Abstract
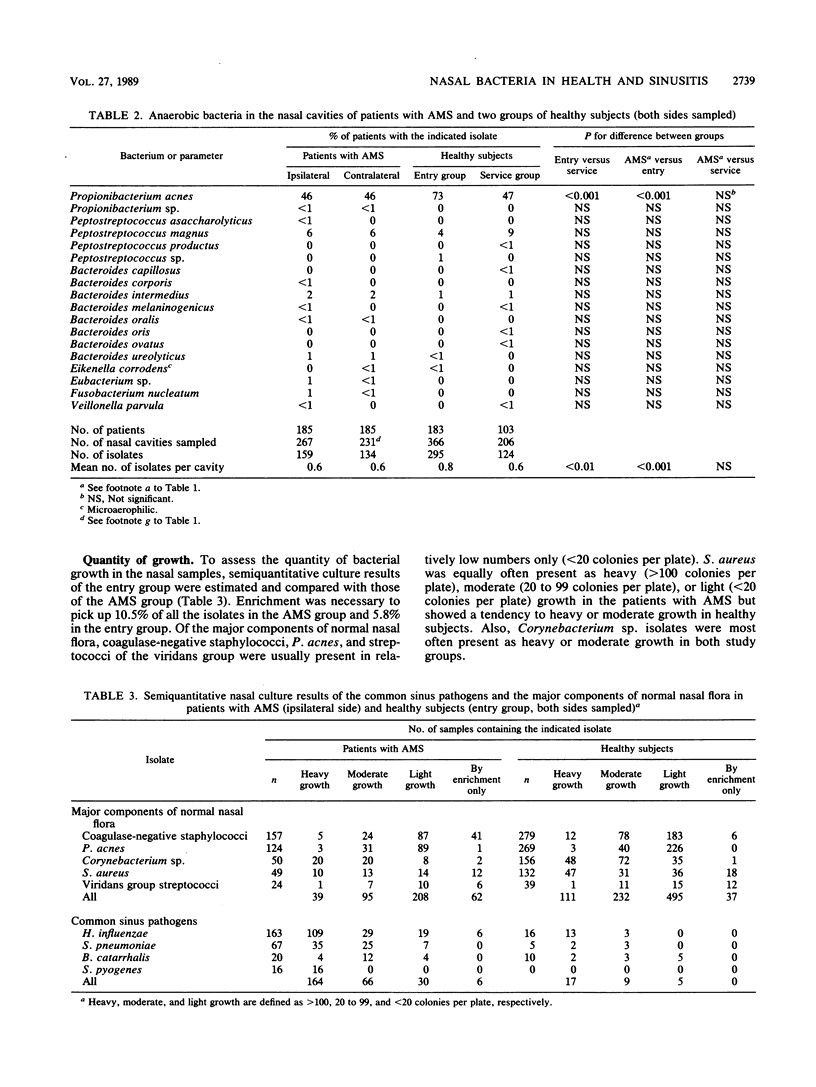
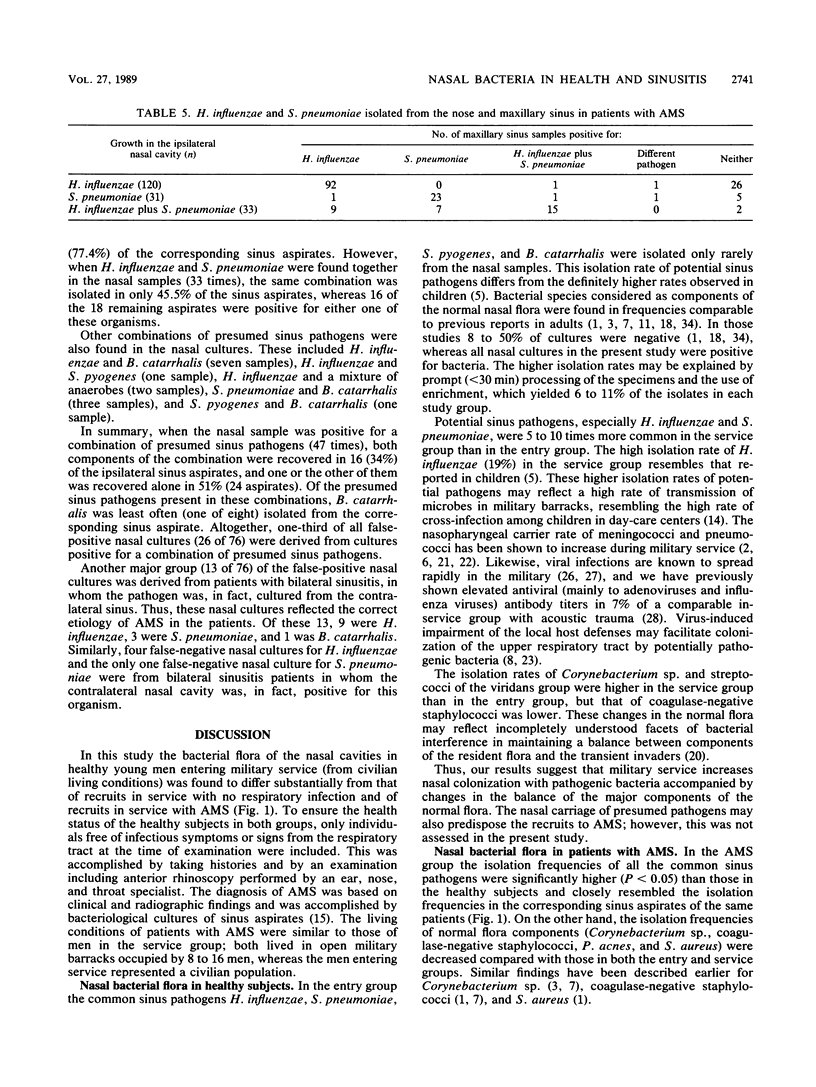
The nasal bacterial flora was studied in 183 healthy men entering military service (entry group), 103 healthy recruits in service (service group), and 185 recruits with acute maxillary sinusitis. The 267 nasal and ipsilateral sinus aspirate findings in the same patients with acute maxillary sinusitis were compared pairwise. In the entry group presumed sinus pathogens were only rarely isolated from the nasal cavities: Haemophilus influenzae in 4%, Streptococcus pneumoniae in 1%, Branhamella catarrhalis in 3%, and Streptococcus pyogenes in 0%. The corresponding isolation frequencies in the service group were 19, 13, 3, and less than 1%, respectively, and those in the group with acute maxillary sinusitis were 61, 25, 7, and 6%, respectively. Suppression of the major components of the normal nasal flora, Corynebacterium sp., coagulase-negative staphylococci, Propionibacterium acnes, and Staphylococcus aureus, was seen in the group with acute maxillary sinusitis and also occasionally in the service group. When a sinus aspirate culture yielded a presumed sinus pathogen, the same pathogen was found in the nasal samples in 91% of the cases. The predictive value of a pathogen-positive nasal finding was highest (93.8%) for S. pyogenes, followed by 77.7% for H. influenzae and 68.7% for S. pneumoniae, and lowest (20%) for B. catarrhalis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYCOCK W. L., MUELLER J. H. Meningococcus carrier rates and meningitis incidence. Bacteriol Rev. 1950 Jun;14(2):115–160. doi: 10.1128/br.14.2.115-160.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson A., Brorson J. E. The correlation between bacteriological findings in the nose and maxillary sinus in acute maxillary sinusitis. Laryngoscope. 1973 Dec;83(12):2003–2011. doi: 10.1288/00005537-197312000-00011. [DOI] [PubMed] [Google Scholar]
- Bovre K. Oxidase positive bacteria in the human nose incidence and species distribution, as diagnosed by genetic transformation. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(6):780–784. [PubMed] [Google Scholar]
- Catlin F. I., Cluff L. E., Reynolds R. C. The bacteriology of acute and chronic sinusitis. South Med J. 1965 Dec;58(12):1497–1502. doi: 10.1097/00007611-196512000-00006. [DOI] [PubMed] [Google Scholar]
- Couch R. B. The effects of influenza on host defenses. J Infect Dis. 1981 Sep;144(3):284–291. doi: 10.1093/infdis/144.3.284. [DOI] [PubMed] [Google Scholar]
- Evans F. O., Jr, Sydnor J. B., Moore W. E., Moore G. R., Manwaring J. L., Brill A. H., Jackson R. T., Hanna S., Skaar J. S., Holdeman L. V. Sinusitis of the maxillary antrum. N Engl J Med. 1975 Oct 9;293(15):735–739. doi: 10.1056/NEJM197510092931502. [DOI] [PubMed] [Google Scholar]
- Gwaltney J. M., Jr, Sydnor A., Jr, Sande M. A. Etiology and antimicrobial treatment of acute sinusitis. Ann Otol Rhinol Laryngol Suppl. 1981 May-Jun;90(3 Pt 3):68–71. doi: 10.1177/00034894810903s216. [DOI] [PubMed] [Google Scholar]
- Hays G. C., Mullard J. E. Can nasal bacterial flora be predicted from clinical findings? Pediatrics. 1972 Apr;49(4):596–599. [PubMed] [Google Scholar]
- Heczko P. B., Höffler U., Kasprowicz A., Pulverer G. Quantitative studies of the flora of the nasal vestibule in relation to nasal carriage of Staphylococcus aureus. J Med Microbiol. 1981 Aug;14(3):233–241. doi: 10.1099/00222615-14-3-233. [DOI] [PubMed] [Google Scholar]
- Istre G. R., Conner J. S., Broome C. V., Hightower A., Hopkins R. S. Risk factors for primary invasive Haemophilus influenzae disease: increased risk from day care attendance and school-aged household members. J Pediatr. 1985 Feb;106(2):190–195. doi: 10.1016/s0022-3476(85)80285-7. [DOI] [PubMed] [Google Scholar]
- Jousimies-Somer H. R., Savolainen S., Ylikoski J. S. Bacteriological findings of acute maxillary sinusitis in young adults. J Clin Microbiol. 1988 Oct;26(10):1919–1925. doi: 10.1128/jcm.26.10.1919-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler L. Die Bakterienflora Nasenhaupt- und Nasennebenhöhlen bei chronischen Sinuitiden und ihre Beziehung zueinander. HNO. 1968 Feb;16(2):35–39. [PubMed] [Google Scholar]
- LYSTAD A., BERDAL P., LUND-IVERSEN L. THE BACTERIAL FLORA OF SINUSITIS WITH AN IN VITRO STUDY OF THE BACTERIAL RESISTANCE TO ANTIBIOTICS. Acta Otolaryngol Suppl. 1964;188:SUPPL 188–188:390+. doi: 10.3109/00016486409134592. [DOI] [PubMed] [Google Scholar]
- Linoli O., Marconi S., Garaffa M. Ecologia batterica quantitativa della mucosa nasale normale. Ann Sclavo. 1981 Mar-Apr;23(2):151–161. [PubMed] [Google Scholar]
- Mackowiak P. A. The normal microbial flora. N Engl J Med. 1982 Jul 8;307(2):83–93. doi: 10.1056/NEJM198207083070203. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Käyhty H., Weckström P., Sivonen A., Renkonen O. V. Effect of group-A meningococcal vaccine in army recruits in Finland. Lancet. 1975 Nov 8;2(7941):883–886. doi: 10.1016/s0140-6736(75)92125-x. [DOI] [PubMed] [Google Scholar]
- Nichols T., Freeman R. A new selective medium for Streptococcus pneumoniae. J Clin Pathol. 1980 Aug;33(8):770–773. doi: 10.1136/jcp.33.8.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylén O., Jeppsson P. H., Branefors-Helander P. Acute sinusitis. A clinical bacteriological and serological study with special reference to Haemophilus influenzae. Scand J Infect Dis. 1972;4(1):43–48. doi: 10.3109/inf.1972.4.issue-1.09. [DOI] [PubMed] [Google Scholar]
- Penttinen K., Cantell K., Somer P., Poikolainen A. Mumps vaccination in the Finnish defense forces. Am J Epidemiol. 1968 Sep;88(2):234–244. doi: 10.1093/oxfordjournals.aje.a120882. [DOI] [PubMed] [Google Scholar]
- Pyhälä R., Aho K., Visakorpi R. Seroepidemiology of H1N1 influenza: striking differences in the attack rate among young people. Acta Pathol Microbiol Scand B. 1979 Jun;87B(3):161–164. doi: 10.1111/j.1699-0463.1979.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Savolainen S., Jousimies-Somer H., Kleemola M., Ylikoski J. Serological evidence of viral or Mycoplasma pneumoniae infection in acute maxillary sinusitis. Eur J Clin Microbiol Infect Dis. 1989 Feb;8(2):131–135. doi: 10.1007/BF01963896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen S., Ylikoski J., Jousimes-Somer H. Predictive value of nasal bacterial culture for etiological agents in acute maxillary sinusitis. Rhinology. 1987 Mar;25(1):49–55. [PubMed] [Google Scholar]
- Savolainen S., Ylikoski J., Jousimies-Somer H. The bacterial flora of the nasal cavity in healthy young men. Rhinology. 1986 Dec;24(4):249–255. [PubMed] [Google Scholar]
- Vecchio T. J. Predictive value of a single diagnostic test in unselected populations. N Engl J Med. 1966 May 26;274(21):1171–1173. doi: 10.1056/NEJM196605262742104. [DOI] [PubMed] [Google Scholar]
- WATSON E. D., HOFFMAN N. J., SIMMERS R. W., ROSEBURY T. Aerobic and anaerobic bacterial counts of nasal washings: presence of organisms resembling Corynebacterium acnes. J Bacteriol. 1962 Jan;83:144–148. doi: 10.1128/jb.83.1.144-148.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther B., Brofeldt S., Grønborg H., Mygind N., Pedersen M., Vejlsgaard R. Study of bacteria in the nasal cavity and nasopharynx during naturally acquired common colds. Acta Otolaryngol. 1984 Sep-Oct;98(3-4):315–320. doi: 10.3109/00016488409107569. [DOI] [PubMed] [Google Scholar]
- Ylikoski J., Savolainen S., Jousimies-Somer H. Bacterial flora in the nasopharynx and nasal cavity of healthy young men. ORL J Otorhinolaryngol Relat Spec. 1989;51(1):50–55. doi: 10.1159/000276031. [DOI] [PubMed] [Google Scholar]



