Abstract
OBJECTIVE
Upon diagnosis of type 1 diabetes, patients are usually symptomatic, and many have ketoacidosis. Screening for islet autoantibodies (IAs) has been shown to decrease A1C level and rate of hospitalization at diabetes onset. Metabolic tests and the presence of symptoms were described at diabetes onset during the Diabetes Prevention Trial–Type 1 (DPT-1).
RESEARCH DESIGN AND METHODS
The DPT-1 screened relatives of patients with type 1 diabetes for islet cell autoantiobodies (ICAs). Those with positive ICAs had intravenous and oral glucose tolerance tests (IVGTTs and OGTTs) and were randomized into one of two prevention trials. Throughout the DPT-1 parenteral and oral insulin study, 246 people were diagnosed with type 1 diabetes.
RESULTS
Of the 246 subjects diagnosed with diabetes, 218 had data regarding the presence of symptoms, and 138 (63.3%) reported no symptoms suggestive of diabetes. Eight subjects (3.67%) presented with ketosis. Subjects presented with a mean ± SD A1C of 6.41 ± 1.15%. At diagnosis, 90 subjects (50.8%) had A1C in the normal range (<6.2%). OGTT data at the time of diagnosis indicate that 35.4% had a glucose result of <100 mg/dl at 0 min.
CONCLUSIONS
The majority of subjects diagnosed with type 1 diabetes through the DPT-1 were asymptomatic at onset and had normal fasting glucose and A1C levels. This suggests that intermittent screening (IA followed by OGTT) may allow diagnosis of diabetes before severe metabolic decompensation. Screening with A1C will miss identifying many of the subjects with newly diagnosed type 1 diabetes in this cohort.
Type 1 diabetes affects ∼15–30 million people worldwide and 1.4 million people in the U.S. (1) and is responsible for significant morbidity, premature mortality, and financial burden (2). Onset of disease is usually preceded by a preclinical period that can last for months or years. Pre-diabetes can often be detected by the presence of autoantibodies to islet antigens such as GAD65, insulin, insulinoma-associated protein 2 (IA-2), and the recently identified ZnT8 (3). These antibodies can be highly predictive of diabetes risk in children with (4) and without (5) a first-degree relative with type 1 diabetes. First-degree relatives of patients with type 1 diabetes carry a 10- to 20-fold higher risk for diabetes than the general population (6).
The onset of type 1 diabetes is often marked by the presence of symptoms including polyuria, polydipsia, polyphagia, weight loss, and diabetic ketoacidosis (DKA). DKA is the leading cause of morbidity and mortality in children with type 1 diabetes. Approximately 25% of newly diagnosed cases present with DKA in the U.S. (7). Cerebral edema complicates 0.5–1% of cases of DKA in children, and there is a 50% risk for significant morbidity and mortality in patients with cerebral edema. New-onset diabetes is a risk factor for DKA (8). Therefore, preventing DKA at onset may have a significant impact on diabetes-related mortality in childhood. A prospective follow-up of children through the Diabetes Autoimmunity Study in the Young (DAISY) for islet autoantibodies (IAs) and diabetes showed that children followed in DAISY were hospitalized less often and had milder metabolic abnormalities at diagnosis compared with a control population of children diagnosed in the community (9).
The Diabetes Prevention Trial–Type 1 (DPT-1) parenteral and oral insulin trials (10,11) screened relatives of people with type 1 diabetes with islet cell autoantibodies (ICAs). Subjects positive for these antibodies were invited for further testing including oral and intravenous glucose tolerance testing (OGTT and IVGTT). If eligible, subjects were enrolled in one of the two prevention trials and were followed with serial OGTTs for the diagnosis of type 1 diabetes. By studying those diagnosed with diabetes during the DPT-1, metabolic characteristics and the presence of symptoms were evaluated in those enrolled in prevention and screening trials. Previous reports have focused on a subset of asymptomatic individuals who were diagnosed solely by 2-h OGTT (12). Metabolic tests and the presence of symptoms were described in the individuals diagnosed through DPT-1 during the two clinical trials.
RESEARCH DESIGN AND METHODS
The DPT-1 initially screened 103,391 relatives of patients with type 1 diabetes for ICAs. Of these, 97,273 were eligible because they did not have diabetes, provided an adequate blood sample, were in the required age range (3–45 years for first degree-relatives and 3–20 years for second-degree relatives), and had a qualifying relative. There were 3,483 individuals who were ICA positive, and 2,423 had an IVGTT. A total of 771 were randomized to the parenteral (n = 339) or oral insulin (n = 372) prevention trials.
The staging of the study confirmed ICA positivity, measured insulin autoantibody (IAA) status, and assessed first-phase insulin response (FPIR) to IVGTT. Glucose tolerance was assessed based on an OGTT and the presence or absence of the protective haplotype HLA-DQA1*0102/DQB*0602 (subjects with this haplotype were excluded from participation) (10). In addition to ICA and IAA positivity, eligibility was based on the level of FPIR (the sum of insulin levels at 1 and 3 min) for IVGTTs and glucose abnormalities for OGTTs.
If the subject was ICA positive, had an FPIR less than threshold (as previously described) on two occasions, or had impaired fasting plasma glucose (FPG) or impaired glucose tolerance as determined by an OGTT, he or she was eligible for the parenteral insulin trial. If none of the metabolic abnormalities were present but IAA was positive, participants were eligible for the oral insulin trial (10).
Subjects enrolled in the parenteral and oral insulin trials were seen every 6 months for an OGTT to assess glycemic status (10,11). Neither intervention therapy showed a significant overall effect. Thus, participation in either of these studies would not be expected to substantially alter the manner in which diabetes presented. In total, 931 people (26.7% of ICA-positive individuals) were diagnosed with type 1 diabetes throughout participation in DPT-1 and follow-up. Individuals were diagnosed during staging (n = 512), during participation in one of the two intervention trials (n = 241), after completion of the parenteral study (n = 17), through a follow-up questionnaire (n = 156), or as part of a high-risk follow-up study (n = 5). Metabolic data were available for analysis on the 241 individuals diagnosed in the intervention studies and the 5 individuals diagnosed as part of the high-risk follow-up group assembled to study the affect of follow-up over time on the presence of symptoms and metabolic parameters. Other subjects did not have available metabolic data and are not included in this report.
Tolerance test procedures
Tolerance tests were performed after an overnight fast and insertion of an intravenous line. IGTTs were performed as described (13). The dose of oral glucose was 1.75 g/kg (maximum, 75 g of carbohydrate). Plasma glucose values were interpreted according to the guidelines of the American Diabetes Association. An FPG of ≥126 mg/dl or a glucose level of ≥200 mg/dl after 120 min was diagnostic for diabetes. In asymptomatic patients, diagnosis was confirmed by a second OGTT. If the glucose values were not confirmed at the follow-up visit, participants continued to be followed at 6-month intervals with additional OGTTs. Random plasma glucose >200 mg/dl in the presence of symptoms of diabetes was also considered diagnostic of diabetes. Previous studies reported a subset of 61 individuals who were diagnosed solely by 2-h OGTT throughout enrollment in the DPT-1 (12). This analysis examined 241 subjects diagnosed with diabetes during one of the intervention trials and five diagnosed as part of a high-risk follow-up study.
Laboratory measures
All assays were performed as previously described (10). Plasma glucose was measured by the glucose oxidase method. C-peptide levels were determined by radioimmunoassay. Measurable C-peptide was considered ≥0.2 ng/ml because this was the lowest level detectable by this method. A1C was measured using the VARIANT (Bio-Rad) instrument. The measurable range for this instrument was 3.6–17.0%, with normal levels <6.2%.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 5. Continuous variables are reported as mean ± SD unless otherwise noted. Categorical variables are reported with percent. A1C within 30 days of diagnosis was analyzed for those diagnosed with type 1 diabetes. In order to define the sensitivity and specificity of A1C as a measurement of progression to diabetes, analysis was performed by receiver operating characteristic (ROC) curve. The onset of type 1 diabetes was confirmed using one of three glucose values: FPG, 2-h OGTT, or random plasma glucose test. OGTT glucose and C-peptide results within 2 months of diagnosis were analyzed. Area under the curve for C-peptide on OGTT was calculated. Comparisons were made with subjects who had not developed diabetes by study end using the last available laboratory tests. Continuous variables were compared using Student's t test, and categorical variables were compared using the χ2 test. Differences were considered significant at P < 0.05.
RESULTS
Demographic characteristics
The 246 subjects diagnosed through follow-up in the study were analyzed (98 in the oral insulin trial, 143 in the parenteral insulin trial, and 5 during the high-risk follow-up study). Of the 246 people diagnosed throughout the study, 167 (67.9%) were diagnosed by an OGTT, 42 (17.1%) were diagnosed by a random plasma glucose test, and 29 (11.8%) were diagnosed by FPG. The remaining eight (3.25%) diagnoses occurred outside of routine study evaluation by a nonstudy physician, and diagnostic glucose data were not available.
Baseline characteristics of those diagnosed through the study (n = 246) were compared with those of individuals enrolled in the study who did not develop diabetes (n = 453) (Table 1). There were no significant differences between the two groups for race, sex, or relationship to the index relative with type 1 diabetes. Children diagnosed with diabetes were younger at randomization compared with those not diagnosed by study end (P = 0.03).
Table 1.
Individuals diagnosed with type 1 diabetes during the study and as part of the high-risk follow-up compared with individuals randomized in the DPT-1 but not diagnosed with type 1 diabetes
| Diagnosed with type 1 diabetes | Not diagnosed with type 1 diabetes | P | |
|---|---|---|---|
| n | 246 | 453 | |
| Race (white) | 224 (91.87) | 407 (89.8) | 0.07 |
| Sex (male) | 133 (54.07) | 258 (56.9) | 0.58 |
| First-degree relative with type 1 diabetes | 228 (92.68) | 410 (90.5) | 0.40 |
| Age at randomization (years) | 11.41 ± 7.07 | 15.25 ± 10.39 | 0.03* |
Data are n (%) or means ± SD unless otherwise indicated.
Symptoms at presentation of type 1 diabetes
Of the 246 people analyzed, 28 (11.4%) did not have data regarding the presence of symptoms at diagnosis. These individuals were excluded from analysis of symptoms. For the remaining subjects, 80 (36.70%) reported a presence of at least one symptom (71 [32.57%] reported polyuria, 54 [24.77%] polydipsia, and 20 [9.17%] polyphagia), and 58 (26.6%) reported the presence of more than one symptom. Weight loss was reported in 21 subjects (9.63%). DKA was reported in eight subjects (3.67%).
Laboratory tests at diagnosis
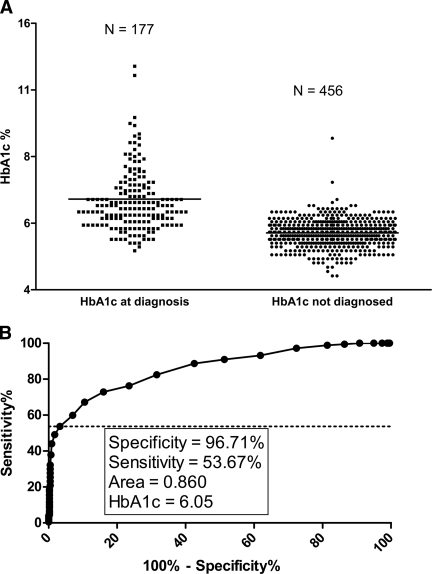
Several laboratory results were examined for those diagnosed during the study. Of the 246 people diagnosed, 177 (72.0%) had an A1C within 30 days of diagnosis, and 158 (89.3%) of these A1C tests were within 1 week of diagnosis. The mean A1C was 6.41 ± 1.15%, with 2 of 177 (1.13%) having an A1C >10%, and 90 subjects (50.8%) had a A1C in the normal range (<6.2%) near the time of diagnosis. The mean A1C of the group with normal A1C at onset of diabetes was 5.7% and ranged from 4.9–6.1%. A1C at diagnosis was normal for 53% percent of subjects less than 18 years of age and 39% of those greater than 18 years of age. A1C at diagnosis was compared with the last reported A1C of individuals randomized in the study but not diagnosed with diabetes. The mean A1C for this group was 5.38 ± 0.41% (P < 0.0001) compared with those diagnosed with diabetes (Fig. 1A). ROC analysis identified an A1C of 6.05% as achieving the optimal sensitivity (53.7%) and specificity (96.7%) (Fig. 1B).
Figure 1.
A1C at onset for those diagnosed with type 1 diabetes during the DPT-1 and the last reported A1C for those randomized in DPT-1 but not diagnosed with type 1 diabetes. A: A1C for those diagnosed with type 1 diabetes (n = 177) compared with the last reported A1C for those randomized into the trial but not diagnosed with type 1 diabetes (n = 456). These groups were significantly different (P < 0.0001). B: ROC analysis of those diagnosed with type 1 diabetes compared with those randomized but not diagnosed with type 1 diabetes.
The onset of type 1 diabetes was confirmed using one of three glucose tests: FPG, 2-h OGTT, or random plasma glucose test. The mean ± SD glucose value for those diagnosed based on an FPG test (n = 29) was 209.2 ± 84.9 mg/dl, 2-h glucose OGTT (n = 167) was 289.3 ± 71.3 mg/dl, and random plasma glucose (n = 42) was 406.6 ± 200.3 mg/dl. A glucose result >500 mg/dl was present for 14 subjects (5.7%).
OGTTs obtained within 2 months of diagnosis were also evaluated. The median date of this OGTT was 0 days (range 50 days before diagnosis to 50 days after diagnosis), and 91% of samples were on the day of diagnosis. Many subjects had FPG results <100 mg/dl (35.4%), although 2-h glucose results <200 mg/dl are less frequent.
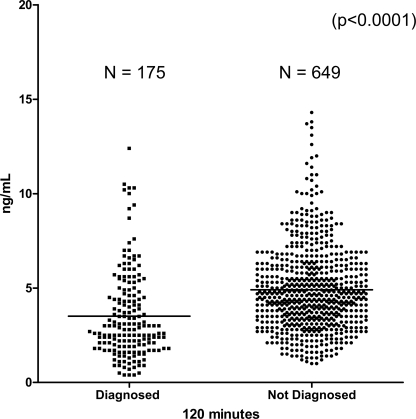
The mean C-peptide level at time 0 min was 1.35 ± 1.05 ng/ml (range 0.09–8.3) compared with 1.34 ± 0.69 ng/ml (0.10–4.8) in those not diagnosed with type 1 diabetes (P = 0.89). At 120 min, the mean C-peptide level was 3.51 ± 2.29 ng/ml (0.4–12.40) compared with 4.91 ± 2.21 ng/ml (1.0–14.3) in those randomized in the DPT-1 but not diagnosed with type 1 diabetes (P < 0.0001) (Fig. 2). At 0 min, 96.6% (169/175) of subjects diagnosed with type 1 diabetes had a C-peptide level of ≥0.2 ng/ml.
Figure 2.
OGTT C-peptide results at 120 min for those diagnosed with type 1 diabetes during the trial (n = 175) compared with the last reported OGTT for those not diagnosed with diabetes (n = 649). For those diagnosed with diabetes, all OGTT tests were within 50 days of diagnosis (P < 0.0001).
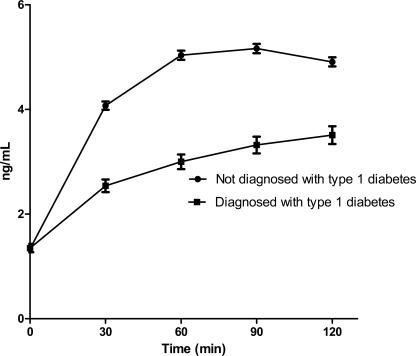
The C-peptide area under the curve was 338.6 ± 205.1 ng/ml at 120 min for individuals diagnosed with diabetes compared with 529.3 ± 212.1 ng/ml at120 min for subjects randomized in the DPT-1 but not diagnosed with diabetes (P < 0.0001). Mean ± SE C-peptide levels are provided in Fig. 3.
Figure 3.
Mean ± SE OGTT C-peptide for those diagnosed with type 1 diabetes versus those randomized in DPT-1 but not diagnosed with type 1 diabetes. Mean values are shown at 0, 30, 60, 90, and 120 min of 2-h OGTT within 50 days of diagnosis of type 1 diabetes for those diagnosed and the last reported OGTT for those randomized but not diagnosed with diabetes (P < 0.0001).
CONCLUSIONS
Although DPT-1 was unsuccessful in preventing type 1 diabetes, the study provided valuable information about metabolic progression to diabetes (14) and has set the standard for future prevention trials. Participation in prevention trials has the potential to benefit subjects with a diabetes diagnosis before the development of significant symptoms and DKA. Early diagnosis has the potential to identify subjects before the complete loss of endogenous insulin production, with the theoretical potential to preserve the remaining β-cells through intensive diabetes management or immunologic intervention.
DKA is a potentially life-threatening complication at the onset of type 1 diabetes, because it accounts for 0.15% of mortality at onset, with the majority of deaths due to cerebral edema (15). Reported frequencies of DKA at diagnosis range between 15 and 67% in Europe and North America and may be more common in developing countries (16,17). Consistent with the experience in the DAISY study, very few subjects (<4%) participating in the DPT-1 presented with ketosis at onset of diabetes, much lower than reported frequencies of DKA in community populations. Therefore, high-risk relatives may benefit from close attention or screening in order to prevent DKA at diagnosis.
Laboratory results from DPT-1 indicate that individuals diagnosed throughout the study had a lower average A1C of 6.41% than the average A1C of 10.9% reported for children diagnosed in community groups without periodic screening, and A1C for the DPT-1–diagnosed subjects was also lower for children who reported a first-degree relative diagnosed with type 1 diabetes outside of a research protocol (7.2%) (9). Over 53% of children less than 18 years of age diagnosed through the DPT-1 had a normal A1C at onset of type 1 diabetes. Using ROC analysis to identify the optimal A1C cutoff for both sensitivity and specificity failed to identify a level with an acceptable sensitivity (53.7% at A1C of 6%), although specificity was high (96.7% at A1C of 6%). These data suggest that A1C is not an effective marker for screening for early diagnosis of type 1 diabetes.
It is widely accepted that measurement of C-peptide is an appropriate outcome measure for type 1 diabetes trials to preserve β-cell function (18). Studies have shown that patients diagnosed around puberty or as adults show consistent baseline and stimulated C-peptide levels ranging from 0.9–2.7 ng/ml and 1.8–3.9 ng/ml, respectively (19). In prepubertal children, however, several studies have demonstrated that average C-peptide levels at diagnosis are <0.6 ng/ml, indicating more extensive destruction of β-cells (20,21). The vast majority (96.6% [169/175]) of subjects followed through the DPT-1 had C-peptide levels >0.2 ng/dl at diagnosis. It has been shown that sustaining higher levels of C-peptide secretion is associated with fewer microvascular complications of diabetes and fewer episodes of hypoglycemia (22).
Arguments against screening for diabetes-related antibodies are based upon the psychological stress that may accompany the knowledge of increased diabetes risk. The BABYDIAB study showed that parents who receive an IA-negative result for their child are often happy, whereas parents that receive an IA-positive result are often unhappy. However, given that the majority of subjects receive an IA-negative result, the effect of participation in a research protocol is to reduce overall anxiety. Most parents of IA-negative and -positive children wish to know the diabetes risk of their child, and both groups are glad to be informed about their child's status (23). In contrast, some have suggested that until a reliable method of preventing diabetes is identified, screening should be reserved for the realm of research (24).
Subjects diagnosed with type 1 diabetes through the DPT-1 had few symptoms, normal A1C, rare ketosis, and evidence for insulin production at the time of diagnosis. This suggests that intermittent screening and follow-up of IA-positive subjects may allow for early diagnosis and prevent severe metabolic decompensation at onset and also that screening with A1C alone may not be adequate.
Acknowledgments
The DPT-1 was supported by the National Institutes of Health, National Center for Research Resources, American Diabetes Association, and Juvenile Diabetes Research Foundation. J.M.B. is supported by a Juvenile Diabetes Research Foundation Early Career Development award.
No potential conflicts of interest relevant to this article were reported.
The authors acknowledge the study participants and their families for their contributions, which made this research possible.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Libman I, Songer T, LaPorte R: How many people in the U.S. have IDDM? Diabetes Care 1993; 16: 841– 842 [DOI] [PubMed] [Google Scholar]
- 2. Songer TJ, LaPorte R, Lave JR, Dorman JS, Becker DJ: Health insurance and the financial impact of IDDM in families with a child with IDDM. Diabetes Care 1997; 20: 577– 584 [DOI] [PubMed] [Google Scholar]
- 3. Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC: The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007; 104: 17040– 17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS: Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996; 45: 926– 933 [DOI] [PubMed] [Google Scholar]
- 5. Bingley PJ, Bonifacio E, Williams AJK, Genovese S, Bottazzo GF, Gale EAM: Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997; 46: 1701– 1710 [DOI] [PubMed] [Google Scholar]
- 6. Allen C, Palta M, D'Alessio DJ: Risk of diabetes in siblings and other relatives of IDDM subjects. Diabetes 1991; 40: 831– 836 [DOI] [PubMed] [Google Scholar]
- 7. Rewers A, Klingensmith G, Davis C, Petitti DB, Pihoker C, Rodriguez B, Schwartz ID, Imperatore G, Williams D, Dolan LM, Dabelea D: Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics 2008; 121: e1258– e1266 [DOI] [PubMed] [Google Scholar]
- 8. Wolfsdorf J, Glaser N, Sperling MA: Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care 2006; 29: 1150– 1159 [DOI] [PubMed] [Google Scholar]
- 9. Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M: Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004; 27: 1399– 1404 [DOI] [PubMed] [Google Scholar]
- 10. Diabetes Prevention Trial–Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002; 346: 1685– 1691 [DOI] [PubMed] [Google Scholar]
- 11. Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E: Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005; 28: 1068– 1076 [DOI] [PubMed] [Google Scholar]
- 12. Greenbaum CJ, Cuthbertson D, Krischer JP: Type 1 diabetes manifested solely by 2-h oral glucose tolerance test criteria. Diabetes 2001; 50: 470– 476 [DOI] [PubMed] [Google Scholar]
- 13. Bingley PJ, Colman P, Eisenbarth GS, Jackson RA, McCulloch DK, Riley WJ, Gale EAM: Standardization of IVGTT to predict IDDM. Diabetes Care 1992; 15: 1313– 1316 [DOI] [PubMed] [Google Scholar]
- 14. Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: The Diabetes Prevention Trial–Type 1 Study Group. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2006; 29: 643– 649 [DOI] [PubMed] [Google Scholar]
- 15. Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TP, Glaser NS, Hanas R, Hintz RL, Levitsky LL, Savage MO, Tasker RC, Wolfsdorf JI: European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics 2004; 113: e133– e140 [DOI] [PubMed] [Google Scholar]
- 16. Levy-Marchal C, Papoz L, de Beaufort C, Doutreix J, Froment V, Voirin J, Czernichow P: Clinical and laboratory features of type I diabetic children at time of diagnosis. Diabet Med 1992; 9: 279– 284 [DOI] [PubMed] [Google Scholar]
- 17. Komulainen J, Lounamaa R, Knip M, Kaprio EA, Akerblom HK: Ketoacidosis at the diagnosis of type 1 (insulin dependent) diabetes mellitus is related to poor residual beta cell function: Childhood Diabetes in Finland Study Group. Arch Dis Child 1996; 75: 410– 415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW: C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 2004; 53: 250– 264 [DOI] [PubMed] [Google Scholar]
- 19. Pozzilli P, Visalli N, Buzzetti R, Cavallo MG, Marietti G, Hawa M, Leslie RD: Metabolic and immune parameters at clinical onset of insulin-dependent diabetes: a population-based study: IMDIAB Study Group: immunotherapy diabetes. Metabolism 1998; 47: 1205– 1210 [DOI] [PubMed] [Google Scholar]
- 20. Knip M, Ilonen J, Mustonen A, Akerblom HK: Evidence for an accelerated B-cell destruction in HLA-Dw3/Dw4 heterozygous children with type I (insulin-dependent) diabetes. Diabetologia 1986; 29: 347– 351 [DOI] [PubMed] [Google Scholar]
- 21. Snorgaard O, Lassen LH, Binder C: Homogeneity in pattern of decline of β-cell function in IDDM: prospective study of 204 consecutive cases followed for 7.4 yr. Diabetes Care 1992; 15: 1009– 1013 [DOI] [PubMed] [Google Scholar]
- 22. Steffes MW, Sibley S, Jackson M, Thomas W: β-Cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care 2003; 26: 832– 836 [DOI] [PubMed] [Google Scholar]
- 23. Hummel M: Psychological impact of childhood islet autoantibody testing in families participating in the BABYDIAB study. 2004; 21: 324– 328 [DOI] [PubMed] [Google Scholar]
- 24. Ross LF: Minimizing risks: the ethics of predictive diabetes mellitus screening research in newborns. Arch Pediatr Adolesc Med 2003; 157: 89– 95 [DOI] [PubMed] [Google Scholar]