Abstract
OBJECTIVE
In this study, we sought to determine whether postprandial insulin secretion, insulin action, glucose effectiveness, and glucose turnover were abnormal in type 2 diabetes.
RESEARCH DESIGN AND METHODS
Fourteen subjects with type 2 diabetes and 11 nondiabetic subjects matched for age, weight, and BMI underwent a mixed-meal test using the triple-tracer technique. Indexes of insulin secretion, insulin action, and glucose effectiveness were assessed using the oral “minimal” and C-peptide models.
RESULTS
Fasting and postprandial glucose concentrations were higher in the diabetic than nondiabetic subjects. Although peak insulin secretion was delayed (P < 0.001) and lower (P < 0.05) in type 2 diabetes, the integrated total postprandial insulin response did not differ between groups. Insulin action, insulin secretion, disposition indexes, and glucose effectiveness all were lower (P < 0.05) in diabetic than in nondiabetic subjects. Whereas the rate of meal glucose appearance did not differ between groups, the percent suppression of endogenous glucose production (EGP) was slightly delayed and the increment in glucose disappearance was substantially lower (P < 0.01) in diabetic subjects during the first 3 h after meal ingestion. Together, these defects resulted in an excessive rise in postprandial glucose concentrations in the diabetic subjects.
CONCLUSIONS
When measured using methods that avoid non–steady-state error, the rate of appearance of ingested glucose was normal and suppression of EGP was only minimally impaired. However, when considered in light of the prevailing glucose concentration, both were abnormal. In contrast, rates of postprandial glucose disappearance were substantially decreased due to defects in insulin secretion, insulin action, and glucose effectiveness.
Glucose concentrations are determined by the balance between the rate of glucose entering and leaving the systemic circulation. Fasting hyperglycemia in type 2 diabetes occurs when endogenous glucose production (EGP) is inappropriately increased and glucose disappearance (Rd) inappropriately decreased when considered in light of prevailing glucose and insulin concentrations (1–4). The cause of the excessive rise in glucose that occurs after mixed-meal ingestion is not well established. Whereas postprandial suppression of EGP has consistently been reported to be delayed (5–7), rates of appearance of glucose in the meal (MRa) have been reported to be increased, decreased, or not different from those observed in nondiabetic subjects (5,6,8). Similarly, postprandial Rd has been reported to be increased or not different compared with that observed in nondiabetic subjects (5–10).
However, all of the above studies have used a dual-tracer approach in which one tracer is added to ingested glucose and another is infused intravenously. The intravenously infused tracer is used to trace the rate of appearance of both ingested tracer and rate of total glucose appearance (Ra). MRa is calculated by multiplying the rate of appearance of ingested tracer by its enrichment in the meal, and EGP is calculated by subtracting MRa from Ra. Rd is calculated by subtracting the change in plasma glucose mass from Ra. Unfortunately, the validity of all of these calculations is jeopardized by the marked change in tracer-to-tracee ratios that occurs after carbohydrate ingestion with the dual-tracer approach (11,12). Perhaps even more problematic, differences in MRa and Ra can lead to differences in tracer-to-tracee ratios (12). Depending on the magnitude of change in glucose concentration and turnover, non–steady-state error could account for the discrepant results among previous studies that have used the dual-tracer method to compare the pattern of postprandial glucose metabolism in diabetic and nondiabetic subjects (5–10).
The present studies were undertaken to reassess the relative contribution of alterations in EGP, MRa, and Rd to postprandial hyperglycemia in individuals with diabetes using a triple (rather than dual)-tracer approach (11) that is designed to minimize postprandial changes in tracer-to-tracee ratios that are used to measure MRa and EGP. We also used the oral “minimal” and C-peptide models (13,14) to determine whether changes in pattern of postprandial glucose metabolism are accompanied by alterations in insulin secretion, insulin action, and glucose effectiveness.
RESEARCH DESIGN AND METHODS
After approval from the Mayo Clinic Institutional Review Board, 14 diabetic and 11 nondiabetic subjects provided written informed consent to participate in the study. Subject characteristics are provided in supplementary Table A1 (available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc08-1826/DC1).
All participants were in good health, and none were regularly engaged in vigorous physical exercise. Oral antihyperglycemic medications were discontinued 3 weeks before the study. Two diabetic subjects and one nondiabetic subject were receiving thyroxine replacement therapy but had normal thyroid-stimulating hormone levels. All participants were instructed to follow a weight maintenance diet for 3 days before the study. At screening, body composition and visceral fat were measured using dual-energy X-ray absorptiometry and a single-cut computed tomographic scan (15).
Subjects were admitted to the Mayo Clinical Research Unit at 1700 h on the evening before the study. After eating a 10 kcal/kg meal (55% carbohydrate, 30% fat, and 15% protein), subjects received nothing by mouth except water until the following morning. At ∼0600 h on the morning of the study, an 18-G cannula was inserted in a hand vein, and the hand was placed in a heated Plexiglas box (∼55°C) to obtain arterialized venous blood samples. Another 18-G cannula was inserted into the opposite forearm for tracer infusion. A primed-continuous infusion of [6,6-2H2]glucose (14.8 mg/kg prime and 0.148 mg/kg continuous; MassTrace, Woburn, MA) was started at 0700 h (time −120) and continued until the end of the study. For diabetic individuals, the prime dose was adjusted upward, depending on the ambient glucose concentration. At 0900 h (time 0) a mixed meal (10 kcal/kg; 45% carbohydrate, 40% fat, and 15% protein) consisting of scrambled eggs, Canadian bacon, 100 ml water, and Jell-O (1.2 g/kg body weight of glucose) containing [1-13C]glucose was consumed within 15 min. An infusion of [6-3H]glucose also was started at time 0, and the rate was varied to mimic the anticipated rate of appearance of [1-13C]glucose contained within the meal (11,14). Simultaneously, the [6,6-2H2]glucose infusion rate was altered to mimic the anticipated fall in the rate of EGP.
Analytical techniques
Arterialized samples were placed on ice, centrifuged at 4°C, separated, and stored at −20°C until assay. The plasma glucose concentration was measured using a glucose oxidase method (YSI, Yellow Springs, OH). The plasma insulin concentration was measured using a chemiluminescence assay (Access Assay; Beckman Coulter, Chaska, MN), and C-peptide was measured using a radioimmunoassay (Linco Research, St. Louis, MO). Plasma [6-3H]glucose–specific activity was measured by liquid scintillation counting as previously described (11). Plasma enrichment of [1-13C]glucose and [6,6-2H2]glucose was measured using gas chromatography–mass spectrometry (Thermoquest, San Jose, CA) to simultaneously monitor the C1,2 and C3–6 fragments (16).
Calculations
Fasting and postprandial rates of glucose turnover were calculated as detailed elsewhere (11). Briefly, the systemically infused [6-3H]glucose was used to trace the systemic rate of appearance of [1-13C]glucose that was contained in the meal, whereas [6,6-2H2]glucose was used to trace the rate of appearance of endogenously produced glucose. The ratio of the plasma concentration of [6-3H]glucose to the plasma concentration of [1-13C]glucose was used to calculate the rate of appearance of ingested [1-13C]glucose, and the ratio of plasma concentration of [6,6-2H2]glucose to the plasma concentration of endogenously produced glucose was used to calculate EGP. The plasma concentration of endogenously produced glucose was calculated by subtracting the concentration of exogenously derived (ingested) glucose (i.e., plasma [1-13C]glucose concentration times meal [1-13C]glucose enrichment) from the total plasma glucose concentration (11).
Indexes of net insulin action (Si), glucose effectiveness (Sg), and the ability of insulin (Si*) and glucose (Sg*) to stimulate glucose disposal were calculated using the oral minimal model as described previously (13,14,17). Indexes of insulin secretion including the total response to the glycemic stimuli (Φtotal), the response to a change in glucose concentration (Φdynamic), and the response to a given glucose concentration(Φstatic) were calculated using the oral C-peptide secretion model as described previously (14). Disposition indexes were calculated by multiplying indexes of insulin secretion times indexes of insulin action to determine whether insulin secretion was appropriate for the degree of insulin resistance.
Statistics
All results are expressed as means ± SEM. Student's two-tailed unpaired t tests were performed to compare normally distributed glucose turnover data, and nonparametric Mann-Whitney tests were performed to compare the indexes. P < 0.05 was considered statistically significant.
RESULTS
Plasma glucose, insulin, C-peptide, and glucagon concentrations
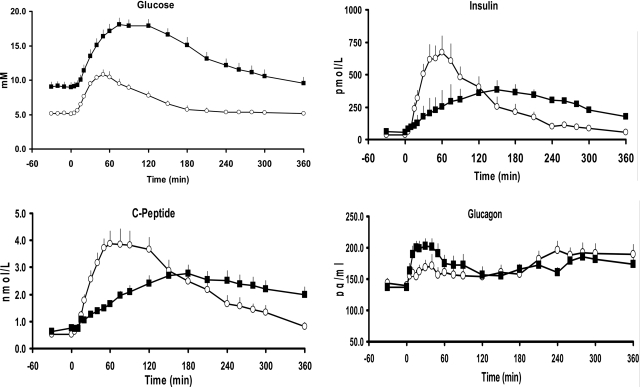
Plasma glucose concentrations were higher (P < 0.001) in diabetic than in nondiabetic individuals before the meal (9.1 ± 0.7 vs. 5.2 ± 0.1 mmol/l) and increased to a higher peak (P < 0.001) after the meal (18.1 ± 0.9 vs. 10.8 ± 0.7 mmol/l), resulting in a greater (P < 0.0001) postprandial integrated response above basal in diabetic than in nondiabetic subjects (1,671 ± 125 vs. 557 ± 92 mmol/l over 6 h) (Fig. 1).
Figure 1.
Glucose, insulin, C-peptide, and glucagon concentrations observed in diabetic (■) and nondiabetic (□) subjects before and after ingestion of a mixed meal at time 0.
Fasting insulin concentrations (59 ± 9 vs. 37 ± 6 pmol/l) did not differ between diabetic and nondiabetic individuals. However, peak postprandial plasma insulin concentrations were lower (382 ± 54 vs. 673 ± 126 pmol/l; P < 0.02) and occurred later (156 ± 16 vs. 62 ± 7 min; P < 0.001) in diabetic than in nondiabetic subjects. This led to a smaller (P < 0.001) increase in insulin above basal (21 ± 4 vs. 52 ± 8 nmol/l over 2 h) in diabetic subjects during the first 2 h after the meal but no difference in the integrated insulin response above basal for the entire 6 h of the study (79 ± 14 vs. 80 ± 13 nmol/l over 6 h).
C-peptide concentrations did not differ between diabetic and nondiabetic individuals before the meal (0.7 ± 0.1 vs. 0.5 ± 0.1 nmol/l). Peak postprandial C-peptide concentrations (2.8 ± 0.3 vs. 3.9 ± 0.5 nmol/l; P < 0.07) tended to be lower and occur later (191 ± 13 vs. 81 ± 10 min; P < 0.001) in diabetic than in nondiabetic subjects. As for insulin, the C-peptide response above basal was lower (P < 0.001) in diabetic than in nondiabetic subjects for the first 2 h after meal ingestion (113 ± 14 vs. 305 ± 35 nmol/l over 2 h) but did not differ over the entire 6 h of the study (527 ± 76 vs. 637 ± 83 nmol/l over 6 h).
Plasma glucagon concentrations did not differ in the two groups before meal ingestion but increased more (P < 0.05) in diabetic than in nondiabetic subjects during the first 2 h after meal ingestion (5.1 ± 0.9 vs. 2.1 ± 1.0 ng · ml−1 · 2 h−1). Glucagon concentrations did not differ in the two groups from 2 h onward, resulting in a comparable integrated response over the 6 h of the study (13.2 ± 1.8 vs. 11.3 ± 2.1 ng/ml over 6 h).
Indexes of insulin action
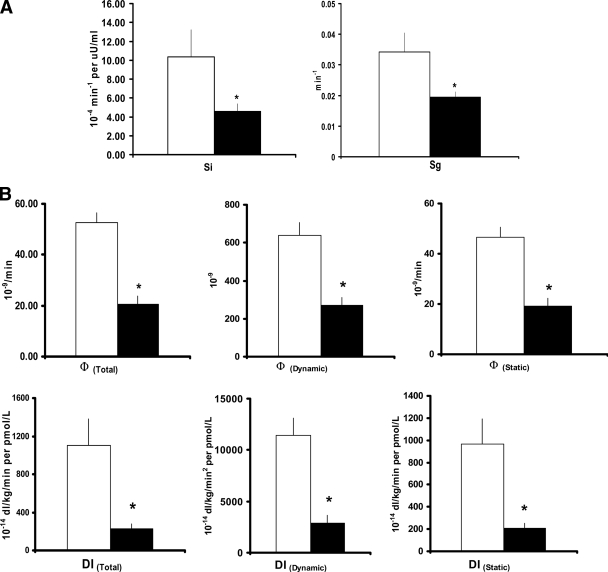
Si (4.6 ± 0.8 vs. 10.4 ± 2.9 × 10−4 min−1 · μU−1 · ml−1) and Sg (0.019 ± 0.002 vs. 0.034 ± 0.006 min−1) were lower (P < 0.03) in diabetic than in I nondiabetic subjects after meal ingestion (Fig. 2A). Likewise, Si* (1.25 ± 0.25 vs. 4.78 ± 1.53 × 10−4 min−1 per μU/ml) and Sg* (0.0016 ± 0.0006 vs. 0.172 ± 0.001 min−1) were lower (P < 0.02) in diabetic than in nondiabetic subjects (data not shown).
Figure 2.
A: Indexes of net insulin action (Si) and net glucose effectiveness (Sg) observed in diabetic (■) and nondiabetic (□) subjects after ingestion of a mixed meal. *P < 0.05 versus nondiabetic. B: Indexes of insulin secretion (Φtotal, Φdynamic, and Φstatic) and disposition indexes (DItotal, DIdynamic, and DIstatic) observed in diabetic (■) and nondiabetic (□) subjects after ingestion of a mixed meal. *P < 0.05 versus nondiabetic.
Indexes of insulin secretion
Φtotal was lower (P < 0.01) in diabetic than in nondiabetic subjects (20.7 ± 3.0 vs. 52.6 ± 4.1 × 10−9/min) (Fig. 2B). This result was due to a decrease (P < 0.0001) in Φdynamic (269.5 ± 44.0 vs. 638.1 ± 69.1 × 10−9) and a decrease (P < 0.001) in Φstatic (19.3 ± 2.9 vs. 46.6 ± 3.8 10−9 min−1).
Disposition indexes, calculated to adjust insulin secretion for the prevailing level of insulin action, all were lower (P < 0.002) in diabetic than in nondiabetic subjects including DItotal (227 ± 51 vs. 1,104 ± 276 × 10−14 dl · kg−1 · min−1 · pmol−1 · l−1), DIdynamic (2,897 ± 748 vs. 11,403.4 ± 1,700 × 10−14 · kg−1 · min−1 · pmol−1 · l−1), and DIstatic (209 ± 47 vs. 964 ± 22.8 × 10−14 · kg−1 · min−1 · pmol−1 · l−1).
Tracer-to-tracee ratios
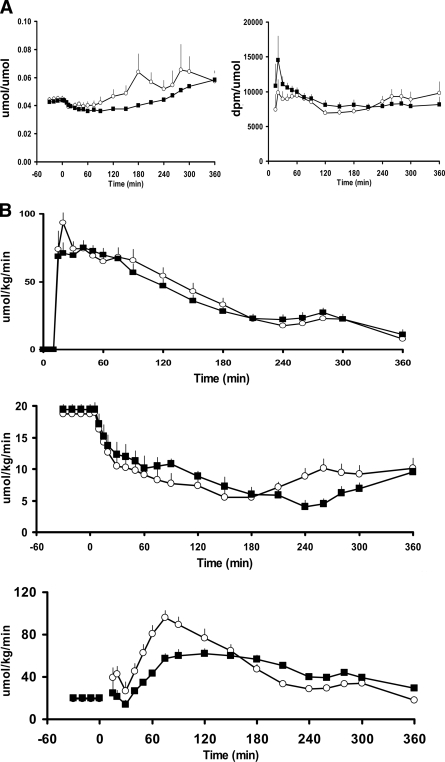
The plasma ratio of [6-3H]glucose to [1-13C]glucose (used to calculate MRa) increased during the first 10 min after the start of the meal in both groups and then changed minimally thereafter (Fig. 3A). The plasma ratio of [6,6-2H2]glucose to plasma endogenous glucose concentration (used to calculate EGP) decreased slightly but equally in both groups during the first hour after meal ingestion. With the exception of a marked increase that occurred in one nondiabetic subject, this ratio then gradually rose from 60 min onward in both groups.
Figure 3.
A: Pattern of change in the plasma [6-3H]glucose-to-[1-13C]glucose ratio (used to calculate the rate of meal appearance) (right panel) and the [6,6-2H2]glucose–to–endogenous glucose ratio (used to calculate endogenous glucose production) (left panel) observed in diabetic (■) and nondiabetic (□) subjects before and after ingestion of a mixed meal at time 0. B: Pattern of change of the rate of appearance of meal glucose (top panel), endogenous glucose production (middle panel), and the rate of glucose disappearance (lower panel) observed in diabetic (■) and nondiabetic (□) subjects before and after ingestion of a mixed meal at time 0.
MRa, EGP, and Rd
MRa increased rapidly, reaching a peak in both groups between 20 and 30 min postprandially and then returned toward baseline over the next 6 h (Fig. 3B). Although peak MRa (74.9 ± 5.4 vs. 93.6 ± 7.0 μmol · kg−1 · min−1) was slightly lower (P < 0.05) in diabetic than in nondiabetic subjects, MRa during the first 2 h (6.7 ± 0.5 vs. 7.3 ± 0.5 mmol/kg over 2 h) or over the 6 h of the study (12.8 ± 0.7 vs. 13.4 ± 0.8 mmol/kg over 6 h) did not differ between groups. Hence, splanchnic extraction of ingested glucose also did not differ between the diabetic and nondiabetic subjects (8.8 ± 0.4 vs. 8.2 ± 0.5 mmol/kg over 6 h).
EGP did not differ in diabetic and nondiabetic subjects before meal ingestion (19.4 ± 0.9 vs. 18.8 ± 0.7 μmol · kg−1 · min−1) and decreased in both groups after meal ingestion. This result produced no difference in suppression below basal over the entire 6 h of the study (−4.0 ± 0.3 vs. −3.6 ± 0.2 mmol/kg over 6 h) but a lower (P < 0.05) percent suppression from baseline in diabetic than in nondiabetic subjects during the first 3 h (45.9 ± 1.8 vs. 53.0 ± 3.8%).
Rd did not differ in diabetic and nondiabetic subjects before meal ingestion (20.2 ± 0.85 vs. 19.6 ± 0.74 μmol · kg−1 · min−1). Although the increment above basal did not differ in diabetic and nondiabetic individuals over the entire 6 h of study (8.7 ± 0.7 vs. 9.9 ± 0.7 mmol/kg over 6 h), it was lower (P < 0.01) in diabetic subjects during the first 3 h after meal ingestion (4.8 ± 0.5 vs. 7.9 ± 0.8 mmol/kg over 3 h).
CONCLUSIONS
The present data indicate that a low Rd is the primary cause of the excessive postprandial rise in glucose concentration in diabetes. Defects in insulin secretion, insulin action, and glucose effectiveness probably contribute to the low rates of postprandial Rd. Although postprandial suppression of EGP is modestly delayed and is not appropriate for the prevailing glucose concentration, the absolute rate of postprandial EGP was only minimally greater in diabetic than in nondiabetic subjects. Therefore, whereas lack of appropriate suppression of EGP may have exacerbated the postprandial rise in glucose, it did not cause it. MRa did not differ between groups.
Despite a greater glycemic excursion, MRa was slightly lower in diabetic than in nondiabetic subjects during the first hour after meal ingestion. Therefore, increased meal appearance was not the cause of excessive postprandial hyperglycemia. This finding is consistent with results from most, but not all, of the previous experiments that have measured meal appearance using the dual-tracer method (5–10). Because the ratio of ingested tracer to infused tracer markedly increases as ingested glucose enters the circulation, the resultant rapid change in the tracer-to-tracee ratio introduces error into the calculation of MRa (12,18). This error does not occur when the plasma ratio of the ingested and infused tracers is kept constant as was done in the present experiments because measurement of meal appearance becomes essentially model independent (13,14). When the rate of intravenously infused tracer is kept constant, the plasma ratio of the ingested to infused tracer is determined solely by the rate of appearance of ingested tracer because both the tracer and tracee are cleared in parallel. However, it is noteworthy that because hyperglycemia is a potent stimulus of hepatic glucose uptake (19), the fact that MRa was comparable in diabetic and nondiabetic subjects despite far higher glucose concentrations in the former is consistent with impaired hepatic glucose uptake (19,20).
The rate of suppression of EGP after meal ingestion was slightly slower in diabetic than in nondiabetic subjects. This is similar to the report by Singhal et al. (21) when postprandial suppression of EGP was measured using a variable intravenous glucose tracer infusion analogous to that used in the present experiments. Delayed suppression of EGP also has been reported in studies using the dual-tracer approach (5,6). EGP measured with this approach is calculated by subtracting MRa from Ra. Because Ra also is measured with the constant intravenous tracer, non–steady-state error caused by the rapid fall of the plasma tracer-to-tracee ratio that occurs immediately after eating results in an underestimation of Ra. This is followed by a rise in the tracer-to-tracee ratio that results in an overestimation of Ra. Therefore, although the absolute rates may be wrong, if the sizes of the errors in both MRa and Ra are the same in both groups, then conclusions regarding the temporal pattern of suppression of EGP of one group relative to the other could be correct. This explanation possibly accounts for the fact that the conclusion in previous studies using the dual-tracer method (5–10) and the present studies as well as those of Singhal et al. (21) was that postprandial suppression of EGP is delayed in diabetes.
EGP did not differ in diabetic and nondiabetic subjects before meal ingestion. However, plasma glucose concentrations were substantially higher in diabetic than in nondiabetic subjects. Because hyperglycemia suppresses EGP, rates were not appropriate for the prevailing glucose concentration (22). We and others (2,3,23,24) have reported that the absolute rate of EGP is increased in individuals with severe diabetes as indicated by marked fasting hyperglycemia. In addition, diabetic subjects in the present study had an A1C of 6.8% at the time of screening, indicating excellent glycemic control. Therefore, it is probable that abnormalities in regulation of EGP would be even more marked in individuals with poor glycemic control. Therefore, excessive hepatic glucose release probably contributes to postprandial hyperglycemia in individuals with substantially elevated preprandial glucose concentrations. Delayed insulin secretion, insulin resistance, and an increase in glucagon concentrations all could have contributed to impaired postprandial suppression of EGP. Furthermore, EGP was lower in diabetic than in nondiabetic subjects during 3 to 6 h postprandially probably because of higher insulin and glucose concentrations and slightly lower glucagon concentrations in diabetic subjects. The present experimental design cannot distinguish between these possibilities.
Rd was substantially lower in diabetic subjects during the first several hours after meal ingestion when the excessive rise in glucose occurred. Because splanchnic glucose clearance and therefore, by implication, hepatic glucose uptake, did not differ between groups, decreased Rd was the primary cause of hyperglycemia in diabetic subjects. The overall pattern of postprandial glucose metabolism, namely a marked decrease in Rd immediately after meal ingestion when glucose concentrations are rising in the presence of normal MRa and minimal changes in postprandial suppression of EGP, strongly resembles that which we have recently reported in individuals with impaired fasting glucose (25). The pattern differs from that in previous studies (5–10) using the dual-tracer approach, in which postprandial Rd has been reported to be unchanged or increased relative to that in nondiabetic subjects. In retrospect, these discrepancies are readily explainable because Rd is calculated by subtracting the change in glucose mass from Ra. Therefore, errors in calculation of Ra result in errors in calculation of Rd.
Multiple factors probably contributed to lower postprandial Rd. The difference in Rd between diabetic and nondiabetic subjects closely paralleled the difference in the pattern in insulin concentrations with both being lower in diabetic subjects during the initial several hours after meal ingestion followed by concentrations that were higher than those observed in nondiabetic subjects from 3 h onward. In addition, postprandial insulin action and glucose effectiveness were impaired in diabetic subjects. These abnormalities all probably contributed to the lower Rd during the initial 2 h after meal ingestion. Factors that contribute to postprandial glucose turnover include meal content, meal size, duration, and severity of diabetes among others. Our experimental design is unable to determine the impact of these factors. Furthermore, although disposition indexes were lower in diabetic subjects, their interpretation needs to be considered in the context of a mixed meal (14).
Although the total 6-h postprandial insulin concentrations were similar between groups, the insulin secretory response in the diabetic subjects was sluggish for the first 2 h probably because of reduced sensitivity of the β-cells to the glucose challenge. Furthermore, our observations are relevant only for those subjects whose type 2 diabetes is relatively early in its natural history and is well controlled with oral antidiabetic agents and therefore cannot be extrapolated to those with grossly impaired β-cell reserves after a longer duration of the disease.
In summary, when measured using methods that avoid non–steady-state error, MRa of ingested glucose was normal and suppression of EGP was only slightly impaired. However, when considered in the light of the prevailing glucose concentration, both are abnormal. In contrast, Rd was lower in diabetic subjects for several hours after meal ingestion owing to a combination of defects in insulin secretion, insulin action, and glucose effectiveness. Therefore, agents that correct only one of these abnormalities are unlikely to fully restore postprandial metabolism to normal in type 2 diabetes.
Supplementary Material
Acknowledgments
This study was supported by U.S. Public Health Service grants (DK29953, R-00585, and U 54RR 24150-1).
This study was also supported by a Takeda research grant. No other potential conflicts of interest relevant to this article were reported.
We thank Cathy Dvorak, RN, Barb Norby, RN, and Jean Feehan, RN, for execution of the studies; Betty Dicke, Robert Rood, and the staff of the Immunochemical Core Laboratory and the Mass Spectroscopy Core for technical assistance; and the staff of the Mayo Clinical Research Unit and Center for Clinical and Translation Science Activities for assistance with the studies.
Parts of this study were presented in abstract form at the 64th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 4–8 June 2004.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Basu A, Alzaid A, Dinneen S, Caumo A, Cobelli C, Rizza RA: Effects of a change in the pattern of insulin delivery on carbohydrate tolerance in diabetic and nondiabetic humans in the presence of differing degrees of insulin resistance. J Clin Invest 1996; 97: 2351– 2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basu R, Schwenk WF, Rizza RA: Both fasting glucose production and disappearance are abnormal in people with “mild” and “severe” type 2 diabetes. Am J Physiol Endocrinol Metab 2004; 287: E55– E62 [DOI] [PubMed] [Google Scholar]
- 3. Boden G, Chen X, Stein TP: Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2001; 280: E23– E30 [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Ferrannini E, Simonson DC: Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 1989; 38: 387– 395 [DOI] [PubMed] [Google Scholar]
- 5. Ferrannini E, Simonson DC, Katz LD, Reichard G, Jr, Bevilacqua S, Barrett EJ, Olsson M, DeFronzo RA: The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988; 37: 79– 85 [DOI] [PubMed] [Google Scholar]
- 6. Mitrakou A, Kelley D, Veneman T, Jenssen T, Pangburn T, Reilly J, Gerich J: Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes 1990; 39: 1381– 1390 [DOI] [PubMed] [Google Scholar]
- 7. McMahon M, Marsh HM, Rizza RA: Effects of basal insulin supplementation on disposition of mixed meal in obese patients with NIDDM. Diabetes 1989; 38: 291– 303 [DOI] [PubMed] [Google Scholar]
- 8. Firth RG, Bell PM, Marsh HM, Hansen I, Rizza RA: Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1986; 77: 1525– 1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelley D, Mokan M, Veneman T: Impaired postprandial glucose utilization in non-insulin-dependent diabetes mellitus. Metabolism 1994; 43: 1549– 1557 [DOI] [PubMed] [Google Scholar]
- 10. Fery F, Melot C, Balasse EO: Glucose fluxes and oxidation after an oral glucose load in patients with non-insulin-dependent diabetes mellitus of variable severity. Metabolism 1993; 42: 522– 530 [DOI] [PubMed] [Google Scholar]
- 11. Basu R, DiCamillo B, Toffolo G, Basu A, Shah P, Vella A, Rizza RA, Cobelli C: Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 2003; 284: E55– E69 [DOI] [PubMed] [Google Scholar]
- 12. Toffolo G, Basu R, Dalla Man C, Rizza R, Cobelli C: Assessment of postprandial glucose metabolism: conventional dual- vs. triple-tracer method. Am J Physiol Endocrinol Metab 2006; 291: E800– E806 [DOI] [PubMed] [Google Scholar]
- 13. Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA: Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003; 52: 1738– 1748 [DOI] [PubMed] [Google Scholar]
- 14. Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA: Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 2006; 55: 2001– 2014 [DOI] [PubMed] [Google Scholar]
- 15. Jensen MD, Kanaley JA, Reed JE, Sheedy PF: Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995; 61: 274– 278 [DOI] [PubMed] [Google Scholar]
- 16. Beylot M, Previs SF, David F, Brunengraber H: Determination of the 13C-labeling pattern of glucose by gas chromatography-mass spectrometry. Anal Biochem 1993; 212: 526– 531 [DOI] [PubMed] [Google Scholar]
- 17. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C: Measurement of selective effect of insulin on glucose disposal from labeled glucose oral test minimal model. Am J Physiol Endocrinol Metab 2005; 289: E909– E914 [DOI] [PubMed] [Google Scholar]
- 18. Livesey G, Wilson PD, Dainty JR, Brown JC, Faulks RM, Roe MA, Newman TA, Eagles J, Mellon FA, Greenwood RH: Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am J Physiol 1998; 275: E717– E728 [DOI] [PubMed] [Google Scholar]
- 19. Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA: Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes 2000; 49: 272– 283 [DOI] [PubMed] [Google Scholar]
- 20. Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA: Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes 2004; 53: 2042– 2050 [DOI] [PubMed] [Google Scholar]
- 21. Singhal P, Caumo A, Carey PE, Cobelli C, Taylor R: Regulation of endogenous glucose production after a mixed meal in type 2 diabetes. Am J Physiol Endocrinol Metab 2002; 283: E275– E283 [DOI] [PubMed] [Google Scholar]
- 22. Basu A, Caumo A, Bettini F, Gelisio A, Alzaid A, Cobelli C: Impaired basal glucose effectiveness in NIDDM: contribution of defects in glucose disappearance and production, measured using an optimized minimal model independent protocol. Diabetes 1997; 46: 421– 432 [DOI] [PubMed] [Google Scholar]
- 23. Gastaldelli A, Baldi S, Pettiti M, Toschi E, Camastra S, Natali A, Landau BR, Ferrannini E: Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes 2000; 49: 1367– 1373 [DOI] [PubMed] [Google Scholar]
- 24. Radziuk J, Pye S: Production and metabolic clearance of glucose under basal conditions in type II (non-insulin-dependent) diabetes mellitus. Diabetologia 2001; 44: 983– 991 [DOI] [PubMed] [Google Scholar]
- 25. Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R: Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006; 55: 3536– 3549 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.