Abstract
OBJECTIVE
The purpose of this study was to examine prospectively whether renal hyperfiltration is associated with the development of microalbuminuria in patients with type 1 diabetes, after taking into account known risk factors.
RESEARCH DESIGN AND METHODS
The study group comprised 426 participants with normoalbuminuria from the First Joslin Kidney Study, followed for 15 years. Glomerular filtration rate was estimated by serum cystatin C, and hyperfiltration was defined as exceeding the 97.5th percentile of the sex-specific distribution of a similarly aged, nondiabetic population (134 and 149 ml/min per 1.73 m2 for men and women, respectively). The outcome was time to microalbuminuria development (multiple albumin excretion rate >30 μg/min). Hazard ratios (HRs) for microalbuminuria were calculated at 5, 10, and 15 years.
RESULTS
Renal hyperfiltration was present in 24% of the study group and did not increase the risk of developing microalbuminuria. The unadjusted HR for microalbuminuria comparing those with and without hyperfiltration at baseline was 0.8 (95% CI 0.4–1.7) during the first 5 years, 1.0 (0.6–1.7) during the first 10 years, and 0.8 (0.5–1.4) during 15 years of follow-up. The model adjusted for baseline known risk factors including A1C, age at diagnosis of diabetes, diabetes duration, and cigarette smoking resulted in similar HRs. In addition, incorporating changes in hyperfiltration status during follow-up had minimal impact on the HRs for microalbuminuria.
CONCLUSIONS
Renal hyperfiltration does not have an impact on the development of microalbuminuria in type 1 diabetes during 5, 10, or 15 years of follow-up.
The glomerular filtration rate (GFR), the volume of water filtered out of the plasma per unit of time, is indicative of overall kidney function. However, measuring GFR with the gold standard technique is an intensive process and difficult for both the operator and the participant. Thus, it has not been practical to determine GFR in large epidemiological studies. Instead, serum creatinine has been widely used to estimate low levels of GFR when loss of kidney function has already occurred. However, serum creatinine is not sensitive enough to detect changes when renal function is normal or abnormally elevated (1). A laboratory test to estimate GFR based on serum cystatin C levels has been developed recently. Cystatin C assays are easy to perform and have been shown to yield accurate estimates even in the normal or elevated ranges of filtration (2,3). This development has created a new opportunity for studying early diabetic renal function abnormalities in large epidemiological studies.
Hyperfiltration has been suggested as a risk factor for the development of microalbuminuria (4). The increase in pressure and flow may lead to functional and structural changes in the kidney (5,6). In several small studies, hyperfiltration was associated with the development of microalbuminuria in type 1 diabetes, but results have been inconsistent. Some studies were conducted in children beginning at diagnosis or early in the course of diabetes, and usually a few events of microalbuminuria were observed (7–11). Yip et al. (12) found no association between hyperfiltration and microalbuminuria in a 10-year prospective case control study of 25 adult pairs who had diabetes duration between 1 and 19 years. None of these studies adequately addressed confounders. Little subsequent research in large cohorts has been conducted on the role of hyperfiltration, primarily due to difficulties in determining GFR.
Scott et al. (13) studied microalbuminuria onset in the First Joslin Study on the Natural History of Microalbuminuria (First Joslin Kidney Study) during the first 4 years of follow-up (13). They found that younger age at diabetes diagnosis, longer diabetes duration, poorer glycemic control, and cigarette smoking were associated with the development of microalbuminuria. Serum cystatin C measurements (to estimate GFR) were not available at the time of that work. The current project builds upon this prior study by examining whether hyperfiltration, as measured by cystatin C, is associated with the development of microalbuminuria during 15 years of follow-up, after taking into account known risk factors.
RESEARCH DESIGN AND METHODS
The study group is derived from the cohort of the First Joslin Kidney Study. Enrollment was as follows. From January 1991 to April 1992, every other Joslin Clinic patient with type 1 diabetes aged 15–44 years who resided in Massachusetts had his or her urine examined for microalbuminuria using an albumin-to-creatinine ratio (ACR). Based on the initial screening and additional measurements obtained over a 2-year baseline interval, patients (n = 1,602) were categorized as having normoalbuminuria (n = 1,080), microalbuminuria (n = 312), or proteinuria (n = 210). Men with a median ACR <17 mg/g or women with a median ACR <25 mg/g were classified as having normoalbuminuria, and men with median ACR between 17 and 250 mg/g and women with median ACR between 25 and 355 mg/g were classified as having microalbuminuria. Patients with proteinuria were not followed for assessments of albumin excretion rate (AER). All participants with microalbuminuria and a 50% sample of participants with normoalbuminuria were invited to participate in an entry examination during the first 2 years of the study. The participants with normoalbuminuria who still had stored blood samples for measuring cystatin C were the focus of the current investigation.
Entry examination and measurements of characteristics and exposures
At the entry examination, a trained study recruiter administered a questionnaire to obtain medical and diabetes history, collected samples of blood and urine, and measured seated blood pressures twice, separated by a 5-min rest. Chart review supplemented the questionnaire information as needed.
Electronic medical record information captured clinical characteristics such as repeated measures of A1C and ACR. Details of these assays have been published previously (14). The equation for the conversion of ACR to AER was log10(AER) = 0.44 + (0.85)log10(ACR) − (0.13)sex, where sex = 1 for women and 0 for men (14). Baseline A1C was the mean of all A1C measurements over the year before entry examination including A1C measured at the examination. Baseline exposures and characteristics measured for study-specific reasons (such as cystatin C) or related to calendar time (such as age and duration of diabetes) were measurements from the date of entry examination.
Serum cystatin C has been shown to estimate GFR well in diabetic populations with normal or elevated renal function (2,3,15). The equation to estimate GFR from cystatin C was developed by Macisaac et al. (3) (cystatin C-GFR = [86.7/cystatin C] − 4.2). All serum samples were stored at −85°C until the day of assay. Samples were thawed, vortexed for 5 s, and microcentrifuged at 13,200 rpm for 10 min. Samples were then analyzed for cystatin C concentration (Dade Behring, Newark, DE) on a BN ProSpec System nephelometer (Dade Behring). The reported reference interval for cystatin C is 0.53–0.95 mg/l for young, healthy individuals. Factory-provided controls were measured on the day of each run for quality control.
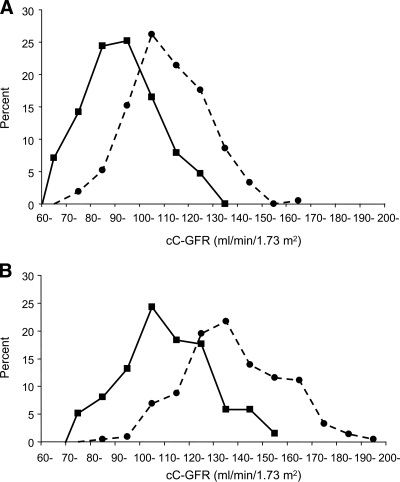
We estimated cystatin C-GFR in healthy nondiabetic individuals aged 18–44 years. They were the nondiabetic relatives examined for our family-based study of the genetics of diabetic nephropathy in type 2 diabetes (16). Their serum cystatin C concentrations were determined in the same laboratory and by the same method as those for the current study. In these nondiabetic individuals, the distribution of cystatin C-GFR was higher for women than for men (Fig. 1). We defined hyperfiltration as a cystatin C-GFR exceeding the sex-specific 97.5th percentile in nondiabetic subjects: >149 and >134 ml/min per 1.73 m2 for women and men, respectively. In previous studies authors used similar definitions (125–140 ml/min per 1.73 m2), but the definitions were not sex-specific (6–8).
Figure 1.
A: Distribution of cystatin C-GFR measurements at baseline in men with (●, n = 210) and without (■, n = 127) diabetes of similar age (18–44 years). B: Distribution of cystatin C-GFR measurements at baseline in women with (●, n = 216) and without (■, n = 136) diabetes of similar age (18–44 years). Measurements of serum cystatin C concentrations in diabetic subjects and nondiabetic subjects were performed in the same laboratory and according to the same method, and the same formula was used to estimate cystatin C-GFR (see research design and methods).
Follow-up of eligible study group
Patients were followed for the development of microalbuminuria over 15 years through routine clinic appointments, home visits by patient recruiters, and mailed urine kits. Of the 502 patients with a study examination, 473 participants qualified by having samples remaining for the measurement of cystatin C-GFR. We excluded participants who developed microalbuminuria before the first examination and those with <1 year AER follow-up after examination (n = 17). Also excluded were 30 participants who did not have A1C measurements within 1 year before the cystatin C-GFR measurement and those who did not have information on smoking status. Thus, 426 participants remained eligible for this study.
Outcome: time to onset of microalbuminuria
The outcome was time to development of microalbuminuria. The onset of microalbuminuria occurred when two consecutive AER measurements reached the microalbuminuria range (AER >30 μg/min). The date of the first AER of the pair was the date of the onset.
Statistical methods
All statistical analyses were conducted in SAS (version 9.1; SAS Institute, Cary, NC). Descriptive analyses (mean ± SD for continuous variables and percentage and counts for categorical variables) compared clinical characteristics among those with and without hyperfiltration during baseline.
The hazard ratio (HR) of developing microalbuminuria during 5, 10, and 15 years of follow-up and the corresponding 95% CIs were calculated using Cox proportional hazards modeling (PROC PHREG). Next, adjusted HRs were calculated, comparing participants who were and were not hyperfiltering at baseline. The potential confounders (baseline A1C, age at diabetes diagnosis, diabetes duration, and cigarette smoking status) were entered into the multivariate model. To assess effect measure modification, we stratified on sex, baseline A1C, age at diabetes diagnosis, and diabetes duration.
Changes in the hyperfiltration status of patients with multiple cystatin C-GFR measures over time were determined. To be eligible for this analysis, patients had at least two determinations at least 2 years apart. The median was three determinations and median follow-up of cystatin C-GFR was 9 years. Over time, hyperfiltration could have been consistently present, consistently absent, or inconsistent. These results were incorporated into an analysis that allowed hyperfiltration status to vary over time. Last, as in the analysis of baseline hyperfiltration status, unadjusted and adjusted HRs for microalbuminuria were calculated during 5, 10, and 15 years of follow-up.
RESULTS
Characteristics of study participants with and without renal hyperfiltration at baseline are displayed in Table 1. Renal hyperfiltration was defined as a cystatin C-GFR exceeding 134 and 149 ml/min per 1.73 m2 for men and women, respectively, the sex-specific 97.5th percentiles in nondiabetic individuals (Fig. 1). There were 24% of patients with renal hyperfiltration in the study group. Those with hyperfiltration were older, were more likely to be female, and had shorter diabetes duration, later age of onset of diabetes, and slightly higher A1C levels. Systolic and diastolic blood pressure, BMI, and percentage of current smokers were similar among those with and without hyperfiltration. Microalbuminuria developed in 23% (74 of 322) of participants without and 19% (20 of 104) of those with hyperfiltration at baseline.
Table 1.
Selected baseline characteristics of participants according to baseline renal hyperfiltration status
| No hyperfiltration | Hyperfiltration | |
|---|---|---|
| n | 322 | 104 |
| Women (%) | 48 | 61 |
| Age (years) | 29 ± 8 | 31 ± 7 |
| Diabetes duration (years) | 14 ± 8 | 12 ± 7 |
| Age of diabetes diagnosis (years) | 15 ± 8 | 19 ± 8 |
| A1C (%) | 8.1 ± 1.3 | 8.6 ± 1.7 |
| Systolic blood pressure (mmHg) | 119 ± 13 | 117 ± 14 |
| Diastolic blood pressure (mmHg) | 71 ± 8 | 72 ± 8 |
| Current smoking (%) | 17 | 18 |
| BMI (kg/m2) | 24.3 ± 3.0 | 23.1 ± 2.6 |
| Cystatin C-GFR (ml/min per 1.73 m2) | 122 ± 13 | 155 ± 13 |
| Developed microalbuminuria (%)* | 23 | 19 |
Data are means ± SD or %.
*Developed confirmed microalbuminuria during 15 year follow-up.
HRs for developing microalbuminuria comparing participants with and without hyperfiltration at baseline are shown in Table 2. Hyperfiltration did not increase the rate of developing microalbuminuria. The unadjusted HR was 0.8 (95% CI 0.4–1.7) during the first 5 years, 1.0 (0.6–1.7) during the first 10 years, and 0.8 (0.5–1.4) during 15 years of follow-up. In a model adjusting for known risk factors for microalbuminuria (A1C, age at diabetes diagnosis, diabetes duration, and current cigarette smoking), the HRs were little changed: 0.8 (0.4–1.7) during the first 5 years, 1.0 (0.5–1.7) during the first 10 years, and 0.9 (0.6–1.4) during 15 years of follow-up. There was also no effect measure modification due to sex, baseline A1C, age at diabetes diagnosis, and diabetes duration. Results were identical to those in Table 2, when we performed a sensitivity analysis using lower (95th percentile) or higher (99th percentile) cutoffs for renal hyperfiltration (data not shown).
Table 2.
Unadjusted and adjusted HRs of developing microalbuminuria comparing individuals with and without renal hyperfiltration at baseline
| Events of microalbuminuria | Total person-years | HR (95% CI) |
||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| 5-year HR | ||||
| No hyperfiltration | 35 | 1,500 | 1 (Ref) | 1 (Ref) |
| Hyperfiltration | 9 | 486 | 0.8 (0.4–1.7) | 0.8 (0.4–1.7) |
| 10-year HR | ||||
| No hyperfiltration | 53 | 2,744 | 1 (Ref) | 1 (Ref) |
| Hyperfiltration | 17 | 888 | 1.0 (0.6–1.7) | 1.0 (0.5–1.7) |
| 15-year HR | ||||
| No hyperfiltration | 74 | 3,574 | 1 (Ref) | 1 (Ref) |
| Hyperfiltration | 20 | 1,145 | 0.8 (0.5–1.4) | 0.9 (0.6–1.4) |
Hyperfiltration is defined as exceeding the 97.5th percentile of cystatin C-GFR in a nondiabetic, similarly aged population. For women this cutoff was 149 ml/min per 1.73 m2 and for men 134 ml/min per 1.73 m2.
*Adjusted by baseline mean A1C, age at diabetes diagnosis, diabetes duration, and current cigarette smoking. Ref, referent.
There were 243 participants with multiple cystatin C-GFR measures over follow-up. Hyperfiltration was absent throughout follow-up (median 11 years) in 69% (167 of 243). Hyperfiltration was consistently present in 4% (9 of 243); however, they had shorter follow-up than those whose hyperfiltration status changed. Hyperfiltration status changed in 28% (67 of 243). In the majority of these individuals, baseline hyperfiltration resolved during follow-up (37 of 67). In a few instances, baseline hyperfiltration resolved only to return (n = 9). In the remainder, hyperfiltration developed during follow-up and remained (n = 13) or resolved to normal (n = 8). Similar to the consistently hyperfiltering group, the group with hyperfiltering that developed during follow-up and remained had shorter follow-up than other groups. This suggests that if follow-up were longer, these participants may have returned to normal filtration levels, albeit this was a small proportion of the total group with follow-up cystatin C-GFR (19 of 243).
Incorporating changes in hyperfiltration status over follow-up (time-varying analysis) had minimal impact on the HR. The unadjusted HR for microalbuminuria comparing those with and without hyperfiltration at baseline was 0.6 (95% CI 0.3–1.4) during the first 5 years, 1.0 (0.6–1.7) during the first 10 years, and 1.0 (0.6–1.7) during 15 years of follow-up. Similar to the analysis of baseline hyperfiltration, there was minimal confounding due to the other risk factors. The adjusted HR during 5, 10, and 15 years of follow-up was 0.7 (0.3–1.5), 1.0 (0.6–1.8), and 1.1 (0.7–1.8), respectively.
CONCLUSIONS
In our 15-year follow-up of AER in a cohort of young adults with type 1 diabetes, neither the presence of hyperfiltration at baseline nor its development subsequently was a risk factor for the development of microalbuminuria within 5, 10, or 15 years. There was very little confounding by A1C, age at diabetes diagnosis, diabetes duration, and current cigarette smoking in the relation between hyperfiltration and microalbuminuria development. There was also no effect measure modification on the relation between hyperfiltration and microalbuminuria due to sex, baseline A1C, age at diabetes diagnosis, and diabetes duration.
In the subset of the cohort with multiple measurements of cystatin C-GFR during follow-up, we characterized the patterns of change in hyperfiltration status. It did not change in the majority of individuals. Hyperfiltration was never present in 66% and always present in 4%. In the remaining 30% in whom the status changed, the change was resolution of hyperfiltration in the majority and development of hyperfiltration in a small minority.
Most data on the biological mechanisms underlying the impact of hyperfiltration on the kidney come from animal models (17). In those models, hyperfiltration increases glomerular pressure and flow, which initiate destructive processes in the kidney (5,6). However, hyperfiltration is benign in some human conditions other than diabetes, so hyperfiltration cannot be the problem by itself (18). Under experimental conditions, induction of hyperglycemia in humans with diabetes increases the GFR in those with hyperfiltration but not in those with normal GFR or in individuals without diabetes (19). In our study, hyperglycemia predicted the onset of microalbuminuria, but hyperfiltration did not, so the effect of glycemic control is not through an effect on GFR.
Our findings differ from the results of several studies, but the disagreements may be due to methodological limitations in those studies. For instance, in the study by Chiarelli et al. (7), hyperfiltration predicted microalbuminuria over a 10-year follow-up in a prospective case-control study of children and young adults aged 9–19 years. However, with only eight cases of microalbuminuria divided between 23 individuals with hyperfiltration and 23 without, their result has large statistical uncertainty. Moreover, the analysis did not control for confounding by A1C. Amin et al. (8) tested the hyperfiltration and microalbuminuria hypothesis in a 5-year follow-up study of 273 children with diabetes duration of 5 years. Microalbuminuria developed in 30 children. After controlling for A1C, the estimated HR of 1.02 per unit of GFR was statistically significant. The clinical meaningfulness, however, of such a small effect is questionable.
On the other hand, our findings are consistent with a number of studies. In the late 1980s, Lervang et al. (20) studied 29 patients with type 1 diabetes who had been studied 18 years previously when diabetes duration averaged 2 years (range 0–9). The AER did not differ according to hyperfiltration status at baseline. They suggested that the disagreement with other study findings might have been due to their population's older age at onset of diabetes (age 19 on average) (21,22). Steinke et al. (11) did not find compelling evidence that hyperfiltration predicted microalbuminuria and suggested that differences among studies may be due to variable definitions of hyperfiltration and AER progression and the inclusion of patients with very short durations of diabetes. Levine (17) hypothesized that the duration of hyperfiltration, which is not usually taken into consideration, may be an important factor in kidney damage.
One 5-year prospective case-control study of adults with type 1 diabetes and without proteinuria or hypertension found no difference in AER according to hyperfiltration status at baseline (23). However, in this study the rate of renal function decline was faster in those with hyperfiltration than in those without. In a study of the same patients at 10 years of follow-up, the rate of decline continued to differ according to hyperfiltration status at baseline. However, the absolute GFR remained higher in the hyperfiltration group (12).
The First Joslin Kidney Study has many strengths, including a large, well-characterized cohort followed prospectively over 15 years. The availability of a detailed entry questionnaire and repeated visits that generated laboratory data and stored specimens allowed the examination of novel definitions of exposures and outcomes. Moreover, frequent AER measurements yielded more reliable determinations of renal status than studies based on single measurements, and the sample size enabled us to control for confounders and assess effect measure modification.
One limitation of the current study, however, is that an individual with hyperfiltration occurring and resolving before entry into the study was misclassified. Similarly, we were unable to assess the effect of hyperfiltration occurring and resolving very soon after diabetes onset due to the small number of individuals studied with <5 years duration of diabetes. It is possible that renal hyperfiltration has a more immediate impact on the development of microalbuminuria, and studies started during childhood have captured this information, but follow-up was short so outcomes were few.
In summary, this study provides evidence that hyperfiltration is not a risk factor for the development of microalbuminuria in type 1 diabetes. There was little change in the HR at 5, 10, or 15 years when known risk factors and potential confounders were accounted for, and there was no effect measure modification by sex, baseline A1C, age at diabetes diagnosis, and diabetes duration.
Acknowledgments
This research was supported by National Institutes of Health Grant DK 041526.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Viberti GC, Bilous RW, Mackintosh D, Keen H: Monitoring glomerular function in diabetic nephropathy: a prospective study. Am J Med 1983; 74: 256– 264 [DOI] [PubMed] [Google Scholar]
- 2. Tan GD, Lewis AV, James TJ, Altmann P, Taylor RP, Levy JC: Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care 2002; 25: 2004– 2009 [DOI] [PubMed] [Google Scholar]
- 3. Macisaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, Poon A, Jenkins MA: Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia 2006; 49: 1686– 1689 [DOI] [PubMed] [Google Scholar]
- 4. Mogensen CE: Glomerular hyperfiltration in human diabetes. Diabetes Care 1994; 17: 770– 775 [DOI] [PubMed] [Google Scholar]
- 5. Brenner BM, Meyer TW, Hostetter TH: Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 1982; 307: 652– 659 [DOI] [PubMed] [Google Scholar]
- 6. Brenner BM: Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int 1983; 23: 647– 655 [DOI] [PubMed] [Google Scholar]
- 7. Chiarelli F, Verrotti A, Morgese G: Glomerular hyperfiltration increases the risk of developing microalbuminuria in diabetic children. Pediatr Nephrol 1995; 9: 154– 158 [DOI] [PubMed] [Google Scholar]
- 8. Amin R, Turner C, van Aken S, Bahu TK, Watts A, Lindsell DR, Dalton RN, Dunger DB: The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: The Oxford Regional Prospective Study. Kidney Int 2005; 68: 1740– 1749 [DOI] [PubMed] [Google Scholar]
- 9. Dahlquist G, Stattin EL, Rudberg S: Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy: a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Transplant 2001; 16: 1382– 1386 [DOI] [PubMed] [Google Scholar]
- 10. Rudberg S, Persson B, Dahlquist G: Increased glomerular filtration rate as a predictor of diabetic nephropathy—an 8-year prospective study. Kidney Int 1992; 41: 822– 828 [DOI] [PubMed] [Google Scholar]
- 11. Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M: International Diabetic Nephropathy Study Group. The early natural history of nephropathy in type 1 diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 2005; 54: 2164– 2171 [DOI] [PubMed] [Google Scholar]
- 12. Yip JW, Jones SL, Wiseman MJ, Hill C, Viberti G: Glomerular hyperfiltration in the prediction of nephropathy in IDDM: a 10-year follow-up study. Diabetes 1996; 45: 1729– 1733 [DOI] [PubMed] [Google Scholar]
- 13. Scott LJ, Warram JH, Hanna LS, Laffel LM, Ryan L, Krolewski AS: A nonlinear effect of hyperglycemia and current cigarette smoking are major determinants of the onset of microalbuminuria in type 1 diabetes. Diabetes 2001; 50: 2842– 2849 [DOI] [PubMed] [Google Scholar]
- 14. Krolewski AS, Laffel L, Krolewski M, Quinn M, Warram JH: Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 1995; 332: 1251– 1255 [DOI] [PubMed] [Google Scholar]
- 15. Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G: Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care 2008; 31: 971– 973 [DOI] [PubMed] [Google Scholar]
- 16. Placha G, Poznik GD, Dunn J, Smiles A, Krolewski B, Glew T, Puppala S, Schneider J, Rogus JJ, Rich SS, Duggirala R, Warram JH, Krolewski AS: A genome-wide linkage scan for genes controlling variation in renal function estimated by serum cystatin C levels in extended families with type 2 diabetes. Diabetes 2006; 55: 3358– 3365 [DOI] [PubMed] [Google Scholar]
- 17. Levine DZ: Can rodent models of diabetic kidney disease clarify the significance of early hyperfiltration? Recognizing clinical and experimental uncertainties. Clin Sci (Lond) 2008; 114: 109– 118 [DOI] [PubMed] [Google Scholar]
- 18. Nenov VD, Taal MW, Sakharova OV, Brenner BM: Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens 2000; 9: 85– 97 [DOI] [PubMed] [Google Scholar]
- 19. Wiseman MJ, Mangili R, Alberetto M, Keen H, Viberti G: Glomerular response mechanisms to glycemic changes in insulin-dependent diabetics. Kidney Int 1987; 31: 1012– 1018 [DOI] [PubMed] [Google Scholar]
- 20. Lervang HH, Jensen S, Brøchner-Mortensen J, Ditzel J: Early glomerular hyperfiltration and the development of late nephropathy in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1988; 31: 723– 729 [DOI] [PubMed] [Google Scholar]
- 21. Mogensen CE, Christensen CK: Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 1984; 311: 89– 93 [DOI] [PubMed] [Google Scholar]
- 22. Mogensen CE: Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand J Clin Lab Invest 46: 201– 206, 1986 [DOI] [PubMed] [Google Scholar]
- 23. Jones SL, Wiseman MJ, Viberti GC: Glomerular hyperfiltration as a risk factor for diabetic nephropathy: five year report of a prospective study. Diabetologia 1991; 34: 59– 65 [DOI] [PubMed] [Google Scholar]