Abstract
Purification of lymphocytes, particularly T cells, is commonly performed using nylon wool. This enrichment method selectively retains B cells and some myeloid cells allowing a significantly more pure T cell population to flow through a nylon wool column. T cells purified in this fashion are assumed to be unaltered and functionally naïve, however some studies have suggested aberrant in vitro T cell responses after nylon wool treatment. We found that nylon wool purification significantly altered T cell proliferation, expression of activation markers and production of cytokines. Our results suggest that nylon wool treatment modifies T cell activation responses and that caution should be used when choosing this purification method.
Keywords: Nylon wool, T cells, Lymphocyte activation, Cytokines
1. Introduction
Adherence properties of immune cells such as T cells, B cells and antigen presenting cells (APCs) vary widely and can be used to enrich for cellular subsets. Nylon wool purification is a long established cell separation method, although the mechanistic basis for differential cellular retention on nylon wool is unclear (Eisen et al., 1972; Julius et al., 1973). This method has been widely used due to its convenience and cost effectiveness to purify a variety of leukocyte subsets including T cells, B cells and even stem cells (Wong and Mittal, 1981; Gunzer et al., 2001; Kwekkeboom et al., 1998). Despite its widespread use, there are reports suggesting alterations in T cell function after nylon wool enrichment. These alterations include changes in proliferative capacity (Gunzer et al., 2001; Hrabak et al., 1985), and changes in lymphocyte membrane composition (Corrigan et al., 1979). In the course of studies examining the role of complement receptors on T cells, we found that nylon wool purification of T cells altered T cell responses in a variety of assays. These studies led us to examine the effects of nylon wool on T cell functions. We found that nylon wool enrichment markedly altered T cell proliferation, activation marker expression, and cytokine production. Our studies suggest that caution is required when using nylon wool-purified T cells due to the potential for atypical activation responses.
2. Materials and Methods
2.1 Mice
C57Bl/6 mice were used for all experiments. All procedures were performed with approval from the UAB Institutional Animal Care and Use Committee.
2.2 Leukocyte Isolation and stimulation
Scrubbed nylon fibers (Zeptometrix Corporation) were put into a 10ml syringe and then autoclaved for sterility. Before use, the columns were equilibrated by washing with 20ml RPMI tissue culture media, sealed, and incubated for 30 minutes at 37°C and 5% CO2. Spleens and lymph nodes were removed, ground through a 70μm cell strainer, washed with Hanks balanced salt solution and red blood lysed (Sigma RBC lysis buffer). Cells (1.5 × 108) subjected to nylon wool purification were resuspended in 2 ml of warm RPMI, loaded onto the column, and washed with 2 ml warm RPMI. The column was sealed and incubated at 37°C and 5% CO2 for 45 minutes. Cells were then eluted with 10 ml warm RPMI. In some experiments B cells were removed from spleen and lymph node cell preparations by magnetic bead separation using MACS anti-B220 magnetic beads according to the manufacturers instructions (Miltenyi Biotec). Greater than 99% of B cells were removed by this procedure as assessed by flow cytometry. Flat-bottom 96 well plates were coated with anti-CD3e antibody (145-2C11, eBiosciences, 1μg/ml) overnight at 4°C. Cells were plated at a concentration of 1×106/ml and incubated at 37°C and 5% CO2 for 72 hours. Proliferation, cytokine production, and activation marker expression were then analyzed.
2.3 Flow Cytometry
Samples of cell preparations before and after nylon wool purification were characterized by flow cytometry using anti-CD3e (FITC 145-2C11), γδ TCR (FITC GL3), CD11b (PE M1/70), CD11c (APC HL3), CD19 (PE MB19-1), Pan-NK cells (APC DX5, all from eBiosciences). In other experiments, cells were stimulated with anti-CD3 for 72 hours and then stained with CD4 FITC (GK1.5), CD25 PE (PC61.5), CD69 Biotin (H1.2F3), and CD8 APC (53-6.7, all from eBioscience). Biotin antibodies were visualized with streptavidin-PerCP (BD Pharmingen). Stained cells were run on a FACSCalibur and data was analyzed with CellQuest software (BD biosciences).
2.4 Proliferation
Cell Titer 96 AQueous One Solution Cell Proliferation Assay (Promega) was used to measure cell proliferation. This assay is a colorimetric method used to determine the number of viable cells in culture. All assays were performed according to manufacturer’s instructions. Briefly, after 72 hours of anti-CD3 stimulation, 40 μl of MTS was added to cultures (10μl/100μl media). Cultures were then incubated for 3 hours and absorbance was measured at 450nm.
2.5 Cytokine Production
Cytokine production was analyzed in culture supernatants from cells (1 × 106/well for untreated and nylon wool-treated) stimulated for 72 hours with anti-CD3 antibody. Enzyme-linked immunosorbent assay (ELISA) kits were used to measure IFN-γ (eBioscience) IL-2 and IL-4 (R&D systems). All assays were performed according to manufacturer’s instructions.
2.6 Statistics
Cell proliferation, ELISA analysis and flow cytometric analysis of untreated and nylon wool-treated samples were analyzed by student’s unpaired. A p value less than 0.05 was considered significant.
3. Results
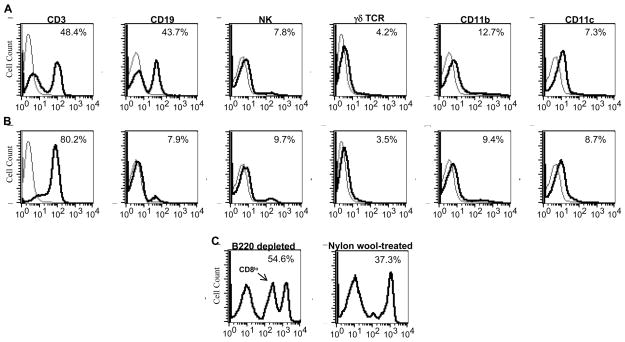
We first determined how nylon wool treatment altered leukocyte subpopulation purification. For these experiments we performed flow cytometry on spleen and lymph node cell preparations before and after nylon wool purification examining for changes in T cell (CD3), γδ T cell, B cell (CD19), NK cell, macrophages (CD11b) and dendritic cell (CD11c) populations. As shown in figure 1, nylon wool removed most B cells (>80%), enhancing the T cell purity of the samples, but had little effect on the purity of any other leukocyte subpopulation examined. We also depleted B cells using B220 magnetic beads in an effort to determine if the effects we observed on nylon wool treatment were due to interaction with the nylon wool or to the loss of B cell interactions. Flow cytometric analysis of the B220-depleted cell population revealed distinct differences in leukocyte subpopulations compared to nylon wool treated cells. For example, B cells numbers in the B220-depleted preparations were as expected markedly lower than in nylon wool-treated preparations. Furthermore, we observed the selective loss of CD8lo T cells on treatment with nylon wool not seen on B220 depletion (Fig 1C). These data indicate that nylon wool treatment produces subtle alterations in leukocyte populations and, depletion of B cells alone may not account for the functional differences seen between untreated and nylon wool-treated leukocytes.
Figure 1.
Nylon wool purification enriches T cells through retention of B cells. Cells from lymph nodes of C57Bl/6 mice were isolated and either untreated (A) or purified using nylon wool columns (B) as described in materials and methods and stained for FACS analysis of T cell, B cell, NK cell, macrophage, and dendritic cell populations. C, Lymph node cells were purified using B220 magnetic beads or nylon wool and stained for FACS analysis of CD8 cells. Bold line represents cell type specific staining, thin line represents negative control. Data shown is from one representative experiment of three.
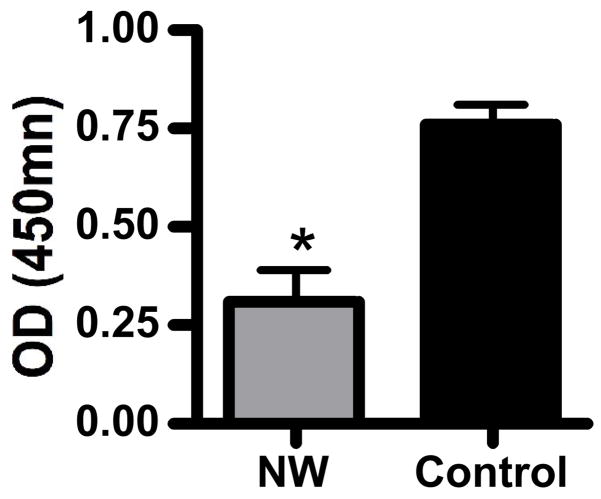
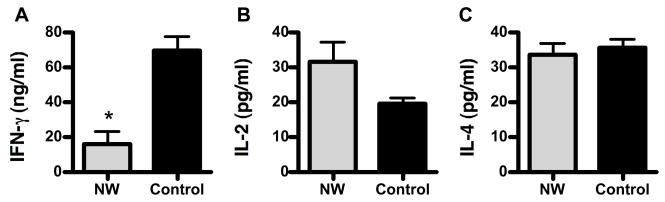
To assess the effects of nylon wool purification on T cell activation, we first measured proliferation after cross-linking the T cell receptor. Primary leukocytes from C57Bl/6 mice were either activated directly ex vivo with immobilized anti-CD3 antibody or after nylon wool purification. Stimulated cells were cultured for 72 hours and proliferation was measured using the MTS assay. Proliferation of cells purified with nylon wool was significantly reduced (p<0.05, student’s t-test) compared to that of control cells (Figure 2). To further characterize the activation deficit in nylon wool-treated T cells, we examined for activation marker expression (CD25 and CD69) on cultured T cells. After 72 hours of anti-CD3 stimulation cells were harvested and stained for FACS analysis. Nylon wool-treated T cells showed a trend towards increased CD25 and decreased CD69 expression compared to control T cells, however these differences were not statistically significant (Table 1). We also found that cytokine production was altered by the purification of T cells with nylon wool. For these studies, we measured the production of IFN-γ, IL-2, and IL-4 in the supernatants of control and nylon wool purified cells stimulated with anti-CD3 for 72 hours. Nylon wool purified cells produced significantly less IFN-γ (p<0.05) and elevated levels of IL-2 compared to that of control cells (Figure 3, panels A and B respectively). Production of IL-4 was not altered by nylon wool purification (Figure 3C).
Figure 2.
Proliferation of leukocytes is reduced following nylon wool purification. Cells from lymph nodes of C57Bl/6 mice were isolated and either untreated or purified using nylon wool columns as described in materials and methods. Cells were then stimulated with immobilized anti-CD3 antibodies for 72 hours. Proliferation was assessed using an MTS assay system as described in materials and methods. Results shown are mean ± SE for three independent experiments. *, p<0.05, student’s t-test.
Table 1.
Activation marker expression on untreated and nylon wool-treated T cells
| CD4+CD25+CD69− | CD4+CD25+CD69+ | CD8+CD25+CD69− | CD8+CD25+CD69+ | |
|---|---|---|---|---|
| Untreated | 13.1 +/− 8.3% | 84.5 +/− 8.2% | 7.6 +/− 5.1% | 89 +/− 4.3% |
| Nylon wool | 29.9 +/− 2.5% | 64 +/− 3.5% | 22.2 +/− 3% | 77 +/− 3.5% |
Figure 3.
T cell cytokine production is altered by nylon wool purification. Lymph node cells were isolated and either untreated or purified using nylon wool as described in materials and methods. Cells were then stimulated with immobilized anti-CD3 antibodies for 72 hours. Production of IFN-γ (A) IL-2 (B), and IL-4 (C) was measured from supernatants using ELISAs. Results shown are mean ± SE for three independent experiments. *, p<0.05, student’s t-test.
4. Discussion
Nylon wool enrichment is a widely used method to purify T cells. T cell enrichment by this method relies on preferential B cell and some APC adherence to nylon wool and provides only modest T cell purification (2-fold or less in our hands). Within a few years of the introduction of this purification method, several reports suggested adverse effects on T cell functions when using nylon wool (Corrigan et al., 1979; Tardieu et al., 1975). Chief among these effects was reduced T cell proliferation. The earliest report to note this effect found that T cells passed through nylon wool columns failed to respond to ConA stimulation (Tardieu et al., 1975). Subsequently it was shown that treatment of nylon wool-enriched T cells with anti-CD3 and anti-CD28 antibodies resulted in an approximately five-fold reduction in proliferation compared to red blood cell-depleted T cell preparations (Gunzer et al., 2001). Surprisingly, these results were interpreted as “pronounced proliferation responses”. In our hands, nylon wool-purified T cells treated with either anti-CD3 or the combination of anti-CD3 and anti-CD28 antibodies (data not shown) proliferated poorly compared to untreated cells. Furthermore, expression of CD69, an early T cell activation marker, was reduced and CD25 expression was markedly increased on both CD4+ and CD8+ T cells purified by nylon wool. It is interesting to note that nylon wool treatment strikingly alters lymphocyte membranes depleting phospholipids, cholesterol and sialic acid (Corrigan et al., 1979). These results raise the possibility that the unusual T cell activation profile we observed on nylon wool treatment may be due to changes in the membrane composition of lipid rafts where T cell activation components are concentrated (Manes and Viola, 2006).
We also observed for the first time an altered production of cytokines in nylon wool-treated T cells compared to untreated T cells. In these studies, IFN-γ production was significantly reduced on nylon wool treatment (over 4-fold), a finding consistent with the altered CD25/CD69 activation profile (Table 1). In addition, we noted the loss of a CD8+ subset in nylon wool treated preparations (Fig. 1C). The loss of these cells may also contribute to the observed reduction in IFN-γ as well. In contrast, IL-2 production was elevated and this may contribute to the elevated expression of CD25 we observed on nylon wool-enriched T cells. Interestingly, IL-4 production was unaffected in nylon wool-enriched T cells. The apparently normal level of IL-4 production by these cells may help explain why nylon wool-enriched T cells, when combined with B cells, provide sufficient help for antibody production (Trizio and Cudkowicz, 1974; Indiveri et al., 1980). We also observed unusual cytokine production profiles on cross-linking of complement receptors after nylon wool-purification of T cells, including reduced IFN-γ but elevated TGF-β levels (data not shown). Taken together, our results indicate that T cells purified using nylon wool may not be suitable for activation studies and that common readout parameters such as activation marker expression and cytokine production do not mimic in vivo activation events. Our study indicates that nylon wool enrichment is not a benign purification technique and has unexpected effects on early T cell activation events. These effects need to be considered before using this method to enrich for T cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Corrigan A, O’Kennedy R, Smyth H. Lymphocyte membrane alterations caused by nylon wool column separation. J Immunol Methods. 1979;31:177–82. doi: 10.1016/0022-1759(79)90298-9. [DOI] [PubMed] [Google Scholar]
- Eisen SA, Wedner HJ, Parker CW. Isolation of pure human peripheral blood T-lymphocytes using nylon wool columns. Immunol Commun. 1972;1:571–7. doi: 10.3109/08820137209022965. [DOI] [PubMed] [Google Scholar]
- Gunzer M, Weishaupt C, Planelles L, Grabbe S. Two-step negative enrichment of CD4+ and CD8+ T cells from murine spleen via nylon wool adherence and an optimized antibody cocktail. J Immunol Methods. 2001;258:55–63. doi: 10.1016/s0022-1759(01)00466-5. [DOI] [PubMed] [Google Scholar]
- Hrabak A, Szabo MT, Antoni F. Characteristic biochemical differences in human T and B lymphocytes separated on nylon wool. Int J Biochem. 1985;17:113–7. doi: 10.1016/0020-711x(85)90094-1. [DOI] [PubMed] [Google Scholar]
- Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–9. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kwekkeboom J, Buurman DE, Ploemacher RE, Baars JW, Loos HA, Slaper-Cortenbach IC. A nylon wool filter coated with human immunoglobulin for rapid depletion of monocytes and myeloid cells from peripheral blood stem cell transplants. Exp Hematol. 1998;26:400–8. [PubMed] [Google Scholar]
- Manes S, Viola A. Lipid rafts in lymphocyte activation and migration. Mol Membr Biol. 2006;23:59–69. doi: 10.1080/09687860500430069. [DOI] [PubMed] [Google Scholar]
- Tardieu M, Crchova V, Daguillard F. Immunological activities of rat lymphocytes. III. --Isolation of differing subpopulations of lymphocytes by two techniques of T cell separation. Ann Immunol (Paris) 1975;126:281–9. [PubMed] [Google Scholar]
- Trizio D, Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974;113:1093–7. [PubMed] [Google Scholar]
- Wong DW, Mittal KK. HLA-DR typing: a comparison between nylon wool adherence and T cell rosetting in the isolation of B cells. J Immunol Methods. 1981;46:177–86. doi: 10.1016/0022-1759(81)90134-4. [DOI] [PubMed] [Google Scholar]