Abstract
OBJECTIVE
Because declines in acute insulin response (AIR) and insulin action (M) predict development of type 2 diabetes, we sought to determine childhood factors that predict insulin action and AIR using longitudinal data from young Pima Indian adults with normal glucose regulation.
RESEARCH DESIGN AND METHODS
Predictors of adult M, measured by the euglycemic-hyperinsulinemic clamp, and AIR, measured after a 25-g glucose bolus, were assessed in 76 individuals from a set of childhood data (BMI, systolic blood pressure [sBP] and diastolic blood pressure, cholesterol, fasting and 2-h insulin, and glucose levels during an oral glucose tolerance test).
RESULTS
After adjustment for sex, adult percent body fat, adult and childhood age, childhood BMI, and sBP were negative and independent predictors of adult M. A 5 kg/m2 increase in childhood BMI was associated with a 7.4% decrease in adult insulin action (95% CI −12.7 to −1.8%, P = 0.01) and a 10-mmHg increase in childhood sBP with a 5.0% decrease in adult M (95% CI −8.4 to −1.4%, P = 0.007). After a similar adjustment with M as an additional covariate, childhood 2-h insulin was a positive predictor of adult AIR such that a 25% increase predicted a 7.3% increase in adult AIR (95% CI 1.5–13.5%, P = 0.014).
CONCLUSIONS
Childhood insulin response during an oral glucose challenge predicts adult AIR, indicating that β-cell capacity may be set early in life. Childhood measures related to adiposity predict adult insulin action, which may reflect common underlying mechanisms that may be amenable to modification through programs targeting prevention or treatment of childhood obesity.
Type 2 diabetes is a lifelong process progressing from normal glucose regulation to impaired glucose regulation and ultimately to diabetes (1). If lifestyle interventions are initiated in people with impaired glucose regulation, type 2 diabetes can be prevented, at least in the short term (2). However, over longer periods, once impaired glucose regulation has occurred, a large proportion of people may eventually develop type 2 diabetes despite preventive efforts (3). Development of type 2 diabetes is preceded by declines in insulin release from pancreatic islet cells and insulin sensitivity such that even in people with normal glucose regulation, these two measures predict subsequent type 2 diabetes (4). It has been shown that defects in these two factors occur early in the natural history of diabetes before clinically apparent changes in glucose handling (1).
Risk factors including obesity, dyslipidemia, hypertension, and glucose intolerance are known to predict type 2 diabetes (5). Many of these also track from childhood into adulthood (6–8), and it has been shown that early life events such as in utero exposure to diabetes and childhood obesity predict future risk of type 2 diabetes (9,10). The relation of these risk factors during childhood to later adult measures of acute insulin release (AIR) and insulin sensitivity before the development of diabetes is not well defined but may lead to greater understanding of the pathogenesis of type 2 diabetes and point to targets for early intervention.
We evaluated a subset of childhood metabolic measures including blood pressure, BMI, cholesterol, and fasting and 2-h glucose (2hG) and insulin (2hIns) levels during an oral glucose tolerance test in the Pima Indians of the Gila River Community of Arizona, a population with a high prevalence of type 2 diabetes. These measures were assessed as potential predictors of insulin action (M) and AIR in adults with normal glucose regulation, before potential pathologic declines in β-cell function.
RESEARCH DESIGN AND METHODS
Data were collected prospectively in 76 full-heritage Pima Indians from the Gila River Indian Community. Since 1965, all residents of the community age 5 years or older have been invited to participate in a longitudinal study of health that included a biennial health exam as well as a 75-g oral glucose tolerance test (OGTT). For children under the age of 18 years, written informed consent was provided by a parent or guardian and the child assented. For this subset analysis, we selected the earliest exam in those participants who were evaluated between their 5th and 16th birthdays, with all measures recorded for BMI, systolic blood pressure (sBP), diastolic blood pressure (dBP), cholesterol, and fasting and 2-h plasma glucose and insulin levels during the OGTT (n = 1,435) and excluded those children with type 2 diabetes, as determined by the 2003 American Diabetes Association (ADA) criteria (11) (n = 27). Of the remaining 1,408 individuals, 198 participated as nonsmoking healthy adults in our study investigating metabolic determinants of type 2 diabetes (12) with complete data for M and AIR measurements. All adults provided written informed consent. For the purposes of ensuring adequate separation between childhood and adult measures, only adults 21 years or older were included in this analysis. As adults, we excluded those individuals with impaired glucose regulation (n = 117) and those with in utero exposure to diabetes (n = 5), a factor known to dramatically increase the risk of developing diabetes by the third decade (13) and to reduce acute insulin release in Pima Indians (14). In utero exposure to diabetes was defined by a confirmed date of diabetes diagnosis in the mother that preceded the child's birth date or an OGTT during pregnancy with a 2-h value of ≥11 mmol/l. In the final subset of 76 subjects (38% of eligible adult subjects), the childhood evaluations spanned the years 1979–1994, and the adult visits occurred over the years 1987–2004. Both study protocols were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
Childhood measurements
All individuals were asked to arrive to the visit after an overnight fast. A 75-g OGTT was performed. Fasting and 2-h blood samples were drawn for measurement of glucose and insulin concentrations. Plasma glucose was measured by an autoanalyzer using the potassium ferricyanide method (Technicon Instruments, Tarrytown, NY). Serum insulin concentrations were measured by radioimmunoassay using the Herbert modification of the Yalow and Berson method (15) or an automated analyzer (Concept 4; ICN Radiochemicals, Costa Mesa, CA). All insulin values were converted using regression equations to the earlier radioimmunoassay values for comparability. Height was measured using a stadiometer and weight using calibrated scales by a set of trained observers. No standard measures of puberty were available.
Adult measurements
OGTTs (75 g) were used to exclude people with diabetes before enrollment and to classify glucose tolerance. Normal glucose regulation was determined using 2003 ADA criteria, a fasting glucose <5.6 mmol/l, and a 2hG <7.8 mmol/l (11). All plasma glucose concentrations were determined by the glucose oxidase method (Beckman Instruments, Fullerton, CA). Upon admission to the clinical research unit, participants were given a weight-maintaining diet (50% carbohydrate, 30% fat, and 20% protein) and asked to abstain from strenuous exercise. Body composition was estimated by underwater weighing with determination of residual lung volume by helium dilution (20 participants) or by total-body dual-energy X-ray absorptiometry (DPX-L; Lunar Radiation, Madison, WI) (56 participants). Percent body fat measurements from the dual-energy X-ray absorptiometry scan were converted to underwater values using a conversion equation (16).
AIR was determined using an intravenous glucose tolerance test. A 25-g glucose bolus was given over 3 min, and blood was drawn every minute until the fifth minute for measurement of insulin levels. The AIR was calculated as the average increase in plasma insulin from baseline during this time period. Plasma insulin concentrations were determined by radioimmunoassay via an automated analyzer, either the Concept 4 analyzer described previously or an Access analyzer (Beckman Instruments). All insulin measurements were normalized to the earliest radioimmunoassay using regression equations for comparability with childhood values.
Insulin action was assessed using a euglycemic-hyperinsulinemic clamp. After an overnight fast, a primed continuous insulin infusion was administered through an antecubital vein at 40 mU/m2 per minute for 100 min. The plasma glucose concentrations were measured every 5 min after the start of the insulin infusion, and a variable infusion of 20% dextrose was used to maintain plasma glucose at 5.6 mmol/l. The rate of glucose required to maintain euglycemia, as a measure of insulin sensitivity (M), was calculated for the last 40 min of the insulin infusion and corrected for both steady-state plasma insulin levels and endogenous glucose production. Endogenous glucose production was determined at baseline (120 min) and during the insulin infusion using a primed continuous infusion (0.3 μCi/min) of [3-3H]glucose. M values were normalized to estimated metabolic body size, or fat-free mass +17.7 kg (17). The average coefficient of variation for M was 2.2% in this subset of individuals.
Statistical analysis
Subject characteristics are presented as means ± SD if normally distributed and as median with 25th and 75th percentiles if skewed. M, AIR, and insulin levels were log10 transformed to meet the assumptions of linear regression. Linear regression models were used to determine subsets of childhood predictors of adult M and AIR using backward selection, i.e., all factors were initially placed in the model, and the weakest factors were sequentially rejected until only those with a P < 0.1 remained. Significance level was set at P < 0.05. Adult and childhood age, sex, and percent body fat were not made available to the stepwise procedure and thus, by default, were included in the final model. Additionally, M was forced into the model for AIR. Because of concerns about the small sample size relative to the number of predictor variables, the models were also run using forward selection, i.e., the variables most strongly related to the outcome are entered into the model one at a time until none of the remaining variables improve the model, with identical results. The validity of the selected models was assessed using bootstrapping (18) with 1,000 replicates, each with a sample size of 76, created by sampling with replacement from the original dataset. Bootstrapping provides an estimate of what might be observed with multiple repetitions of a study. The backward selection procedure was applied to each of the 1,000 replicates to verify the stability of the chosen predictors in the final models. The final model for AIR was also applied to adult 30-min insulin levels during the OGTT, a frequently used surrogate for AIR. All analyses were performed using SAS Enterprise Guide 4.
RESULTS
Subject characteristics
Subject characteristics for the 76 individuals (63% male) included in this study during both childhood and adulthood are presented in Table 1. The median duration of follow-up time between the childhood and adult evaluations was 13.4 years (range 6.5–21.0). A total of 55% had at least one parent who developed type 2 diabetes before the age of 45 years. However, parental diabetes before age 45 years, if included in either model, was not a significant predictor of M or AIR in this subset. At the childhood visit, the individuals included in this analysis compared with those 1,332 children not included were of a similar age but had significantly lower average BMI, cholesterol, sBP, fasting insulin, and 2hIns levels. The differences in sBP and 2hIns levels were attributable to the BMI differences. On average, the individuals in this analysis, as adults, were relatively young and obese with an average BMI of 34.2 kg/m2 and body fat of 32.3%. This is not substantially different from the complete group of full heritage adult Pima Indians who have participated in our metabolic studies. By design, all in our study population had normal glucose regulation and normal blood pressure. As expected, there was a high degree of correlation between the childhood factors that were evaluated as predictors of M and AIR (Table 2). The highest correlations were between BMI and insulin levels as well as glucose and insulin levels, with no difference between sexes.
Table 1.
Subject characteristics during childhood and adulthood
| Childhood | Adult | |
|---|---|---|
| Age (years) | 11.7 ± 2.6 | 25.3 ± 3.2 |
| BMI (kg/m2) | 21.3 ± 5.0 | 34.2 ± 7.7 |
| % Body fat | 32.3 ± 8.2 | |
| sBP (mmHg) | 99 ± 15 | 112 ± 11 |
| dBP (mmHg) | 56 ± 10 | 66 ± 9 |
| Cholesterol (mmol/l) | 3.4 ± 0.5 | |
| Fasting glucose (mmol/l) | 5.0 ± 0.4 | 4.7 ± 0.4 |
| 2hG (mmol/l) | 5.5 ± 1.2 | 6.1 ± 0.9 |
| Fasting insulin (pmol/l) | 128 (90–208) | 270 (207–360) |
| 2hIns (pmol/l) | 576 (319–056) | 1,039 (680–1,379) |
| AIR (pmol/l) | 1,787 (1,241–2,387) | |
| Insulin action (mg glucose/kg EMBS per minute) | 2.31 (2.10–2.91) |
Data are means ± SD or medians (25th to 75th percentile). The median duration of follow-up between evaluations was 13.4 years (range 6.5–21.0). EMBS, estimated metabolic body size.
Table 2.
Pearson correlations between childhood metabolic factors evaluated as predictors of adult insulin action and AIR
| Child age | Child BMI | Child sBP | Child dBP | Child cholesterol | Child fasting glucose | Child 2hG | Child fasting insulin | Child 2hIns | Adult insulin action | Adult AIR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Child age | 1.00 | 0.47 (<0.0001) | 0.55 (<0.0001) | 0.45 (<0.0001) | −0.03 (0.80) | 0.3 (<0.01) | 0.26 (<0.05) | 0.36 (<0.01) | 0.34 (<0.01) | 0.09 (0.46) | 0.09 (0.45) |
| Child BMI | 1.00 | 0.47 (<0.0001) | 0.26 (<0.05) | 0.15 (0.18) | 0.38 (<0.001) | 0.42 (<0.001) | 0.68 (<0.0001) | 0.53 (<0.0001) | −0.43 (<0.001) | 0.32 (<0.01) | |
| Child sBP | 1.00 | 0.51 (<0.0001) | −0.10 (0.40) | 0.26 (<0.05) | 0.25 (<0.05) | 0.50 (<0.0001) | 0.36 (<0.01) | −0.30 (<0.01) | 0.18 (0.12) | ||
| Child dBP | 1.00 | −0.01 (0.91) | 0.20 (0.08) | 0.18 (0.12) | 0.35 (<0.01) | 0.24 (<0.05) | −0.09 (0.45) | −0.07 (0.53) | |||
| Child cholesterol | 1.00 | −0.11 (0.35) | 0.20 (0.08) | 0.19 (0.10) | 0.26 (<0.05) | −0.06 (0.61) | −0.03 (0.77) | ||||
| Child fasting glucose | 1.00 | 0.40 (<0.001) | 0.44 (<0.0001) | 0.16 (0.18) | −0.15 (0.19) | −0.05 (0.67) | |||||
| Child 2hG | 1.00 | 0.41 (<0.001) | 0.70 (<0.0001) | −0.08 (0.51) | 0.09 (0.43) | ||||||
| Child fasting insulin | 1.00 | 0.70 (<0.0001) | −0.35 (<0.01) | 0.24 (<0.05) | |||||||
| Child 2hIns | 1.00 | −0.25 (0.04) | 0.34 (<0.01) | ||||||||
| Adult insulin action | 1.00 | −0.33 (<0.01) | |||||||||
| Adult AIR | 1.00 |
Data are Pearson correlation coefficient (P value). Insulin action, AIR, and insulin levels were log10-transformed to approximate a normal distribution.
Childhood predictors of adult insulin action
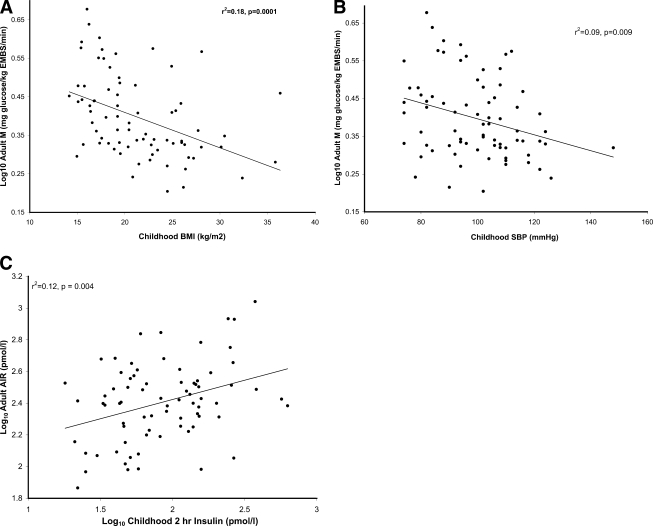
The final regression model included childhood sBP (P = 0.007) and childhood BMI (P = 0.01) as negative predictors of insulin action after controlling for sex, adult percentage body fat, and child and adult age (F = 9.62, r2 = 0.46, P < 0.0001) (Fig. 1). These two childhood measures explained an additional 14% of the variance in adult M over the reduced model. Because insulin action was log transformed, the clinical meaning of the relationship between M and the childhood factors is reported in terms of percent change in M. For every 5 kg/m2 increase in childhood BMI, adult M decreased by 7.4% (95% CI −12.7 to −1.8%). For every 10 mmHg increase in childhood sBP, adult M decreased by 5.0% (95% CI −8.4 to −1.4%). Bootstrapping confirmed that childhood BMI and sBP were the two strongest childhood predictors of M (chosen in 812 and 768 of the 1,000 replicates). The other six predictors were chosen <40% of the time.
Figure 1.
Simple linear regression plots between childhood predictors and adult measures; r2 and P values are indicated on the graphs. A: Negative association between childhood BMI and log10 adult insulin action (M). M was log10 transformed to meet the assumptions of linear regression. B: Negative association between childhood sBP and log10 adult insulin action (M). C: Positive relationship between log10 of childhood 2-h insulin levels during an OGTT and log10 adult AIR. All measures of insulin in this study were log10-transformed to meet the assumptions of linear regression. EMBS, estimated metabolic body size.
We did not have enough individuals or pubertal information to be able to separate children into pre- and postpubertal developmental stages; however, in the small group of children with data before the age of 12 years (n = 37), the relationships between childhood factors and adult measurements maintain the same directionality as those in the larger group, but only the association between childhood BMI and adult M was significant (data not shown).
Childhood predictors of adult acute insulin secretion
Both childhood 2hG (P = 0.086) and 2hIns (P = 0.014) were chosen for the final model of AIR, but only 2hIns was statistically significant (F = 2.68, r2 = 0.23, P = 0.017) (Fig. 1). In a reduced model without 2hIns, neither the model (P = 0.09) nor 2hG (P = 0.85) were statistically significant. Additionally, without 2hG in the model, 2hIns was no longer significant (P = 0.08), indicating that 2hG is a suppresser confounder for 2hIns, i.e., a variable associated with both the risk factor and the outcome, which when adjusted for, allows the relationship between the factor and the outcome to become apparent (22). The insulin sensitivity index combining glucose and insulin measurements into a single factor was not significant in the model and did not improve the fit of the model. Childhood 2hIns explained an additional 8.6% of variance in adult AIR over the reduced model. Adjusting for childhood BMI in the final model also did not alter the results. For every 25% increase in childhood 2hIns, adult AIR increased 7.3% (95% CI 1.5–13.5%). Bootstrapping also confirmed that childhood 2hIns was the strongest childhood predictor of adult AIR (810 of 1,000 replicates). The other six factors were chosen in less than half the replicates. Notably, childhood fasting insulin was only chosen in 177 out of the 1,000 replicates. To further corroborate these findings, the final model was applied to adult 30-min insulin levels from the OGTT, a commonly used surrogate for AIR. Childhood 2hIns was significant in the model (β = 0.7, 95% CI 0.2–1.2) with (P = 0.007) and without (P = 0.04) 2hG, but the association was stronger when 2hG was included. Again, 2hG was not quite significant (P = 0.06).
CONCLUSIONS
In this longitudinal observational study, childhood factors predicted adult insulin action and secretion in adults with normal glucose regulation, i.e., before clinical evidence of glucose dysregulation. The main findings are first that childhood BMI and sBP are negatively associated with adult M and, second, that 2hIns during a childhood OGTT is positively associated with adult AIR after adjustment for childhood 2hG.
Both M and AIR predict type 2 diabetes in the Pima population (12), even in individuals with normal glucose regulation (4). In the development of type 2 diabetes, both insulin secretion and insulin action have already deteriorated by the time impaired glucose regulation manifests (1). These prior studies indicate that preventing diabetes should begin at an early stage and target both insulin resistance and insulin secretion. Our work expands on this idea by identifying early life measures that are associated with these pre-diabetic elements. We have previously shown that childhood metabolic factors predict development of type 2 diabetes at a young age in Pima Indians (5). This study found that 2hG, waist circumference, and BMI were the strongest predictors, whereas sBP and dBP, although predictors, were relatively weak. This study did not include 2hIns (5). An earlier study which did include 2hIns measurements found that in Pima Indians, parental diabetes, weight relative to height, and 2hG predicted adult risk of type 2 diabetes (10). In this study, weight relative to height and fasting and 2hG and insulin levels were associated with parental diabetes. We did not find parental diabetes to be an important contributor to either M or AIR in our study, but this may be due to use of M and AIR as outcomes in the present analysis and diabetes in the previous articles (5,10). Our analysis separates the physiologic components of diabetes to determine individual predictors in this population based dataset of longitudinal data. We show that these childhood factors are predictive even across life stages, with a minimum separation of 6.5 years. We purposefully only looked at children between the ages of 5 and 16 years before the completion of growth and development and reevaluated these individuals after the age of 21 years when development was complete. Our data indicate that changes contributing to later risk of diabetes may begin during childhood.
Childhood sBP and BMI, both measures related to adiposity (23), independently predict adult insulin action. While lifelong obesity may be a causative factor as childhood obesity is strongly associated with adult obesity (6,7), the relationships in this study were independent of adult percent body fat. This implies that excess adiposity during development may have adverse effects independent of later body size. The association of childhood sBP with adult M may reflect common underlying mechanisms that begin early in life and may be as simple as a sedentary lifestyle or more complex such as sympathetic hyperfunction or increased inflammation. Others have found that sBP in childhood, but not dBP, predicts metabolic syndrome, a component of which is impaired fasting glucose, with or without hypertension later in life (8). Our study adds to this previous study by separating the effects of insulin action from insulin release. In the Bogalusa Heart Study, the corollary was found where people who developed hypertension as adults were more likely to have had insulin resistance during childhood (19). Also, in the Bogalusa Heart Study, development of insulin resistance syndrome as an adult was strongly predicted by childhood BMI (20). In another study, childhood BMI and sBP were related to adult insulin sensitivity; however, these associations were attenuated when adult waist circumference and maximal aerobic capacity were included as confounders (21).
We also found that childhood insulin response during OGTT is a predictor of adult AIR independent of childhood BMI and adult percent body fat. 2hG was found to be a suppressor variable for 2hIns. The explanation for 2hG as a suppressor may be that a less robust insulin response is required to maintain a relatively low glucose level in an insulin-sensitive child. The association between 2hIns and future AIR implies that β-cell capacity may be set early in life. Whether this capacity is determined by genetic factors, in utero exposures (14), or other undiscovered reasons is unclear. It has been shown that insulin release in Pima Indians is similar to African Americans and exaggerated when compared with Caucasians (23). Although the prevalence of diabetes is much higher in the Pima Indian population, in general, diabetes in this population has been prototypic of type 2 diabetes in other populations.
Overall, our population had lower childhood BMIs and lower average sBP and 2hIns levels than the general Pima Indian population with childhood measures; the lower sBP and insulin levels were attributable to the lower BMI levels. This is likely due to a survivor effect given our selection of adults with normal glucose regulation, who may have had relatively lower childhood BMIs than those excluded for impaired glucose regulation or diabetes. Nevertheless, as M and AIR are predictors of type 2 diabetes, even in adults with normal glucose regulation, our results are still relevant. Another limitation of this study is the small number of participants who met our inclusion criteria for this analysis. This may have resulted in overfitting of our model given the number of childhood factors evaluated relative to our sample size. However, we did rerun the models using forward selection, which is better suited for dealing with small sample sizes (22) with identical results. The results from our analysis are also consistent with our bootstrapped analysis and with the literature previously mentioned. Nevertheless, given the small sample size, the findings should be interpreted cautiously pending replication in other studies.
In conclusion, abnormalities contributing to adult insulin resistance and release and, thus, risk of diabetes appear to begin in childhood or earlier. While it is important to implement preventive lifestyle measures once impaired glucose regulation develops, identifying childhood factors that contribute to or associate with pathological declines in M and AIR may uncover earlier preventive targets in advance of clinical evidence of dysfunction. In particular, our study implies that prevention of future declines in insulin action may be possible through modification of childhood body weight, but insulin secretion may not be modifiable through lifestyle interventions.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
We thank the members of the Gila River Indian Community who volunteered for our studies. We also thank the staff of the Diabetes Epidemiology and Clinical Research Section and the Clinical Research Unit for their contributions.
This study was presented in abstract form at the Endocrine Society meeting, June 2008.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104: 787– 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH: The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008; 371: 1783– 1789 [DOI] [PubMed] [Google Scholar]
- 4. Bunt JC, Krakoff J, Ortega E, Knowler WC, Bogardus C: Acute insulin response is an independent predictor of type 2 diabetes mellitus in individuals with both normal fasting and 2-h plasma glucose concentrations. Diabete Metab Res Rev 2007; 23: 304– 310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, Infante AM, Krakoff J, Looker HC: Childhood predictors of young-onset type 2 diabetes. Diabetes 2007; 56: 2964– 2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Field AE, Cook NR, Gillman MW: Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res 2005; 13: 163– 169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinberger J, Moran A, Hong CP, Jacobs DR, Jr, Sinaiko AR: Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J Pediatr 2001; 138: 469– 473 [DOI] [PubMed] [Google Scholar]
- 8. Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR: Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics 2007; 119: 237– 246 [DOI] [PubMed] [Google Scholar]
- 9. Dabelea D, Pettitt DJ: Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab 2001; 14: 1085– 1091 [DOI] [PubMed] [Google Scholar]
- 10. McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Bennett PH, Knowler WC: Glucose, insulin concentrations and obesity in childhood and adolescence as predictors of NIDDM. Diabetologia 1994; 37: 617– 623 [DOI] [PubMed] [Google Scholar]
- 11. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003; 26( Suppl. 1): S5– S20 [DOI] [PubMed] [Google Scholar]
- 12. Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C: Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med 1993; 329: 1988– 1992 [DOI] [PubMed] [Google Scholar]
- 13. Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC: Congenital susceptibility to NIDDM: role of intrauterine environment. Diabetes 1988; 37: 622– 628 [DOI] [PubMed] [Google Scholar]
- 14. Gautier JF, Wilson C, Weyer C, Mott D, Knowler WC, Cavaghan M, Polonsky KS, Bogardus C, Pratley RE: Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes 2001; 50: 1828– 1833 [DOI] [PubMed] [Google Scholar]
- 15. Herbert V, Lau KS, Gottlieb CW, Bleicher SJ: Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 1965; 1375– 1384 [DOI] [PubMed] [Google Scholar]
- 16. Tataranni PA, Ravussin E: Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 1995; 62: 730– 734 [DOI] [PubMed] [Google Scholar]
- 17. Lillioja S, Bogardus C: Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev 1988; 4: 517– 540 [DOI] [PubMed] [Google Scholar]
- 18. Sauerbrei W, Schumacher M: A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med 1992; 11: 2093– 2109 [DOI] [PubMed] [Google Scholar]
- 19. Srinivasan SR, Myers L, Berenson GS: Changes in metabolic syndrome variables since childhood in prehypertensive and hypertensive subjects: the Bogalusa Heart Study. Hypertension 2006; 48: 33– 39 [DOI] [PubMed] [Google Scholar]
- 20. Srinivasan SR, Myers L, Berenson GS: Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes 2002; 51: 204– 209 [DOI] [PubMed] [Google Scholar]
- 21. Clausen JO, Ibsen H, Ibsen KK, Borch-Johnsen K: Association of body mass index, blood pressure and serum levels of triglycerides and high-density lipoprotein cholesterol in childhood with the insulin sensitivity index in young adulthood: a 13-year follow-up. J Cardiovasc Risk 1996; 3: 427– 433 [DOI] [PubMed] [Google Scholar]
- 22. Katz MH: Multivariable Analysis: A Practical Guide for Clinicians. 2nd ed. New York, NY, Cambridge University Press, 2006 [Google Scholar]
- 23. Lindsay RS, Hanson RL, Roumain J, Ravussin E, Knowler WC, Tataranni PA: Body mass index as a measure of adiposity in children and adolescents: relationship to adiposity by dual energy X-ray absorptiometry and to cardiovascular risk factors. J Clin Endocrinol Metab 2001; 86: 4061– 4067 [DOI] [PubMed] [Google Scholar]
- 24. Stefan N, Stumvoll M, Weyer C, Bogardus C, Tataranni PA, Pratley RE: Exaggerated insulin secretion in Pima Indians and African-Americans but higher insulin resistance in Pima Indians compared to African-Americans and Caucasians. Diabet Med 2004; 21: 1090– 1095 [DOI] [PubMed] [Google Scholar]