Abstract
OBJECTIVE
To assess the relevance of pulse pressure as a predictor of foot ulcers in type 2 diabetic subjects.
RESEARCH DESIGN AND METHODS
A cohort study was performed on a consecutive series of 1,945 type 2 diabetic outpatients without a foot ulcer at baseline. Incident foot ulcers were identified through the regional hospital discharge system, which contains ICD codes of current diagnoses.
RESULTS
During a follow-up of mean ± SD 4.2 ± 2.2 years, 86 ulcers were observed. After adjusting for confounders, the highest quartiles of pulse pressure had a 2.39-fold (95% CI 1.14–5.02) risk of foot ulcers. When ischemic ulcers were considered separately, the highest pulse pressure quartile was associated with an increased age- and sex-adjusted risk (2.08 [95% CI 1.02–4.24]), whereas no increase of risk was observed for neuropathic ulcers.
CONCLUSIONS
Elevated pulse pressure represents an independent predictor of foot ulcers in diabetic patients; this parameter should be considered for the stratification of risk of ischemic or neuroischemic ulcers.
Pulse pressure is a recognized risk factor for cardiovascular disease in nondiabetic (1–4) and diabetic (5,6) subjects. In particular, elevated pulse pressure is associated with increased risk of arteriopathy of the lower limbs, even after adjusting for mean blood pressure (7). This study aimed to assess the relevance of pulse pressure as a predictor of foot ulcers in type 2 diabetic subjects, which has not been specifically investigated so far.
RESEARCH DESIGN AND METHODS
A cohort study was performed on a consecutive series of 1,945 type 2 diabetic outpatients referred to the Diabetes Clinic of the Geriatric Unit of Careggi University Hospital in Florence, Italy, between 1 December 1995 and 1 December 2000. Blood pressure was measured using a mercury sphygmomanometer, with a cuff of appropriate size, considering the mean of three measurements taken 5 min apart in a sitting position. Serum cholesterol, HDL cholesterol, and triglycerides were measured, using an automated method (Aeroset, Abbott Laboratories), with blood samples drawn in the morning after an overnight fast. Neuropathy was ascertained through biothesiometry (>25 V at toes) (8), and lower limb arteriopathy was screened through the ankle-brachial index (ABI <0.8) (9). A1C was determined with high-performance liquid chromatography (Menarini Diagnostics, Florence, Italy) (upper normal limit 5.8%). Comorbidity was assessed through the calculation of Charlson's comorbidity score (CCS), which includes diabetes and its complications, cardiovascular disease, chronic skin ulcers, renal insufficiency, liver diseases, chronic obstructive pulmonary disease, malignancies, arthritis/arthrosis, and HIV infections (10). Pulse pressure was calculated as the difference between systolic and diastolic blood pressure.
Patients were observed until death, incidence of foot ulcer, or 31 December 2005. Foot ulcers were identified through the regional hospital discharge system, which contains ICD codes of current diagnoses (ICD codes 707 or 440.23); ulcers were considered ischemic when ICD code 440 or 250.7 was present and neuropathic with codes 357 or 250.6. This method identified 40 (77%) of 52 consecutive cases of incident ulcers referred to our clinic during 2004.
The χ2 test, Student's unpaired and paired two-sided t tests, and the Mann-Whitney U test were used for comparisons whenever appropriate; Spearman's method was used for correlations. Survival estimates were performed using Kaplan-Meier curves; stepwise Cox regression was used for multivariate analysis.
RESULTS
Patients enrolled (56.7% women) had a mean ± SD age of 64.0 ± 12.7 years, duration of diabetes 10.7 ± 10.5 years, and A1C 8.1 ± 1.9%. Of the 1,945 patients, 50.3, 47.1, and 32.3% received treatment with metformin, insulin secretagogues, or insulin, respectively. The prevalence of neuropathy, arteriopathy of the lower limbs, and retinopathy was 22.2, 10.1, and 8.8%, respectively. Systolic, diastolic, and pulse pressure was 142.4 ± 20.1, 81.3 ± 10.5, and 61.1 ± 16.4 mmHg, respectively; 61.2, 51.6, and 31.1% of subjects were receiving antihypertensive treatment, antiaggregants, or statins, respectively.
Patients in the highest quartile (>70 mmHg) of pulse pressure had a significantly (P < 0.01) higher age (70.5 ± 8.6 vs. 62.5 ± 13.0 years), duration of diabetes (14.1 ± 11.1 vs. 10.0 ± 10.2 years), CCS (2.5 ± 1.5 vs. 2.1 ± 1.5), and prevalence of neuropathy (36.3 vs. 19.2%), arteriopathy (10.9 vs. 7.0%), retinopathy (13.0 vs. 7.9%), microalbuminuria (16.8 vs. 10.2%), and previous stroke (7.4 vs. 3.5%); they also had higher systolic (168 ± 15 vs. 136 ± 16 mmHg) but not diastolic (82 ± 11 vs. 81 ± 10 mmHg) blood pressure. No differences between patients in the highest pulse pressure quartile and the rest of the sample were observed for A1C, lipid profile, and BMI (data not shown). An inverse correlation was observed between pulse pressure and ABI (r = −0.27; P < 0.01).
During a mean follow-up of 4.2 ± 2.2 years, 86 ulcers were observed, with a yearly incidence rate of 1.1%. Of the incident ulcers, 38 were neuropathic, 15 ischemic, 20 neuroischemic, and 13 of other origin. Age was a relevant predictor of incident ulcers (1.05 [95% CI 1.03–1.07]; P < 0.001). After adjusting for sex and age, other predictors included duration of diabetes (1.03 [1.01–1.05]; P < 0.001), A1C (1.20 [1.10–1.32]; P < 0.001), neuropathy (3.93 [2.55–6.05]; P < 0.001), arteriopathy of the lower limbs (4.32 [2.79–7.38]; P < 0.001), retinopathy (1.97 [1.13–3.43]; P = 0.017), and previous foot ulcer (31.53 [20.17–49.29]; P < 0.001) but not cholesterol, microalbuminuria, or renal failure (data not shown).
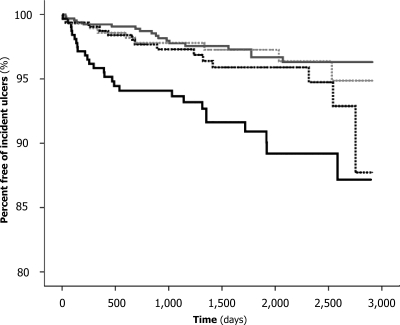
Elevated pulse pressure was associated with a significantly increased risk of foot ulcers (Fig. 1). After adjusting for age, sex, duration of diabetes, systolic blood pressure, and CCS, patients in the highest quartiles of pulse pressure had a 2.39-fold (95% CI 1.14–5.02) risk of foot ulcers in comparison with the rest of the sample (P = 0.022). When ischemic or neuroischemic ulcers were considered separately, the highest pulse pressure quartile was associated with a significantly increased age- and sex-adjusted risk (2.08 [1.02–4.24]; P = 0.043), whereas no increase of risk was observed for neuropathic ulcers (1.33 [0.67–2.65]; P = 0.42).
Figure 1.
Proportion of patients free from incident foot ulcers among those with pulse pressure <50 mmHg (gray line), <60 mmHg (dotted gray line), <70 mmHg (dotted black line), and ≥70 mmHg (black line).
CONCLUSIONS
The present study shows for the first time that pulse pressure is a relevant independent predictor of incident ischemic or neuroischemic foot ulcers in type 2 diabetic patients. The reduction of arterial compliance, which is revealed by the increased pulse pressure, facilitates atherosclerotic lesions in different districts, including the lower limbs (7). Impairment of insulin sensitivity, which has been reported to be associated with elevated pulse pressure (11), could contribute to micro- and macrovascular lesions responsible for foot ulcers. Furthermore, elevated pulse pressure is associated with lower ABI.
The association of elevated pulse pressure with foot ulcers is relevant for identification of patients at higher risk and their subsequent referral to more accurate programs for screening of arteriopathy of the lower limbs and to specific educational interventions. The risk of foot ulcers is more than doubled in patients in the highest quartile of pulse pressure; the increase of risk is comparable to that attributable with known neuropathy or arteriopathy of the lower limbs.
Some limitations should be recognized. Register-based ascertainment of incident ulcers inevitably implies that minor lesions not requiring treatment in hospital-based clinics are missed, which leads to an underestimation, by at least 20%, of the actual incidence of this condition. The register-based identification of ulcers, with no direct observation, does not allow any characterization of cases. Furthermore, pulse pressure was assessed only through office measurement, which, although similarly predictive of cardiovascular events, is less accurate than results of ambulatory monitoring (6).
In conclusion, elevated pulse pressure represents an independent predictor of foot ulcers in diabetic patients. This parameter should be considered for the stratification of risk of ischemic or neuroischemic ulcers.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Franklin SS, Kahn SA, Wong ND, Larson MG, Levy D: Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation 1999; 100: 354– 360 [DOI] [PubMed] [Google Scholar]
- 2. Madhavan S, Ooi WL, Cohen H, Alderman MH: Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension 1994; 23: 395– 401 [DOI] [PubMed] [Google Scholar]
- 3. Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, Grimm R, Cohen J, Stamler J: the MRFIT Research Group. Pulse pressure and cardiovascular disease-related mortality: follow-up study of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 2002; 287: 2677– 2683 [DOI] [PubMed] [Google Scholar]
- 4. Miura K, Dyer AR, Greenland P, Daviglus ML, Hill MA, Liu K, Garside DB, Stamler J: Pulse pressure compared with other blood pressure indexes in the prediction of 25-year cardiovascular and all-cause mortality rates: the Chicago Association Detection Project in Industry Study. Hypertension 2001; 38: 232– 237 [DOI] [PubMed] [Google Scholar]
- 5. Schram MT, Kostense PJ, Van Dijk RA, Dekker JM, Nijpels G, Bouter LM, Heine RJ, Stehouwer CD: Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 2002; 20: 1743– 1751 [DOI] [PubMed] [Google Scholar]
- 6. Mannucci E, Lambertucci L, Monami M, Fedeli A, Chiasserini V, Marchionni N, Masotti G, Ungar A: Pulse pressure and mortality in hypertensive type 2 diabetic patients: a cohort study. Diabetes Metab Res Rev 2006; 22: 172– 175 [DOI] [PubMed] [Google Scholar]
- 7. Pini R, Cavallini MC, Bencini F, Silvestrini G, Tonon E, De Alfieri W, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ: Cardiovascular remodelling is greater in isolated systolic hypertension than in diastolic hypertension in older adults: the Insufficienza Cardiaca negli Anziani Residenti (ICARE) a Dicomano Study. J Am Coll Cardiol 2002; 40: 1283– 1289 [DOI] [PubMed] [Google Scholar]
- 8. Young MJ, Breddy JL, Veves A, Boulton AJM: The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds: a prospective study. Diabetes Care 1994; 17: 557– 560 [DOI] [PubMed] [Google Scholar]
- 9. Winsor T, Simmons EM, Borhani N, Hechter HH: A diagnostic aid for determining peripheral arteriosclerosis obliterans. Dis Chest 1967; 52: 451– 457 [DOI] [PubMed] [Google Scholar]
- 10. Charlson M, Szatrowski TP, Peterson J, Gold J: Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245– 1251 [DOI] [PubMed] [Google Scholar]
- 11. Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, Kamp O, Westerhof N, Bouter LM, Stehouwer CD: Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation 2003; 107: 2089– 2095 [DOI] [PubMed] [Google Scholar]