006 surveys who participated in the mobile examination visit. For the present study, we excluded those individuals who reported that a doctor or health care profession had ever told them they had diabetes (n = 1,900), who were missing information on diabetes status (n = 288), or who were missing A1C data (n = 986). The protocols of conduct for NHANES were approved by the NCHS institutional review board, and informed consent was obtained from all participants.
Fasting plasma glucose subsample
Approximately one-half of NHANES participants were sampled to attend the morning session. These participants were instructed to fast at least 9 hours before the appointment time. Fasting plasma glucose (FPG) values are available for those adults aged ≥20 years who attended the morning examination and were fasting for ≥8 h (n = 9,232). Our analyses of fasting glucose were limited to the fasting subpopulation of adults without diabetes who were not missing A1C data (n = 7,772). In the plasma glucose fasting subsample, we conducted analyses comparing A1C levels among individuals with normal fasting glucose (<100 mg/dl), impaired fasting glucose (IFG) (100–<126 mg/dl), and undiagnosed diabetes (fasting glucose ≥126 mg/dl) (13). In the 2005–2006 survey, an oral glucose tolerance test (OGTT) was added to the laboratory protocol. Thus, OGTT data were available for participants in the morning fasting subsample in the 2005–2006 survey only.
Laboratory measurement of A1C and plasma glucose
A1C measurements for NHANES 1999–2004 were performed by the Diabetes Diagnostic Laboratory at the University of Missouri-Columbia using Primus CLC330 and Primus CLC 385 instruments (Primus, Kansas City, MO). A1C measurements in NHANES 2005–2006 were performed by the Diabetes Laboratory at the University of Minnesota using a Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer (Tosoh Medics, San Francisco, CA). Both assays use a high-performance liquid chromatography system (14). All A1C measurements were standardized to the reference method used for the Diabetes Control and Complications Trial. The plasma glucose concentration was determined by a hexokinase enzymatic method (15).
Other variables of interest
The NHANES examination included measurement of height, weight, and blood pressure. Hypertension was defined as a mean systolic blood pressure of ≥140 mmHg, a mean diastolic blood pressure of ≥90 mmHg, or hypertension medication use. Total cholesterol was measured enzymatically. Hypercholesterolemia was defined as a total cholesterol level of ≥240 mg/dl or lipid medication use. C-reactive protein was measured by latex-enhanced nephelometry, a high-sensitivity assay. Information on age, sex, race/ethnicity, education level, and smoking was based on self-report during the questionnaire portion of the survey. A history of cardiovascular disease was defined on the basis of a self-reported history of coronary heart disease, angina, previous heart attack, or stroke. Smoking status was determined using answers to the questions, “Have you smoked at least 100 cigarettes in your life?” and “Do you now smoke cigarettes?” Alcohol consumption was determined during the computer-assisted personal interview using answers to the questions, “In any one year, have you had at least 12 drinks of any type of alcoholic beverage?” and “In your entire life, have you had at least 12 drinks of any type of alcoholic beverage?” Detailed information regarding the collection of data in NHANES is available elsewhere (12).
Statistical analysis
Analyses were performed incorporating the sampling weights (8-year combined weights) to obtain unbiased estimates from the complex NHANES sampling design using StataSE (version 10.0; StataCorporation, College Station, TX) and R (version 2; Free Software Foundation, Boston, MA). SEs for all estimates were obtained using the Taylor series (linearization) method following NCHS-recommended procedures (16). Analyses of FPG categories were limited to the morning plasma glucose sample and corresponding 8-year fasting subsample weights were used for these analyses. We generated weighted and smoothed histograms (kernel density estimator) to compare the distribution of A1C in individuals with normal fasting glucose, IFG, and undiagnosed diabetes.
For the purposes of this study, we defined “elevated A1C” as A1C >6% in this population without a history of diabetes. However, we also assessed the prevalence of elevated A1C at cut points of 6.0, 6.1, 6.2, 6.3, 6.4, 6.5, 6.6, 6.7, 6.8, 6.9, and ≥7.0%. Adjusted odds ratios (ORs) and their corresponding 95% CIs were estimated from logistic regression models to assess the association between potential risk factors and elevated A1C levels. We conducted multivariable logistic analyses modeling A1C >6% as the outcome in the overall population. In the population of adults with normal A1C (<6%) and normal fasting glucose (<100 mg/dl) levels, we modeled the association between risk factors of interest and A1C level above the weighted median A1C level in this population (>5.2%). Model 1 included age, sex, and race/ethnicity. Model 2 included all variables in model 1 plus hypertension, hypercholesterolemia, BMI, education, history of cardiovascular disease, alcohol consumption, and C-reactive protein categories. Sensitivity analyses were conducting using the OGTT data only available in NHANES 2005–2006 and the appropriate 2-year fasting weights for this survey.
Estimates from this study are nationally representative of the noninstitutionalized population of adults ≥20 years in the U.S. Prevalence estimates were applied to the 2000 U.S. Census to obtain estimates of the number of nondiabetic individuals with elevated A1C in the U.S. in the year 2000.
RESULTS
Mean A1C level and proportion of individuals with elevated A1C (A1C >6%) by population characteristics are displayed in Table 1. The mean ± SE A1C in adults aged ≥20 years without diagnosed diabetes was 5.3 ± 0.01%. Mean A1C level and the proportion of individuals with A1C >6% increased considerably with age. Non-Hispanic blacks also had higher A1C levels than non-Hispanic whites. In this crude comparison, differences in A1C were also observed for hypertension and hypercholesterolemia status, BMI categories, education level, and C-reactive protein quartiles.
Table 1.
A1C and proportions of elevated levels (A1C >6%) by population characteristics in adults aged ≥20 years without diagnosed diabetes, U.S. 1999–2006
| Unweighted n | A1C | A1C >6.0% | |
|---|---|---|---|
| Overall | 15,934 | 5.3 ± 0.01 | 3.8 ± 0.17 |
| Age-group | |||
| 20–39 years | 6,318 | 5.1 ± 0.10 | 1.0 ± 0.13 |
| 40–59 years | 4,789 | 5.4 ± 0.01 | 4.4 ± 0.33 |
| 60–69 years | 2,070 | 5.5 ± 0.03 | 9.1 ± 0.73 |
| ≥70 years | 2,757 | 5.5 ± 0.02 | 8.3 ± 0.59 |
| Sex | |||
| Male | 7,524 | 5.3 ± 0.01 | 4.3 ± 0.26 |
| Female | 8,410 | 5.3 ± 0.01 | 3.4 ± 0.22 |
| Race/ethnicity | |||
| Non-Hispanic white | 8,310 | 5.3 ± 0.01 | 3.2 ± 0.20 |
| Non-Hispanic black | 3,038 | 5.4 ± 0.01 | 6.6 ± 0.45 |
| Mexican American | 3,436 | 5.4 ± 0.01 | 3.9 ± 0.36 |
| Other | 998 | 5.4 ± 0.03 | 6.0 ± 0.83 |
| Hypertension | |||
| No | 10,144 | 5.2 ± 0.97 | 2.2 ± 0.16 |
| Yes | 5,780 | 5.5 ± 0.01 | 7.3 ± 0.41 |
| Hypercholesterolemia | |||
| No | 10,347 | 5.2 ± 0.95 | 2.9 ± 0.18 |
| Yes | 5,494 | 5.4 ± 0.01 | 5.7 ± 0.35 |
| Smoking | |||
| Never smoker | 8,300 | 5.3 ± 0.01 | 3.5 ± 0.23 |
| Former smoker | 4,029 | 5.4 ± 0.02 | 5.1 ± 0.39 |
| Current smoker | 3,583 | 5.3 ± 0.01 | 3.2 ± 0.32 |
| BMI | |||
| <25 kg/m2 | 5,199 | 5.2 ± 0.01 | 1.1 ± 0.14 |
| 25–<30 kg/m2 | 5,578 | 5.3 ± 0.01 | 3.2 ± 0.26 |
| ≥30 kg/m2 | 4,789 | 5.5 ± 0.01 | 7.8 ± 0.46 |
| Education | |||
| Post high school | 7,403 | 5.3 ± 0.01 | 2.8 ± 0.21 |
| High school | 3,799 | 5.3 ± 0.01 | 4.0 ± 0.35 |
| Less than high school | 4,701 | 5.4 ± 0.01 | 6.7 ± 0.45 |
| History of cardiovascular disease | 1,168 | 5.5 ± 0.02 | 8.7 ± 0.91 |
| Alcohol consumption | |||
| Never | 4,553 | 5.4 ± 0.01 | 5.8 ± 0.41 |
| Former | 1,490 | 5.4 ± 0.02 | 6.2 ± 0.73 |
| Current | 8,794 | 5.3 ± 0.01 | 2.7 ± 0.18 |
| C-reactive protein quartiles | |||
| 0.01–<0.08 mg/dl | 3,472 | 5.2 ± 0.01 | 1.1 ± 0.17 |
| 0.08–<0.19 mg/dl | 3,376 | 5.3 ± 0.01 | 2.7 ± 0.29 |
| 0.19–<0.44 mg/dl | 4,065 | 5.4 ± 0.01 | 4.4 ± 0.38 |
| 0.44–29.6 mg/dl | 4,527 | 5.5 ± 0.02 | 7.5 ± 0.47 |
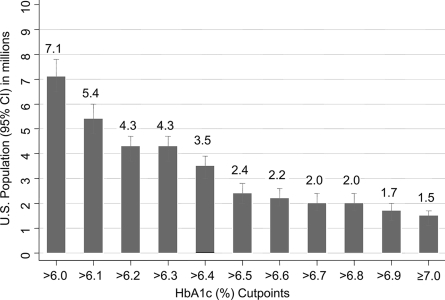
Figure 1 displays the count of individuals (in millions) with elevated A1C at cut points between 6.0 and 7.0%. The prevalence of A1C >6.0% in individuals without a history of diabetes was 3.8% (95% CI 3.5–4.2) (Table 1), corresponding to 7.1 million adults (6.5–7.8) in the U.S (Fig. 1). The prevalence estimates for A1C cut points of >6.1, ≥6.5, and ≥7.0% were 2.9 (2.6–3.2), 1.6 (1.4–1.8), and 0.8 (0.6–0.9). These correspond to 5.4 million (4.8–6.0), 3.0 million (2.6–3.3), and 1.5 million (1.1–1.7) individuals in the U.S. population, respectively. These prevalence estimates suggest the yield of individuals in the U.S. with elevated A1C at different cut points if A1C alone was used to screen for diabetes.
Figure 1.
Count in millions (95% CI) of persons at different A1C cut points in the U.S. 2000 Census population aged ≥20 years without diabetes.
Figure 2 displays the distributions of A1C in individuals with normal fasting glucose (fasting glucose <100 mg/dl), IFG (fasting glucose 100–<126 mg/dl), and undiagnosed diabetes (fasting glucose ≥126 mg/dl), revealing substantially overlapping distributions in individuals with normal glucose and IFG but a right-skewed distribution in individuals with undiagnosed diabetes. The mean A1C levels in individuals with normal fasting glucose, IFG, and undiagnosed diabetes were 5.2 ± 0.01, 5.5 ± 0.01, and 6.9 ± 0.16%, respectively (not shown). Similarly, the distribution of individuals in the fasting glucose categories (<100, 100–125, and ≥126 mg/dl) varied substantially, depending on the A1C cut point. For instance, among individuals with A1C >6.0%, 45.6% had undiagnosed diabetes, 45.3% had IFG, and 9.1% had normal fasting glucose. In contrast, among individuals with an A1C ≥7.0%, 91.7% had undiagnosed diabetes, 6.6% had IFG, and only 1.7% had normal glucose levels. A comparison of the distribution of fasting glucose categories among individuals with A1C >6.1% and A1C ≥6.5% yielded intermediate results. Among individuals with A1C >6%, 53.3% had undiagnosed diabetes, 38.6% had IFG, and 8.1% had normal fasting glucose. Among individuals with A1C ≥6.5%, 76.7% had undiagnosed diabetes, 19.6% had IFG, and 1.7% had normal fasting glucose (supplementary Figure A, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc08-1699/DC1).
Figure 2.
Weighted smoothed histogram comparing distributions of A1C by fasting glucose category, adults aged ≥20 years without diagnosed diabetes, U.S. 1992–2006.
Multivariable logistic regression analysis demonstrated that older age, male sex, non-Hispanic black and Mexican American race/ethnicity, hypertension, higher BMI, less than a high school education, and higher C-reactive protein levels were all associated with the prevalence of elevated A1C (A1C >6%) even after multivariable adjustment in this population of adults without diagnosed diabetes (supplementary Table A). Current alcohol consumption was associated with lower A1C. We next examined the same variables but limited the population to individuals with normal A1C (A1C <6%) and with a fasting glucose <100 mg/dl and assessed the association with having an A1C level above the median in this population (A1C >5.2%) (Table 2). Similar associations as in the model of A1C >6% were observed in the full population. In Table 2, older age, male sex, non-Hispanic black and Mexican-American race/ethnicity, hypercholesterolemia, higher BMI, and lower attained education were significantly associated with having a higher A1C level, even after adjustment. Current smoking was associated with higher A1C and current alcohol consumption with lower A1C in this population with normal glucose levels. Additional adjustment for fasting glucose did not alter these results (data not shown). Our results were also unchanged in sensitivity analyses of NHANES 2005–2006, the only years for which OGTT data were available, in which we further excluded individuals with impaired glucose tolerance (2-h glucose ≥140 mg/dl) from our multivariable models (data not shown).
Table 2.
Adjusted ORs (95% CIs) of A1C above the median (A1C >5.2%) in adults aged ≥20 years without diagnosed diabetes and with normal fasting glucose and normal A1C levels, U.S. 1999–2006
| Variable | OR (95% CI) |
|
|---|---|---|
| Model 1 | Model 2 | |
| n | 4,622 | 4,256 |
| Age-group | ||
| 20–39 years | 1.0 (referent) | 1.0 (referent) |
| 40–59 years | 2.8 (2.4–3.4)* | 2.7 (2.2–3.3)* |
| 60–69 years | 4.6 (3.6–5.8)* | 3.3 (2.4–4.6)* |
| ≥70 years | 6.9 (5.1–9.3)* | 6.0 (3.9–9.1)* |
| Sex | ||
| Female | 1.0 (referent) | 1.0 (referent) |
| Male | 1.4 (1.2–1.7)* | 1.5 (1.2–1.9)* |
| Race/ethnicity | ||
| Non-Hispanic white | 1.0 (referent) | 1.0 (referent) |
| Non-Hispanic black | 2.9 (2.4–3.7)* | 2.4 (1.8–3.1)* |
| Mexican American | 1.7 (1.3–2.2)* | 1.4 (1.0–1.8)* |
| Other | 1.8 (1.3–2.5)* | 1.9 (1.4–2.6)* |
| Hypertension (yes vs. no) | 1.2 (0.9–1.5) | |
| Hypercholesterolemia (yes vs. no) | 1.4 (1.2–1.7)* | |
| Smoking | ||
| Never smoker | 1.0 (referent) | |
| Former smoker | 1.0 (0.8–1.2) | |
| Current smoker | 1.5 (1.2–1.9)* | |
| BMI | ||
| <25 kg/m2 | 1.0 (referent) | |
| 25–<30 kg/m2 | 1.2 (0.9–1.5) | |
| ≥30 kg/m2 | 1.9 (1.4–2.7)* | |
| Education | ||
| Post high school | 1.0 (referent) | |
| High school or equivalent | 1.3 (1.0–1.7)* | |
| Less than high school | 1.5 (1.2–1.9)* | |
| History of cardiovascular disease | 0.9 (0.7–1.3) | |
| Alcohol consumption | ||
| Never | 1.0 (referent) | |
| Former | 0.8 (0.5–1.1) | |
| Current | 0.6 (0.5–0.8)* | |
| C-reactive protein quartiles | ||
| 0.01–<0.08 mg/dl | 1.0 (referent) | |
| 0.08–<0.19 mg/dl | 1.0 (0.8–1.4) | |
| 0.19–<0.44 mg/dl | 1.1 (0.8–1.4) | |
| 0.44–29.6 mg/dl | 1.1 (0.8–1.6) | |
Model 1: age, sex, and race/ethnicity. Model 2: all variables in model 1 plus hypertension, hypercholesterolemia, BMI, education, history of cardiovascular disease, alcohol consumption, and C-reactive protein categories. Absence of diabetes with normal fasting glucose indicates no history of diabetes and FPG <100 mg/dl; normal A1C level indicates A1C <6%.
*P < 0.05.
CONCLUSIONS
This analysis suggests that elevated A1C (>6%) is common in the general population of nondiabetic adults. The overall prevalence of A1C >6% was 3.8%, corresponding to 7.1 million individuals in the U.S. population. Approximately 45% of these individuals have IFG and 45% have fasting glucose ≥126 mg/dl. Elevated A1C levels were particularly common among older adults, non-Hispanic blacks, and obese individuals. We found that demographic characteristics and risk factors for type 2 diabetes and its complications including older age, male sex, nonwhite race/ethnicity, lower attained education level, adiposity, and hypercholesterolemia were associated with elevated A1C even in the presence of normal fasting glucose.
Significant advantages of adopting A1C for the screening and diagnosis of diabetes are the high repeatability of the measurement (17,18) and the high specificity of elevated values for detecting undiagnosed diabetes (19–21). Recent recommendations have stated that diagnosis based on A1C should be confirmed using a glucose-dependent test (FPG or OGTT) or by a second A1C (11). However, glucose-dependent tests are less reliable (repeatable) than A1C (17). Requiring confirmation of a highly reliable test by one that is less reliable poses problems for the interpretation of any discrepancy between the two values. In a previous study, we analyzed repeated measurements taken ∼2 weeks apart on an unselected sample of individuals without diabetes and found that 100% of individuals with A1C ≥7% had a second A1C measurement of ≥7% ∼2 weeks later and 80% of individuals with A1C ≥6.5 had an A1C level ≥6.5% 2 weeks later (Pearson's r = 0.95) (17). There is little marginal gain to repeating the A1C test within a short (several week) time period. Furthermore, we show in the present study that 92% of individuals with A1C ≥7.0% also had FPG ≥126 mg/dl and 77% of individuals with A1C ≥6.5% had FPG ≥126 mg/dl. At the population level, elevated A1C is rare in the absence of elevated fasting glucose. Additional advantages to using A1C for screening and/or diagnosis of diabetes include national standardization of the assay (22,23), the low analytic variability (high methodological quality of the assay, even when compared with glucose) (24), the widespread availability of the A1C test and its current use in the management and treatment of diabetes, and the fact that the patient does not need to fast.
It is unclear why nondiabetic non-Hispanic blacks have consistently higher A1C values even in the setting of normal fasting glucose levels and after adjustment for demographic and clinical characteristics. Further research should be conducted to determine whether this disparity stems from racial differences in postprandial glycemia or from racial differences in the tendency of hemoglobin to undergo glycosylation.
This study has several strengths including the large, nationally representative sample of healthy, nondiabetic individuals. We benefited from the rigorous measurement of risk factors using standardized protocols and strict quality control data collection and laboratory procedures in NHANES. Important limitations include the cross-sectional design, which limits our conclusions regarding the temporality of the observed associations. In addition, we had only a single measurement of fasting glucose. The American Diabetes Association recommends repeating an elevated fasting glucose measurement to confirm the diagnosis of diabetes (13). The use of a single measurement of fasting glucose rather than two will overestimate the prevalence of undiagnosed diabetes (17). Nonetheless, interpretation of single measurements of fasting glucose and A1C as analyzed in this study reflects a common clinical decision-making setting. Although we cannot rule out the possibility of laboratory differences over time, calibration of A1C to account for the change in laboratories in 2005–2006 using a published equation (14) did not appreciably alter our results. The lack of OGTT data in the fasting glucose subsample for all survey years is an important limitation of this study. Nonetheless, similar results were obtained in multivariable models using the OGTT measurements in the subgroup from the 2005–2006 NHANES.
To date, the diagnostic utility of A1C has largely been assessed by its accuracy (as measured by its sensitivity and specificity) to detect glucose-defined cases of diabetes (25). The concordance of A1C with fasting glucose is important and, as confirmed by our data, an A1C ≥6.5% is specific for the detection of undiagnosed diabetes defined by a single fasting glucose level. Thus, it seems reasonable to adopt a single elevated A1C value as being diagnostic for diabetes. However, the real test of utility for A1C as a screening or diagnostic test of diabetes is its association with long-term clinical outcomes in an initially nondiabetic population specifically in comparison with fasting glucose levels. To address this question we need large, observational studies of A1C in populations of individuals without diabetes.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R21 DK080294. E.S. was supported by NIH/NIDDK Grant K01 DK076595. F.L.B. was supported by NIH/NIDDK Grant K24 DK62222.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the American Heart Association 49th Annual Conference on Cardiovascular Disease Epidemiology, Palm Harbor, Florida, 10–14 March 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ: Utility of hemoglobin A1c in predicting diabetes risk. J Gen Internal Med 2004; 19: 1175– 1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ko GT, Chan JC, Tsang LW, Cockram CS: Combined use of fasting plasma glucose and HbA1c predicts the progression to diabetes in Chinese subjects. Diabetes Care 2000; 23: 1770– 1773 [DOI] [PubMed] [Google Scholar]
- 3. Pradhan AD, Rifai N, Buring JE, Ridker PM: Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med 2007; 120: 720– 727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, Eschwege E: the DESIR Study Group. Use of HbA1c in predicting progression to diabetes in french men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2006; 29: 1619– 1625 [DOI] [PubMed] [Google Scholar]
- 5. Inoue K, Matsumoto M, Kobayashi Y: The combination of fasting plasma glucose and glycosylated hemoglobin predicts type 2 diabetes in Japanese workers. Diabetes Res Clin Pract 2007; 77: 451– 458 [DOI] [PubMed] [Google Scholar]
- 6. Levitan EB, Song Y, Ford ES, Liu S: Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med 2004; 164: 2147– 2155 [DOI] [PubMed] [Google Scholar]
- 7. Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N: Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141: 413– 420 [DOI] [PubMed] [Google Scholar]
- 8. Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW: Glycemic control and coronary heart disease risk in persons with and without diabetes: the Atherosclerosis Risk in Communities Study. Arch Intern Med 2005; 165: 1910– 1916 [DOI] [PubMed] [Google Scholar]
- 9. Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR: Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Lancet Neurol 2005; 4: 821– 826 [DOI] [PubMed] [Google Scholar]
- 10. Levitan EB, Liu S, Stampfer MJ, Cook NR, Rexrode KM, Ridker PM, Buring JE, Manson JE: HbA1C measured in stored erythrocytes and mortality rate among middle-aged and older women. Diabetologia 2008; 51: 267– 275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB: A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 2008; 93: 2447– 2453 [DOI] [PubMed] [Google Scholar]
- 12. National Center for Health Statistics, Centers for Disease Control and Prevention. Survey Operations Manuals, Brochures, and Consent Documents: 1999-current NHANES. Available at http://www.cdc.gov/nchs/nhanes.htm [article online], 2007. Accessed 6 March 2009
- 13. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008; 31: S55– S60 [DOI] [PubMed] [Google Scholar]
- 14. National Health and Nutrition Examination Survey 2005–2006. Documentation, Codebook, and Frequencies: glycohemoglobin [article online], 2008. Available from: http://www.cdc.gov/NCHS/data/nhanes/nhanes_05_06/ghb_d.pdf. Accessed 6 March 2009
- 15. National Health and Nutrition Examination Survey 2005–2006. Documentation, Codebook, and Frequencies: plasma fasting glucose and insulin [article online], http://www.cdc.gov/NCHS/data/nhanes/nhanes_05_06/glu_d.pdf. Accessed 6 March 2009
- 16. National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) Analytic Guidelines [article online], 2007. Available from: http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003–2004/analytical_guidelines.htm. Accessed 9 May 2007
- 17. Selvin E, Crainiceanu CM, Brancati FL, Coresh J: Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007; 167: 1545– 1551 [DOI] [PubMed] [Google Scholar]
- 18. Phillipou G, Phillips PJ: Intraindividual variation of glycohemoglobin: implications for interpretation and analytical goals. Clin Chem 1993; 39: 2305– 2308 [PubMed] [Google Scholar]
- 19. Buell C, Kermah D, Davidson MB: Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care 2007; 30: 2233– 2235 [DOI] [PubMed] [Google Scholar]
- 20. Davidson MB, Schriger DL, Peters AL, Lorber B: Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA 1999; 281: 1203 1210 [DOI] [PubMed] [Google Scholar]
- 21. Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberhardt MS, Goldstein DE: Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 2000; 23: 187– 191 [DOI] [PubMed] [Google Scholar]
- 22. Little RR: Glycated hemoglobin standardization—National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med 2003; 41: 1191– 1198 [DOI] [PubMed] [Google Scholar]
- 23. Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE: The national glycohemoglobin standardization program: a five-year progress report. Clin Chem 2001; 47: 1985– 1992 [PubMed] [Google Scholar]
- 24. Rohlfing C, Wiedmeyer HM, Little R, Grotz VL, Tennill A, England J, Madsen R, Goldstein D: Biological variation of glycohemoglobin. Clin Chem 2002; 48: 1116– 1118 [PubMed] [Google Scholar]
- 25. Bennett CM, Guo M, Dharmage SC: HbA1c as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med 2007; 24: 333– 343 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.