SUMMARY
Peptidoglycan recognition proteins (PGRPs) are structurally conserved from insects to mammals. Insect PGRPs have many host defense functions, whereas mammalian PGRPs only have bactericidal and amidase activities. We asked whether mammalian PGRPs have immunomodulating activities in peptidoglycan-induced arthritis and whether they interact with other innate immunity receptors. We demonstrate that PGLYRP-2 and Nod2 are both required for induction of arthritis by peptidoglycan. The sequence of events in peptidoglycan-induced arthritis is activation of Nod2, local expression of PGLYRP-2, chemokine production, and recruitment of neutrophils into the limbs, which induces acute arthritis. This proinflammatory function is unique for PGLYRP-2 and is not exhibited by other PGRPs, of which one (PGLYRP-1) is anti-inflammatory. TLR4 and MyD88 are required for maturation of neutrophils before peptidoglycan challenge. Our results reveal new in vivo functions of PGRPs, Nod2, and TLR4, and demonstrate in vivo interdependence of these three families of pattern recognition molecules in local inflammation.
INTRODUCTION
Innate immunity is crucial for defense against infections, for triggering acquired immune responses, and for maintaining a balance between efficient and prompt protective responses to microbial infections and tolerance to constant exposure to normal flora and environmental microorganisms. Inappropriate deployment of immune effectors may result in pathology, such as septic shock or inflammatory or autoimmune diseases.
Peptidoglycan is an essential and unique component of the cell walls of virtually all bacteria. Because peptidoglycan is not found in eukaryotic cells, it is an excellent target for recognition by the innate immune system. Peptidoglycan is recognized by several mammalian innate immunity proteins, including members of the Nod-like receptors (NLRs), Peptidoglycan Recognition Proteins (PGRPs or PGLYRPs), CD14, Toll-like receptor 2 (TLR2), mannose binding lectin, RegIIIg C-type lectin, and lysozyme (Royet and Dziarski, 2007).
NLRs are intracellular pattern recognition receptors that recognize peptidoglycan fragments, either meso-diaminopimelic acid-containing peptides found in Gram-negative bacteria and Gram-positive bacilli (Nod1) (Chamaillard et al., 2003; Girardin et al., 2003a), or muramyl dipeptide (MDP) found in virtually all bacteria (Nod2, NALP1, and NALP3) (Inohara et al., 2003; Girardin et al., 2003b; Faustin et al., 2007; Martinon et al., 2004). Mutations in Nod2 are associated with Crohn’s disease, an intestinal inflammatory disease likely triggered by bacteria that can be accompanied by arthritis (Hugot et al., 2001; Ogura et al., 2001), and Blau syndrome, a disorder characterized by granulomatous arthritis (Miceli-Richard et al., 2001). However, the role of Nod2 in the development of arthritis is not known.
PGRPs are innate immunity proteins that are conserved from insects to mammals, recognize bacterial peptidoglycan, and function in antibacterial immunity. Insects have many PGRPs with diverse functions, such as activation of Toll or IMD signal transduction pathways (which result in induction of antimicrobial peptides), activation of the prophenoloxidase cascade (which also generates antimicrobial products), induction of phagocytosis, hydrolysis of peptidoglycan, and down-regulation of immune responses (Royet and Dziarski, 2007).
Mammals have four PGRPs, PGLYRP-1, -2, -3, and -4 (that were initially named PGRP-S, -L, -Iα, and -Iβ, respectively) (Kang et al., 1998; Liu et al., 2001). In contrast to insects, there are only two known functions of mammalian PGRPs: PGLYRP-1 (found in PMN’s granules) and PGLYRP-3 and PGLYRP-4 (found in the skin, eyes, salivary glands, throat, tongue, esophagus, stomach, and intestine) are directly bactericidal (Lu et al., 2006; Wang et al., 2007), whereas PGLYRP-2 (which is constitutively expressed in the liver and secreted into blood) is an N-acetylmuramoyl-L-alanine amidase that hydrolyzes peptidoglycan (Gelius et al., 2003; Wang et al., 2003).
Because insect PGRPs with peptidoglycan-hydrolytic activity have anti-inflammatory properties and protect insects from excessive inflammation induced by pro-inflammatory peptidoglycan (Mellroth et al., 2003; Zaidman-Remy et al., 2006; Bischoff et al., 2006), we set out to test the hypothesis whether amidase-active mammalian PGLYRP-2 plays a similar anti-inflammatory role in vivo. To test this hypothesis we selected peptidoglycan-induced arthritis model.
Arthritis is a frequent sequela of bacterial infections, associated with streptococcal rheumatic fever, gastrointestinal infections, urinary tract infections, gonorrhea, bacterial overgrowth in intestinal bypass surgery, inflammatory bowel disease, and Lyme disease. The pathogenesis of these arthritic diseases is still poorly understood, but their unifying feature is the absence of local infection of the joints with bacteria. Thus, this arthritis is likely caused by pro-inflammatory bacterial components deposited in the joints and can be reproduced in a rat model of chronic relapsing arthritis, in which rats are given one systemic injection of bacterial peptidoglycan-polysaccharide complex, which results in initial acute inflammation of the joints, followed by several bouts of chronic, erosive polyarthritis (Cromartie et al., 1977; Lehman et al., 1983). The active component of bacterial cell wall that induces this arthritis is peptidoglycan (Fox et al., 1982; Stimpson et al., 1987), and the severity of arthritis correlates with the persistence of peptidoglycan in the joints (Dalldorf et al., 1980; Eisenberg et al., 1982). Peptidoglycan is also arthritogenic in other animal models of arthritis, such as adjuvant arthritis in rats (Kohashi et al., 1976; Chang et al., 1981), intraperitoneal or intradermal injection model in rats (Speer et al., 1987; Simelyte et al., 2003), intraarticular injection model in mice (Joosten et al., 2003), and intravenous injection model in mice (Koga et al., 1985; Onta et al., 1993). We selected the latter model because its acute phase is likely based on peptidoglycan-induced innate immune response and the role of PGRPs and NLRs in its pathogenesis is unknown.
If the in vivo function of mammalian PGLYRP-2 were similar to the anti-inflammatory properties of amidase-active insect PGRPs, one would expect enhancement of peptidoglycan-induced arthritis in PGLYRP-2-/- mice. Surprisingly, we discovered the opposite effect, i.e., PGLYRP-2 was required for the induction of peptidoglycan-induced arthritis, because PGLYRP-2-/- mice were resistant to peptidoglycan-induced arthritis. Because there are multiple peptidoglycan-recognition mechanisms and because proteins function in vivo in highly interactive modules, we also asked whether PGRPs interact with other innate immunity receptors, such as NLRs and TLRs. We demonstrate that peptidoglycan activates Nod2, which induces local PGLYRP-2 expression. PGLYRP-2 then enhances local production of chemokines, which induce local inflammation. This function is unique for PGLYRP-2 and is not exhibited by other PGLYRPs. TLR4 works independently of peptidoglycan and is required for maturation of neutrophils, which are the main effectors of arthritis and local inflammation. These results reveal new in vivo functions of PGLYRP-2, Nod2, and TLR4, and define several steps in the pathogenesis of peptidoglycan-induced arthritis.
RESULTS
PGLYRP-2 is required for the development of peptidoglycan- or MDP-induced arthritis
Following intravenous injection of Staphylococcus aureus peptidoglycan, wild-type (WT) BALB/c mice developed acute transient arthritis that was most evident in the hind legs and had a gross appearance of swelling and redness of primarily the ankle joint, and in more severe cases of the digits and the entire foot (Figure 1A). The inflammation peaked on day 3 and gradually subsided by the 8th to 12th day post-injection (Figure 1B). The incidence of arthritis in WT mice was ~60% (Figure 1B). Transient acute arthritis also developed in WT mice following intravenous injection of MDP, a synthetic peptidoglycan fragment (Figure 1A,B). The MDP-induced arthritis also primarily involved the ankle joint (Figure 1A) and its incidence was ~80-90%, but it was short-lived – the inflammation peaked on day 1 and quickly subsided (Figure 1B).
Figure 1. PGLYRP-2 and Nod2 are required for the development of peptidoglycan- or MDP-induced acute arthritis.
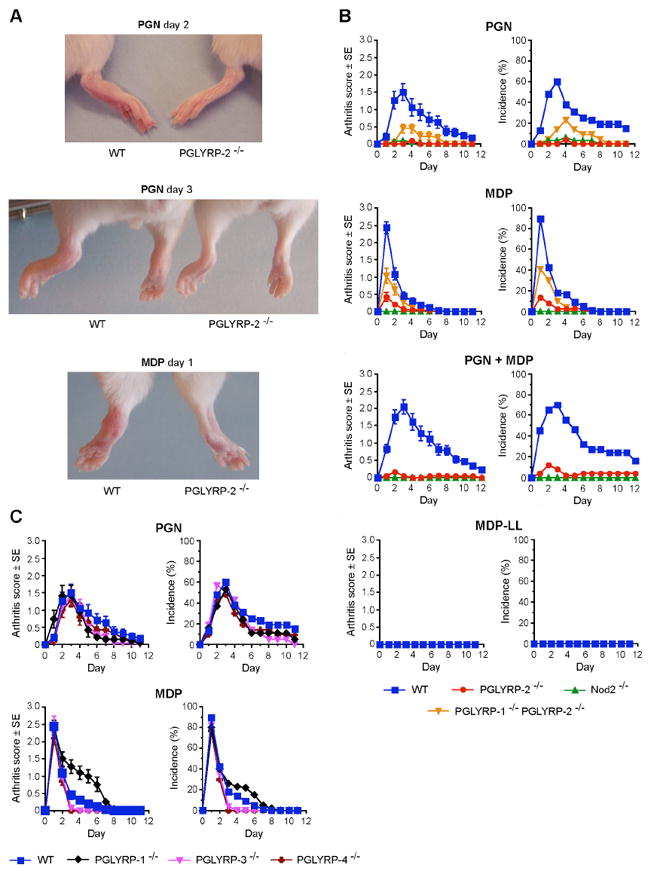
(A) Swelling and erythema of ankles and feet in WT, but not in PGLYRP-2-/- mice after intravenous injection of 200 μg of peptidoglycan (PGN) or 100 μg of MDP. (B) and (C) Arthritis scores and incidence in WT or KO mice after intravenous injection of 200 μg of PGN, 100 μg of MDP, or both PGN and MDP; means ± SE of 19-76 mice/group. Arthritis scores and incidence were significantly higher in: PGN-injected, MDP-injected, and PGN+MDP-injected WT than PGLYRP-2-/- mice and Nod2-/- mice; in PGN-injected and MDP-injected WT than PGLYRP-1-/-PGLYRP-2-/- mice; and also in PGN-injected and MDP-injected PGLYRP-1-/-PGLYRP-2-/- than PGLYRP-2-/- mice (B). Arthritis scores and incidence were not significantly different in PGN-injected or MDP-injected WT than PGLYRP-1-/-, PGLYRP-3-/-, and PGLYRP-4-/- mice, except for PGLYRP-1-/- mice, which after MDP injection had significantly higher scores than WT mice (C). The P values for the significance of all the above differences are listed in Supplemental Data.
By contrast, PGLYRP-2-/- mice were completely resistant to the development of arthritis following injection of peptidoglycan or MDP, or co-administration of peptidoglycan and MDP (Figure 1A,B). Unlike PGLYRP-2-/- mice, PGLYRP-1-/-, PGLYRP-3-/-, and PGLYRP-4-/- mice all developed peptidoglycan- and MDP-induced arthritis of similar severity and incidence as WT mice (Figure 1C). However, MDP-induced arthritis in PGLYRP-1-/- mice lasted significantly longer than in WT mice. These results demonstrate that the requirement for PGLYRP-2 for the development of peptidoglycan- and MDP-induced acute arthritis is unique among the four mammalian PGLYRPs.
Histologically, peptidoglycan-induced inflammation in WT mice was characterized by prominent edema and cellular infiltrations of tendons, tendon sheaths, synovial membrane, subsynovial tissue, synovial space, and connective and subcutaneous tissues (Figure 2A). Polymorphonuclear leukocytes (PMNs) were the predominant cells, but mononuclear cells and proliferating tissue fibroblasts were also present. These changes are characteristic of acute tendonitis and arthritis and they were also observed in WT mice co-injected with peptidoglycan and MDP (Figure 2B). By contrast, these changes were not observed in the limbs in peptidoglycan- or peptidoglycan+MPD-injected PGLYRP-2-/- mice, whose feet appeared normal (Figure 2). MDP-injected WT mice had less severe cellular infiltrates, but numerous inflammatory cells (primarily PMNs) were still seen in synovial and sub-synovial tissues, tendon sheaths and other surrounding tissues. Such infiltrates were not observed in MPD-injected PGLYRP-2-/- mice, whose feet appeared normal (Supplemental Figure S1A). These results demonstrate that PGLYRP-2 is required for the development of pathologic changes in peptidoglycan- and MDP-induced acute tendonitis and arthritis.
Figure 2. PGLYRP-2 is required for the development of peptidoglycan- or peptidoglycan+MDP-induced pathologic changes characteristic of acute arthritis.
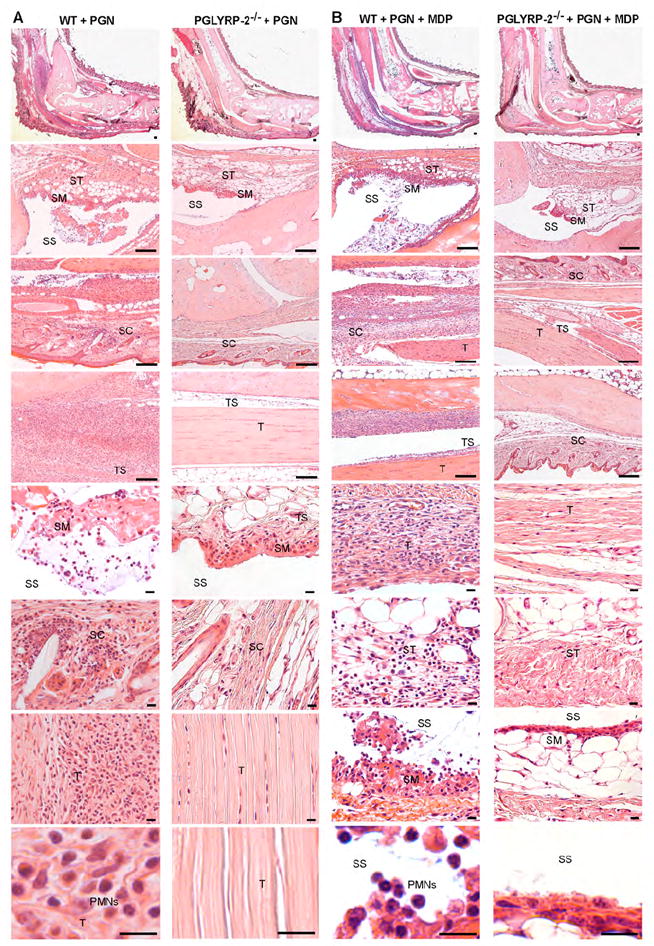
Edema and cellular infiltrations of tendons (T), tendon sheaths (TS), synovial membrane (SM), subsynovial tissue (ST), synovial space (SS), and connective and subcutaneous tissues (SC) in ankles and feet in WT, but not in PGLYRP-2-/- mice, 3 days after intravenous injection of 200 μg of PGN (A) or 200 μg of PGN + 100 μg of MDP (B). PMNs were the predominant infiltrating cells in WT mice (bottom left panels in A and B). Ankles and feet in injected PGLYRP-2-/- mice appeared normal. Bar = 100 μm in four top panels and 10 μm in four bottom panels.
Nod2 is required for the development of peptidoglycan- or MDP-induced arthritis
We next tested whether Nod2 was required for the development of peptidoglycan- and MDP-induced arthritis, because this arthritis was induced by both peptidoglycan and MDP and Nod2 is a well-characterized receptor for MDP, which is the minimum active component of all peptidoglycans (Inohara et al., 2003; Girardin et al., 2003b). In contrast to WT mice, Nod2-/- mice were completely resistant to the development of arthritis following injection of not only MDP, but also peptidoglycan or a mixture of peptidoglycan and MDP (Figure 1B). Because Nod2 is specific for MDP containing L-Ala-D-isoGlu and does not respond to MurNAc-L-Ala-L-isoGlu (MDP-LL), we further confirmed the requirement for Nod2 by demonstrating that MDP-LL was completely inactive and did not induce arthritis in WT mice (Figure 1B). These results demonstrate that Nod2 is required for the development of both peptidoglycan- and MDP-induced arthritis.
PGLYRP-1 opposes the effect of PGLYRP-2 on the development of peptidoglycan- or MDP-induced arthritis
Because MDP-induced arthritis in PGLYRP-1-/- mice lasted significantly longer than in WT mice (Figure 1C), we then tested the possibility that PGLYRP-1 may have an opposite effect to PGLYRP-2 on the development of arthritis using double KO PGLYRP-1-/-PGLYRP-2-/- mice. These mice, following injection of peptidoglycan or MDP, developed arthritis of significantly higher severity and incidence than PGLYRP-2-/- mice, although of significantly lower severity and incidence than peptidoglycan- or MDP-induced arthritis in WT mice (Figure 1B). These results suggest that PGLYRP-1 and PGLYRP-2 may have opposing anti-inflammatory and pro-inflammatory effects, respectively.
MDP strongly induces chemokine and cytokine mRNA in the feet in WT, PGLYRP-1-/-, PGLYRP-3-/-, and PGLYRP-4-/- mice, and but not in PGLYRP-2-/- and Nod2-/- mice
To further define the mechanism of peptidoglycan-induced arthritis, we focused on MDP, because MDP is the smallest synthetic peptidoglycan fragment that induces arthritis and because there is only one class of receptors for MDP (NLRs). Because the arthritic lesions were characterized by acute inflammation and infiltration with PMNs, we next determined whether intravenous injection of MDP induced local production of chemokines and other pro-inflammatory mediators in the foot.
In WT mice, intravenous injection of MDP significantly increased expression of 43 pro-inflammatory genes in the foot out of a total of 84 genes studied, as determined by quantitative real-time PCR (qPCR) of mRNA; 29 of these genes were induced 2 to 20 fold (Figure 3 and Supplemental Table S1). By contrast, intravenous injection of MDP into PGLYRP-2-/- mice significantly increased expression of only 19 out of 84 genes in the foot, 11 of which were induced 2 to 6 fold (Figure 3 and Supplemental Table S1). Overall, 34 out 84 of genes were induced significantly higher in WT than in PGLYRP-2-/- mice, 17 of these genes were induced 2 to 9 fold higher in WT than in PGLYRP-2-/- mice. These genes were primarily chemokines (fold induction in WT/fold induction in PGLYRP-2-/- mice): Ccl7 (MCP-3, 8.9), Ccl2 (MCP-1, 7.3), CCL12 (MCP-5, 6.1), Cxcl1 (KC, 2.9), Cxcl5 (ENA-78, 2.8), Ccl5 (RANTES, 2.7), Cxcl9 (Mig, 2.7), Ccl17 (2.6), Ccl4 (MIP-1β, 2.4), Ccl19 (MIP-3β, 2.4), Ccl20 (MIP-3α, 2.3); chemokine receptors: Ccr5 (2.5) and Ccr4 (2.3); and cytokines and other pro-inflammatory molecules: IL-20 (3.6), IL-1β (3.2), C-reactive protein (2.5), TNF-α (2.1), and IL-6 (5.8, not included in the array). These differences in induction of genes in WT and PGLYRP-2-/- mice were not due to the differences in the basal expression of all tested genes between WT and PGLYRP-2-/- mice, which in general were similar (differed less than 2-fold, Supplemental Figure S2 and Table S4).
Figure 3. MDP strongly induces chemokine and cytokine mRNA in the feet in WT, PGLYRP-1-/-, PGLYRP-3-/-, and PGLYRP-4-/-, but not in PGLYRP-2-/- and Nod2-/- mice.
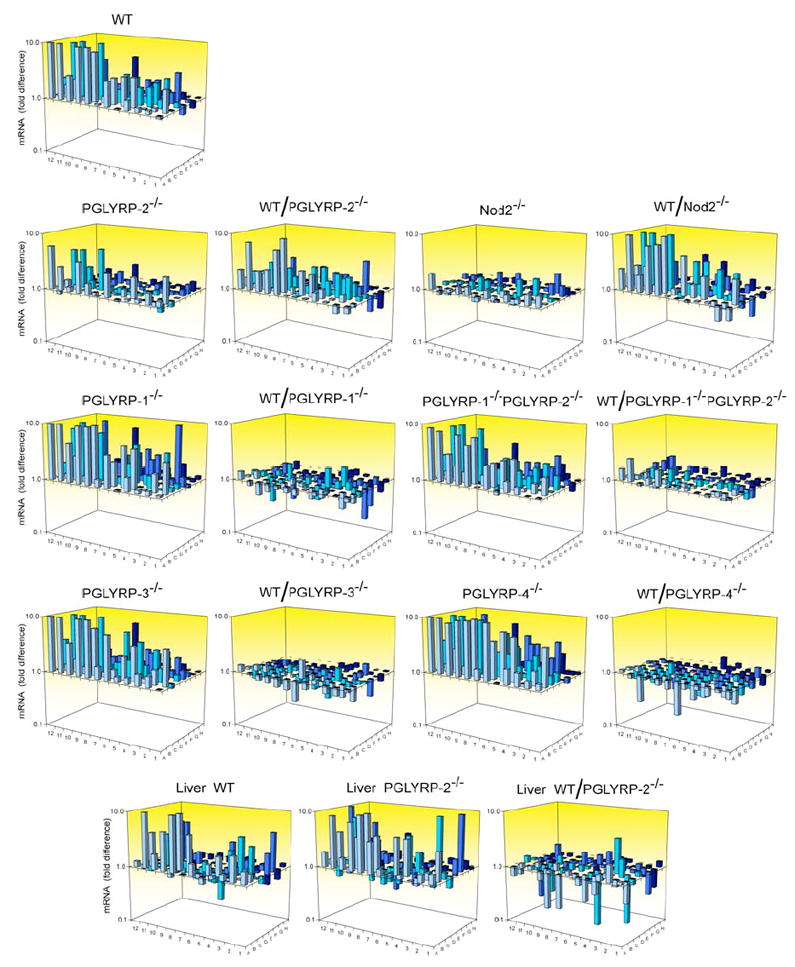
WT or KO mice were injected intravenously with 100 μg MDP or buffer, and after 6 hrs the amounts of mRNA for 84 pro-inflammatory genes and 5 housekeeping genes were measured in feet (or livers, bottom panels) by qPCR and expressed as the ratios of the amount of mRNA in MDP-injected to buffer-injected mice (which represents fold gene induction by MDP), or as the ratios of fold MDP-induced gene activation in WT to KO mice (which represents fold difference in the response to MDP in WT versus KO mice, WT/KO). The results are means of 12 WT or 6 KO mice/group (12 or 6 separate qPCR assays). The means, SE, the significance of differences, and the names of the genes are given in Supplemental Tables S1-3.
These results demonstrate that PGLYRP-2 is required for the full stimulation of expression of chemokines and other pro-inflammatory mediators genes by MDP in the foot. This low induction of pro-inflammatory genes in the foot correlates with the inability of MDP to induce arthritis in PGLYRP-2-/- mice.
By contrast, gene activation in the feet of PGLYRP-1-/-, PGLYRP-3-/-, and PGLYRP-4-/- mice after systemic injection of MDP was generally higher than in WT mice (Figure 3 and Supplemental Table S2). Baseline levels of gene expression in untreated PGLYRP-1-/-, PGLYRP-3-/-, and PGLYRP-4-/- mice were also usually somewhat higher than in WT mice (Supplemental Figure S2 and Table S4). These data suggest that in contrast to PGLYRP-2, which has a pro-inflammatory effect, other PGLYRPs may have opposing anti-inflammatory effects.
We next tested whether Nod2 was the MDP receptor required for the induction of pro-inflammatory genes in the feet of MDP-injected mice. Intravenous injection of MDP into Nod2-/- mice did not significantly increase the expression of pro-inflammatory genes in their feet (except for one out of 84 genes). Thus, 32 out of 84 genes were induced significantly higher in WT than in Nod2-/- mice, 22 of which were induced 2 to 23 fold higher in WT than in Nod2-/- mice (Figure 3 and Supplemental Table S1). These differences in induction of genes in WT and Nod2-/- mice were not due to the differences in the basal expression of all tested genes between WT and Nod2-/- mice, which in general were similar (Supplemental Figure S2 and Table S4). These data demonstrate that Nod2 is required for MDP-induced stimulation of expression of chemokines and other pro-inflammatory genes in the foot and confirm that Nod2 is the main MDP receptor in the foot. This lack of induction of pro-inflammatory genes in the foot of Nod2-/- mice is likely responsible for the inability of MDP and peptidoglycan to induce arthritis in Nod2-/- mice.
PGLYRP-1 opposes the effect of PGLYRP-2 in MDP-induced activation of pro-inflammatory genes
Because PGLYRP-1 opposed the effect of PGLYRP-2 on the development of arthritis (Figure 1B,C), we next tested whether PGLYRP-1 also opposes the effect of PGLYRP-2 on induction of chemokines and other pro-inflammatory genes in the foot. Double KO PGLYRP-1-/- PGLYRP-2-/- mice had significantly higher induction of pro-inflammatory genes than PGLYRP-2-/- mice (Figure 3 and Supplemental Table S3), which is consistent with the hypothesis that PGLYRP-2 has pro-inflammatory and PGLYRP-1 has anti-inflammatory effects.
MDP induces similar chemokine and cytokine mRNA levels in the liver in WT and PGLYRP-2-/- mice
To determine whether this defect in pro-inflammatory gene induction in response to systemic injection of MDP in PGLYRP-2-/- mice was local (in the foot) or general in all tissues, we also measured changes in the expression of the same genes in the same mice in the liver. Intravenous injection of MDP induced similar or higher responses in the livers of PGLYRP-2-/- than WT mice, and several genes were induced significantly higher in PGLYRP-2-/- than in WT mice (Figure 3 and Supplemental Table S3). The basal expression of all 84 genes was similar in the liver in WT and PGLYRP-2 KO mice (Supplemental Figure S2 and Table S4). These results demonstrate that, in contrast to the foot, in the liver PGLYRP-2 is not required for full MDP-induced stimulation of expression of chemokines and other pro-inflammatory mediators genes.
MDP and peptidoglycan induce higher chemokine levels in blood in WT than in PGLYRP-2-/- mice
We next tested whether MDP and peptidoglycan induced higher chemokine levels in blood in WT than in PGLYRP-2-/- mice. We selected three chemokines (CCL-2, CCL-12, and CXCL-1), which are induced in various tissues and whose mRNA was induced significantly higher in the feet in WT than in PGLYRP-2-/- mice, and one cytokine (IL-6), which is highly induced in the liver. Peptidoglycan and MDP (injected intravenously) induced substantial increases in the levels of all three chemokines in blood in WT mice and significantly lower levels of these chemokines in PGLYRP-2-/- mice (Figure 4). Peptidoglycan also induced high level of IL-6, which, however, was similar in WT and PGLYRP-2-/-. MDP did not induce a significant increase in IL-6. These results demonstrate lower production of chemokines in the blood in PGLYRP-2-/- than WT mice following in vivo challenge with MDP or peptidoglycan, further supporting the hypothesis that PGLYRP-2 plays a role in induction of chemokines in vivo.
Figure 4. MDP and peptidoglycan induce higher chemokine levels in blood in WT than in PGLYRP-2-/- mice.
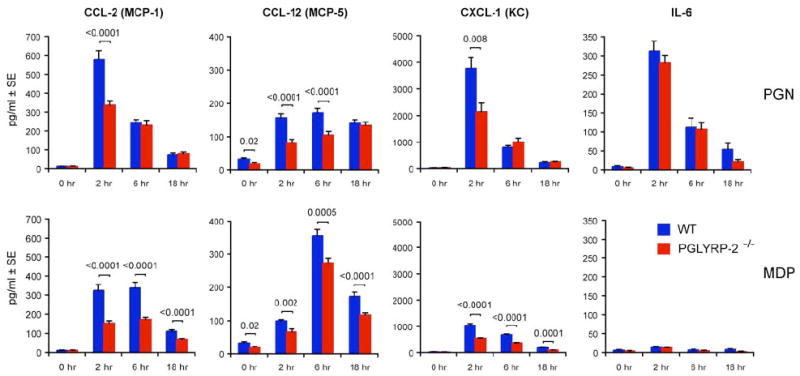
WT or PGLYRP-2-/- mice were injected intravenously with 200 μg PGN or 100 μg MDP, and the concentrations of CCL-2, CCL-12, CXCL-1, and IL-6 were measured in the serum at the indicated times; means of 20-30 mice/group for each time point; the significance of differences between WT and PGLYRP-2-/- mice is indicated.
PGLYRP-2-/- fibroblasts have low responsiveness to bacteria and cytokines, which is reconstituted by recombinant PGLYRP-2
We next compared the responses of paw fibroblasts from WT and PGLYRP-2-/- mice, because fibroblasts are the main local source of chemokines (they produce more chemokines than macrophages), and because we found many proliferating fibroblasts in the inflammatory lesions following peptidoglycan or MDP injection (in addition to infiltrating PMNs). In vitro cultured PGLYRP-2-/- paw fibroblasts, either unstimulated or stimulated with peptidoglycan, ReLPS, bacteria, or IL-1β, secreted significantly less IL-6, CXCL-1, and CCL-12 than WT paw fibroblasts (Figure 5A) and had lower expression of IL-6 mRNA (Figure 5B). Recombinant mouse PGLYRP-2 protein (but not recombinant albumin, added to fibroblast cultures at 1 μg/ml) enhanced expression of IL-6 mRNA induced by bacteria or IL-1β in PGLYRP-2-/- paw fibroblasts to the level seen in WT fibroblasts (Figure 5B), although by itself PGLYRP-2 did not induce IL-6 mRNA. Stimulation with bacteria induced expression of PGLYRP-2 mRNA in WT paw fibroblasts (Figure 5C), and, therefore, the lack of expression of PGLYRP-2 in PGLYRP-2-/- fibroblasts correlated with their low induction of cytokine mRNA and low secretion of cytokines. These results demonstrate that PGLYRP-2 enhances cell responsiveness to bacteria and cytokines, and suggest that PGLYRP-2 serves as an endogenous mediator of inflammation (has a cytokine-like or alarmin-like activity).
Figure 5. PGLYRP-2-/- fibroblasts have low responsiveness to bacteria and cytokines, which is reconstituted by PGLYRP-2; MDP induces expression of PGLYRP-2 in the feet through activation of Nod2; and PGLYRP-2 activates IL-8 and NF-κB and synergizes with TLR2 and TLR4.
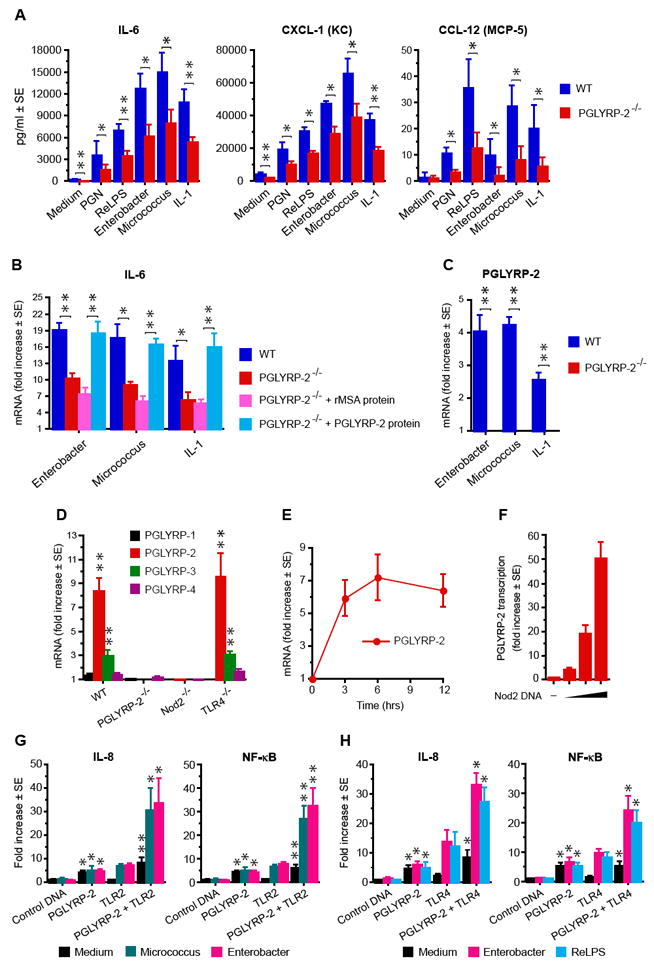
(A) IL-6, CXCL-1, and CCL-12 production by paw fibroblasts from WT and PGLYRP-2-/- mice cultured with the indicated stimulants. (B) IL-6 mRNA in paw fibroblasts from WT and PGLYRP-2-/- mice cultured with the indicated stimulants and reconstitution of the WT-level response in fibroblasts from PGLYRP-2-/- mice cultured with 1 μg/ml of recombinant mouse PGLYRP-2 protein, but not recombinant mouse albumin (rMSA). (C) PGLYRP-2 mRNA in paw fibroblasts from WT and PGLYRP-2-/- mice cultured with the indicated stimulants. (A-C) means ± SE of 3 experiments; significance of differences (WT vs PGLYRP-2-/- cells or rMSA vs PGLYRP-2 protein) is indicated by asterisks. (D) Increase in the amounts of mRNA for PGLYRP-1, PGLYRP-2, PGLYRP-3, and PGLYRP-4 in the feet of WT, PGLYRP-2-/-, Nod2-/-, and TLR4-/- mice 6 hrs after intravenous injection of 100 μg MDP, measured by qPCR and expressed as the ratios of the amount of mRNA in MDP-injected to buffer-injected mice (means ± SE of 6-12 mice/group, significance of increase is indicated by asterisks). (E) Kinetics of induction of PGLYRP-2 mRNA in the feet of WT mice after intravenous injection of 100 μg MDP (means ± SE of 6 mice/group). (F) Induction of PGLYRP-2 transcription in HEK293 cells transfected with PGLYRP-2 promoter-luciferase and control plasmid DNA (-) or increasing concentrations of Nod2 (means ± SE of 4 cultures from 2 experiments). (G, H) Induction of IL-8 transcription or NF-κB in HEK293 cells transfected with control DNA, PGLYRP-2, and TLR2 (G), or control DNA, PGLYRP-2, and TLR4 (plus CD14 and MD-2) (H), alone or in combination, after stimulation with Micrococcus, Enterobacter, or ReLPS, or no stimulation; means ± SE of 7 (G) or 4 (H) experiments, significance of differences of PGLYRP-2 vs control DNA or PGLYRP-2+TLR vs TLR is indicated by asterisks. *, P≤0.05; * *, P≤0.005.
MDP induces expression of PGLYRP-2 in the feet through activation of Nod2
PGLYRP-2 is constitutively expressed in the liver and not in other tissues (Liu et al., 2001), but its expression is induced by bacteria or cytokines in fibroblasts (Figure 5C) and in keratinocytes and other epithelial cells, but not in cells of myeloid origin (monocytes, macrophages, lymphocytes) and endothelial cells (Wang et al., 2005; Li et al., 2006). By contrast, PGLYRP-3 and PGLYRP-4 are constitutively expressed in the skin, and their expression can also be increased by exposure to bacteria or cytokines (Lu et al., 2006). PGLYRP-2 mRNA was not expressed in the feet of untreated WT mice, but its expression was induced following intravenous injection of MDP (Figure 5D,E). Intravenous injection of MDP also significantly increased expression of PGLYRP-3 in the foot (Figure 5D). Stimulation of both PGLYRP-2 and PGLYRP-3 expression by MDP required Nod2, because it was not observed in Nod2-/- mice (Figure 5D). Stimulation of PGLYRP-3 expression by MDP also required PGLYRP-2, because it was not observed in PGLYRP-2-/- mice. Moreover, overexpression of Nod2 (which aggregates and becomes activated) in HEK293 cells activated PGLYRP-2 promoter (Figure 5F), which demonstrates that Nod2 is sufficient for induction of PGLYRP-2 expression. These results indicate that PGLYRP-2 acts downstream of Nod2, because Nod2 induces expression of PGLYRP-2.
PGLYRP-2 activates cells and synergizes with TLR4 and TLR2 in vitro
We next tested whether PGLYRP-2 directly activates cells or cooperates with other receptors in cell activation, especially with TLRs, because Nod2-mediated activation of macrophages by MDP often synergizes with TLRs (Uehara et al., 2005; Kobayashi et al., 2005; Kim et al. 2008). Transfection of HEK293 cells with PGLYRP-2 activated NF-κB transcription factor and IL-8 promoter and this activation was not enhanced by stimulation with bacteria (Figure 5G,H). Co-transfection of HEK293 cells with PGLYRP-2 and TLR2 or TLR4 (plus MD-2 and CD14) enhanced the response of TLR2 or TLR4 to bacteria (Figure 5G,H). These results indicate that PGLYRP-2 directly activates cells and enhances cell activation by TLR2 and TLR4.
TLR4 and MyD88 are required in the effector phase of peptidoglycan- or MDP-induced arthritis
We next tested whether TLR2, TLR4, or MyD88 were required for peptidoglycan- or MDP-induced arthritis because PGLYRP-2 synergized with TLR2 and TLR4 in cell activation. Intravenous injection of peptidoglycan or MDP induced acute arthritis in TLR2-/- mice, manifested by swelling and erythema of ankles and feet (Figure 6A) with similar kinetics, severity, and incidence (Figure 6B) as in WT mice. However, peptidoglycan or MDP did not induce arthritis in TLR4-/- or MyD88-/- mice (Figure 6A,B).
Figure 6. TLR4, MyD88, and PMNs are required for the development of peptidoglycan- or MDP-induced acute arthritis.
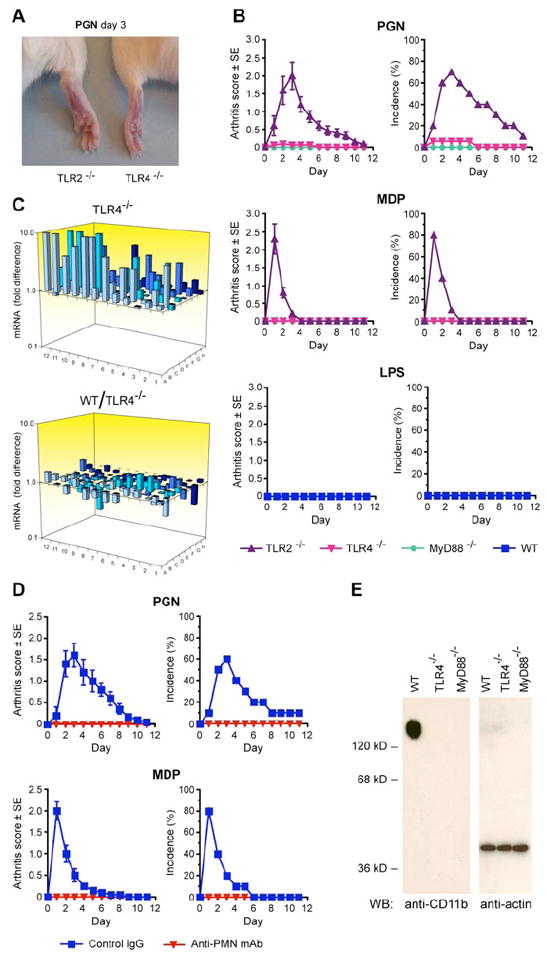
(A) Swelling and erythema of ankles and feet in TLR2-/- but not in TLR4-/- mice after intravenous injection of 200 μg of PGN. (B) Arthritis scores and incidence in TLR2-/-, TLR4-/-, and MyD88-/- mice after intravenous injection of 200 μg of PGN or 100 μg of MDP; or in WT mice after intravenous injection of 10 μg of ReLPS; means ± SE of 12-20 mice/group. (C) Expression of chemokine and cytokine mRNA in the feet of TLR4-/- mice after intravenous injection of 100 μg of MDP, determined as in Figure 3. (D) Arthritis scores and incidence in WT mice treated with control IgG or anti-PMN mAb after intravenous injection of 200 μg of PGN or 100 μg of MDP; means ± SE of 10 mice/group. (E) Blood PMNs from untreated TLR4-/- and MyD88-/- mice, in contrast to WT mice, do not express CD11b integrin detected on Western blot.
Although TLR4 was required for the development of peptidoglycan- or MDP-induced arthritis, activation of TLR4 alone was not sufficient for the development of arthritis, because WT mice injected intravenously with a TLR4 agonist, ReLPS, did not develop arthritis (Figure 6B), despite strong systemic response to ReLPS injection, manifested by high induction of cytokines (TNF-α and IL-6) in their blood (Supplemental Figure S3) and shock-like appearance (lethargy and rough fur).
To determine whether the requirement for TLR4 in the development of MDP-induced arthritis was up-stream or down-stream from Nod2 and PGLYRP-2, we then tested the level of MDP-induced chemokine genes in the feet in TLR4-/- mice. Intravenous injection of MDP induced normal activation of proinflammatory genes (Figure 6C and Supplemental Table S3), indicating that TLR4 is required for the development of MDP-induced arthritis downstream of Nod2, of PGLYRP-2, and of induction of chemokines and other proinflammatory genes (which depend on Nod2 and PGLYRP-2). TLR4 was also not required for MDP-induced expression of PGLYRP-2 in the feet (Figure 5D). These results suggest that TLR4 is mainly required in the effector phase of arthritis, after chemokines and cytokines are generated.
PMNs are required for the development of peptidoglycan- or MDP-induced arthritis and TLR4 is required for expression of CD11b in PMNs and PMNs recruitment into the feet
We next tested whether PMNs were required for the development of peptidoglycan- and MDP-induced arthritis, because the most prominent histological feature of this arthritis is infiltration with PMNs (Figures 2 and S1). Depletion of PMNs in WT mice by intravenous injection of anti-PMN Ly-6G (Gr-1) mAb completely prevented the development of arthritis in peptidoglycan- or MDP-injected mice (Figure 6D), thus showing that PMNs are required for the development of peptidoglycan- and MDP-induced arthritis.
We next tested whether PMNs in our TLR4-/- and MyD88-/- mice express CD11b, because PMNs in TLR4-/- mice have been shown to lack sufficient expression of integrins, which makes them defective in adhering to ICAMs and migrating from blood into the tissues (Zhou et al., 2005). Indeed, PMNs in our untreated TLR4-/- and MyD88-/- mice (in contrast to WT mice) did not express CD11b (Fig. 6E). Furthermore, foot sections from peptidoglycan- or MDP-injected TLR4-/- mice had no PMN infiltrations (Supplemental Figure S4), which confirms that PMNs in TLR4-/- mice are unable to migrate into the tissues. Thus, our results indicate that TLR4 plays a permissive role in this arthritis model (independent of MDP or peptidoglycan), because TLR4 is required in untreated mice for proper maturation of PMNs and expression of integrins, which are needed for PMN migration into the tissues in the effector phase of arthritis.
DISCUSSION
Our results demonstrate that mammalian PGRPs, in addition to their amidase and bactericidal activities, function as modulators of inflammation and cooperate with other innate immunity pattern recognition receptors to induce local inflammation. We show that PGLYRP-2 is required for the development of peptidoglycan- or MDP-induced local inflammation and arthritis, along with Nod2 and TLR4, because PGLYRP-2-/- mice, Nod2-/- mice, and TLR4-/- mice are all resistant to peptidoglycan- or MDP-induced arthritis. The requirement for PGLYRP-2 and Nod2 correlates with their requirement for MDP-induced upregulation of chemokine production in the foot. Nod2 works upstream from PGLYRP-2 and induces expression of PGLYRP-2 in the foot. TLR4 plays a permissive role, as it is required for the expression of CD11b on PMNs, which is peptidoglycan- and MDP-independent (happens before peptidoglycan and MDP challenge). Thus, the sequence of events in peptidoglycan- or MDP-induced arthritis is activation of Nod2 by peptidoglycan fragments, local expression of PGLYRP-2 in the limbs, local chemokine and cytokine production, and then recruitment of PMNs into the limbs, which produces acute local inflammation and arthritis (Figure 7). The chart in Figure 7, however, does not imply one direct signal transduction pathway in which one molecule directly activates the next downstream molecule. The most likely scenario is that after Nod2 induces PGLYRP-2, Nod2 and PGLYRP-2 are in parallel pathways that are synergistic and complementary and that both pathways are needed for full induction of gene expression that eventually manifest themselves in vivo as clinical signs of inflammation. TLR4-mediated maturation of PMNs is independent of peptidoglycan, but it affects the outcome of peptidoglycan-induced pathways, as shown in TLR4-/- mice, whose PMNs do not express CD11b and cannot migrate into tissues.
Figure 7. Proposed sequence of events leading to PGN- or MDP-induced local inflammation and arthritis.
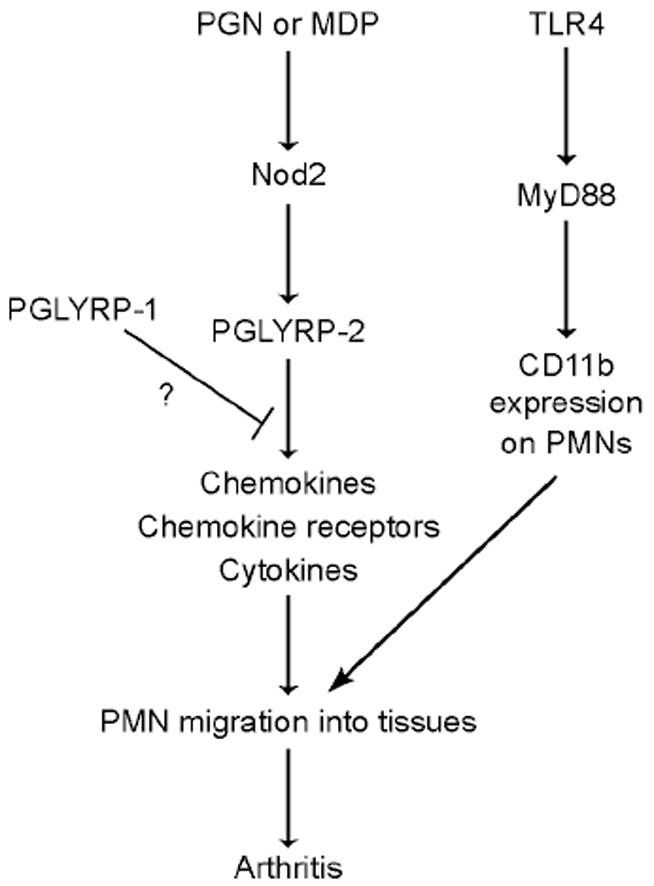
The chart indicates temporal sequence of events and does not imply direct signal transduction pathways. TLR4 permits maturation of PMNs before exposure to PGN or MDP.
Our results, therefore, reveal new in vivo functions of PGLYRP-2, Nod2, and TLR4, define several steps in the pathogenesis of acute peptidoglycan-induced arthritis (Figure 7), and demonstrate in vivo interdependence of these three families of pattern recognition molecules in their requirement for production of local inflammation. These results are consistent with the emerging view of in vivo protein function as regulatory networks that are essential for maintaining immune homeostasis and properly measured response to infections.
PGLYRP-2 is constitutively produced in the liver and is secreted into blood (Xu et al., 2004; Zhang et al., 2005; Royet and Dziarski, 2007). However, PGLYRP-2 from blood does not significantly contribute to the development of MDP-induced arthritis, because intravenous injection of PGLYRP-2 into WT mice, or PGLYRP-2 plus MDP into PGLYRP-2-/- mice, does not induce arthritis (Supplemental Data), and because bacteria or MDP do not increase the expression of PGLYRP-2 in the liver. Thus, local induction of PGLYRP-2 in the feet is required for triggering inflammation.
PGLYRP-2 is not constitutively expressed in extrahepatic tissues, such as skin or the foot, but its expression is induced by bacteria (Wang et al., 2005; Li et al., 2006) or MDP. Induction of PGLYRP-2 expression is mediated through Nod2 (as shown here) and does not involve TLR2 or TLR4 (as shown previously), is limited to non-immune cells (epithelial cells, fibroblasts), and is dependent on the distal regions of the PGLYRP-2 promoter and different transcription factors than the constitutive expression, which is dependent on the proximal region of the PGLYRP-2 promoter (Wang et al., 2005; Li et al., 2006). PGLYRP-2 is not expressed and its expression is not induced in cells of myeloid or lymphoid origin or in endothelial cells (Wang et al., 2005; Li et al., 2006). Therefore, monocytes, macrophages, PMNs, and lymphocytes from PGLYRP-2-/- mice are normal (Supplemental Data). Thus PGLYRP-2-/- mice show a defective inflammatory response (no arthritis and low chemokine production) only locally in the foot, as reported here, but are not deficient in the systemic production of macrophage-derived cytokines, such as TNF-α and IL-6 (Xu et al., 2004).
Although mammalian PGLYRP-2 is an N-acetylmuramoyl-L-alanine amidase that hydrolyzes bacterial peptidoglycan (Gelius et al., 2003; Wang et al., 2003), its amidase activity is not required for its pro-inflammatory effect, because this pro-inflammatory effect is seen with a variety of stimulants that are not amidase substrates (described in detail in Supplemental Data). Thus, in addition to its enzymatic activity, which resides in the C-terminal PGRP domain (Wang et al., 2003), PGLYRP-2 also functions as an alarmin, similar to antimicrobial peptides, such as β-defensins, which besides their antimicrobial activity enhance immune responses and inflammation (Biragyn et al., 2002).
The role of PGLYRP-2 in arthritis is unique, because other mammalian PGLYRPs do not have similar pro-inflammatory effect. Interestingly, they also do not have the N-terminal domain that is unique for PGLYRP-2. Other PGRPs may have an opposite anti-inflammatory effect, because PGLYRP-1-/-, PGLYRP-3-/-, and PGLYRP-4-/- mice all had higher MDP-induced activation of pro-inflammatory genes than WT mice. Moreover, PGLYRP-1-/- mice have longer-lasting MDP-induced arthritis than WT mice, and PGLYRP-1-/-PGLYRP-2-/- double KO mice have arthritis of intermediate severity, i.e., more severe than PGLYRP-2-/- but less severe than WT mice. PGLYRP-1 is only expressed in the PMNs and its expression in other cells is not induced by bacteria or cytokines (Liu et al., 2000, 2001). The anti-inflammatory function of PGLYRP-1 manifests itself only in the later stages of MDP-induced arthritis, which is consistent with the local release of PGLYRP-1 from PMN granules after PMNs’ arrival into the foot. Anti-inflammatory activity of a PMN granule protein (PGLYRP-1) or proteins present on the skin and in secretions of mouth and intestinal cells (PGLYRP-3 and PGLYRP-4), which all come in contact with large numbers of bacteria, would be desirable to limit over-responsiveness of the immune system to bacteria.
The absolute requirement for Nod2 in peptidoglycan- or MDP-induced arthritis and for activation of pro-inflammatory genes in the foot demonstrates that Nod2 is the main and required peptidoglycan and MDP receptor for these responses in the foot, and that this function cannot be performed by other MDP receptors (NALP1 and NALP3) or other peptidoglycan receptors. An important step in this proinflammatory cascade is induction of PGLYRP-2 expression, which, as shown here, is induced by Nod2, but cannot be induced by TLR2 or TLR4 (Wang et al., 2005).
PGLYRP-2 directly enhances cell responsiveness to bacteria and cytokines. Fibroblasts from PGLYRP-2-/- mice have lower in vitro responsiveness than fibroblasts from WT mice, and their responsiveness is reconstituted to the WT level by PGLYRP-2 protein. Moreover, transfection of cells with PGLYRP-2 in vitro activates these cells and co-transfection with TLR2 or TLR4 enhances cell activation. This phenomenon is similar to the synergism between TLRs and Nods (Uehara et al., 2005; Kobayashi et al., 2005; Kim et al. 2008). In vivo, however, TLR2 is dispensable, because TLR2-/- mice develop strong peptidoglycan- or MDP-induced arthritis similar to WT mice. These results suggest that this synergistic interaction between PGLYRP-2 and TLR2 is either not required for the development of arthritis or can be performed by other TLRs. Therefore, our arthritis is different from the intraarticular injection model, which, unlike our model, is macrophage-, TLR2-, and partially Nod2-dependent and mimics joint infection (Joosten et al., 2003, 2008).
TLR4 plays a permissive role for the development of arthritis in our model, independent of peptidoglycan or MDP stimulation. The main requirement for TLR4 is for the maturation of PMNs and expression of CD11b integrin, which happens before peptidoglycan or MDP challenge, but which is essential for PMNs migration into the tissues in the effector phase of peptidoglycan- or MDP-induced arthritis. Our results confirm and extend the results of Zhou et al. (2005), who also showed that TLR4-/- mice have greatly reduced expression of CD11b/CD18 integrin, which is essential for adhesion of PMNs to endothelial cells and transmigration into the tissues. Moreover, TLR4 is also required for the responsiveness of PMNs to chemokines (Fan and Malik, 2003), which could additionally contribute to the permissive role of TLR4 in our arthritis model.
Of note, activation of TLR4 by systemic injection of LPS into WT mice (in contrast to activation of Nod2 by peptidoglycan) does not cause arthritis, despite strong systemic and local cytokine production. Such a response to LPS is mostly systemic with massive production of cytokines in blood and all organs, which produces a systemic shock-like response (rather than local response in the foot), and which diverts the effectors, such as PMNs, to other tissues. By contrast, the in vivo response to peptidoglycan or MDP is mostly local, which locally triggers the inflammatory cascade, which then attracts PMNs to the local sites and results in arthritis.
In summary, our results demonstrate that mammalian PGRPs, in addition to amidase and bactericidal activities, also have pro-inflammatory and anti-inflammatory properties, and that PGLYRP-2 and Nod2 are both required for the development of peptidoglycan-induced arthritis. These results reveal new functions of mammalian PGRPs and Nod2 and demonstrate interdependence of multiple innate immune pattern recognition molecules in producing local pathologic changes.
EXPERIMENTAL PROCEDURES
Mice
We generated PGLYRP-1-/-, PGLYRP-2-/-, PGLYRP-3-/-, and PGLYRP-4-/- mice by deleting exons 1, 1-4, 2-5, or 3-4, respectively, and PGLYRP-1-/-PGLYRP-2-/- double KO mice by crossing PGLYRP-1-/- with PGLYRP-2-/- mice, as described in Supplemental Data. Nod2-/-, TLR-2-/-, TLR-4-/-, and MyD88-/- mice are also described in Supplemental Data. All mice were backcrossed to BALB/c because this strain is sensitive to peptidoglycan- and MDP-induced arthritis (Koga et al., 1985; Onta et al., 1993).
Arthritis model
To induce experimental acute arthritis (Koga et al., 1985; Onta et al., 1993), equal numbers of female and male mice (6 to 12 weeks old) were injected intravenously with 200 μg of insoluble peptidoglycan from S. aureus (or from E. coli or B. subtilis, where indicated, purified as described in Supplemental Data), or with 100 μg of synthetic MDP (N-acetylmuramyl-L-alanyl-D-isoglutamine) or MDP-LL (N-acetylmuramyl-L-alanyl-L-isoglutamine) in endotoxin-free saline. Some mice were injected with Salmonella minnesota Re595 LPS (ReLPS).
The development of arthritis in the hind ankles and feet was evaluated as described in Supplemental Data using: (a) severity of arthritis, based on arthritis scores of 0-4; (b) incidence of arthritis; and (c) histological analysis. The significance of differences in arthritis scores were compared using two-sample t-test and in incidence of arthritis using Chi-square test or Yates test (GB-Stat PPC6.5.6, Dynamic Microsystems).
RNA and quantitative real-time PCR
To measure the expression of pro-inflammatory genes, RNA was isolated from both entire hind feet (including ankles) or livers of mice using the TRIZOL method (InVitrogen), followed by digestion with RNase-free DNase (Qiagen) and purification on RNeasy spin columns using RNeasy Minikit (Qiagen). The amounts of mRNA for 84 pro-inflammatory genes and 5 housekeeping genes were measured by quantitative real-time PCR (qPCR) using the Mouse Inflammatory Cytokines and Receptors RT2 Profiler PCR Array (SA Biosciences) following synthesis of cDNA from 2 μg of RNA using RT2 PCR Array First Strand Kit (SA Biosciences), according to the manufacturer instructions (see Supplemental Data). The list of genes is provided in Supplemental Table S1. In preliminary experiments gene activation at 3, 6 and 12 hrs post MDP injection was evaluated. The reported results are from 6 to 12 mice per group individually assayed 6 hrs post injection (which was the optimal time for gene activation) or from untreated mice, and the means ± SE of the fold increase in each gene expression from 6-12 mice were calculated. The fold differences (ratios) of >1 or <1 reflect higher or lower expression levels of the genes (respectively) in WT than in KO mice. To determine whether gene activation was statistically significant, one-sample t-test was used (mean fold induction vs 1), and to determine the significance of differences in gene activation between two groups of mice (e.g., mean fold induction in WT vs KO mice) two-sample t-test was used.
Chemokine and cytokine assays
The amounts of chemokines and cytokines were measured by ELISA as described in Supplemental Data.
Cell cultures and transfections
Human HEK293 cells and peritoneal or bone marrow macrophages were cultured, transfected, stimulated, and assayed as described in Supplemental Data. Primary mouse paw fibroblasts were isolated, cultured, characterized, and stimulated as described in Supplemental Data.
PMN isolation, CD11b expression, and in vivo depletion
Blood PMNs were isolated and assayed for CD11b expression by Western blot as described in Supplemental Data. To deplete PMNs in vivo, mice were injected intravenously with 200 μg of anti-Ly6G (Gr-1) rat mAb (clone RB6-8C5 from eBioscience) (Tanaka et al., 2006), which decreased the numbers of PMNs in peripheral blood to ~6% for at least 3 days. Control mice were injected with 200 μg rat IgG2bκ (eBioscience). 18 hrs after mAbs mice were injected intravenously with 200 μg peptidoglycan or 100 μg MDP and scored for arthritis.
Acknowledgments
We are grateful to Grégoire Lauvau for providing TLR-2-/-, TLR-4-/-, and MyD88-/- mice, Robert Rukavina for maintaining and breeding our mice, and Patrick Bankston for help in interpreting histology slides. This work was supported by USPHS Grants AI28797 and AI56395 from NIH to R.D. and D.G. and DK61707 to G.N. The authors have no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
References
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J. Down regulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2006;2:e14. doi: 10.1371/journal.ppat.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- Chang YH, Pearson CM, Chedid L. Adjuvant polyarthritis. V. Induction by N-acetylmuramyl-L-alanyl-D-isoglutamine, the smallest peptide subunit of bacterial peptidoglycan. J Exp Med. 1981;153:1021–1026. doi: 10.1084/jem.153.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie WJ, Craddock JG, Schwab JH, Anderle SK, Yang CH. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977;146:1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf FG, Cromartie WJ, Anderle SK, Clark RL, Schwab JH. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980;100:383–402. [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R, Fox A, Greenblatt JJ, Anderle SK, Cromartie WJ, Schwab JH. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982;38:127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Fox A, Brown RR, Anderle SK, Chetty C, Cromartie WJ, Gooder H, Schwab JH. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982;35:1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelius E, Persson C, Karlsson J, Steiner H. A mammalian peptidoglycan recognition protein with N-acetylmuramoyl-L-alanine amidase activity. Biochem Biophys Res Commun. 2003;306:988–994. doi: 10.1016/s0006-291x(03)01096-9. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Travassos LH, Hervé M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Heinhuis B, Abdollahi-Roodsaz S, Ferwerda G, Lebourhis L, Philpott DJ, Nahori MA, Popa C, Morre SA, van der Meer JW, et al. Differential function of the NACHT-LRR (NLR) members Nod1 and Nod2 in arthritis. Proc Natl Acad Sci USA. 2008;105:9017–9022. doi: 10.1073/pnas.0710445105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LA, Koenders MI, Smeets RL, Heuvelmans-Jacobs M, Helsen MM, Takeda K, Akira S, Lubberts E, van de Loo FA, van den Berg WB. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- Kang D, Liu G, Lundstrom A, Gelius E, Steiner H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci USA. 1998;95:10078–10082. doi: 10.1073/pnas.95.17.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Núñez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Koga T, Kakimoto K, Hirofuji T, Kotani S, Ohkuni H, Watanabe K, Okada N, Okada H, Sumiyoshi A, Saisho K. Acute joint inflammation in mice after systemic injection of the cell wall, its peptidoglycan, and chemically defined peptidoglycan subunits from various bacteria. Infect Immun. 1985;50:27–34. doi: 10.1128/iai.50.1.27-34.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O, Pearson CM, Watanabe Y, Kotani S, Koga T. Structural requirements for arthritogenicity of peptidoglycans from Staphylococcus aureus and Lactobacillus plantarum and analogous synthetic compounds. J Immunol. 1976;116:1635–1639. [PubMed] [Google Scholar]
- Lehman TJ, Allen JB, Plotz PH, Wilder RL. Polyarthritis in rats following the systemic injection of Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 1983;26:1259–1265. doi: 10.1002/art.1780261013. [DOI] [PubMed] [Google Scholar]
- Li X, Wang S, Wang H, Gupta D. Differential expression of peptidoglycan recognition protein 2 in the skin and liver requires different transcription factors. J Biol Chem. 2006;281:20738–20748. doi: 10.1074/jbc.M601017200. [DOI] [PubMed] [Google Scholar]
- Liu C, Gelius E, Liu G, Steiner H, Dziarski R. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J Biol Chem. 2000;275:24490–24499. doi: 10.1074/jbc.M001239200. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu Z, Gupta D, Dziarski R. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J Biol Chem. 2001;276:34686–34694. doi: 10.1074/jbc.M105566200. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang M, Qi J, Wang H, Li X, Gupta D, Dziarski R. Peptidoglycan recognition proteins are a new class of human bactericidal proteins. J Biol Chem. 2006;281:5895–5907. doi: 10.1074/jbc.M511631200. [DOI] [PubMed] [Google Scholar]
- Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Mellroth P, Karlsson J, Steiner H. A scavenger function for a Drosophila peptidoglycan recognition protein. J Biol Chem. 2003;278:7059–7064. doi: 10.1074/jbc.M208900200. [DOI] [PubMed] [Google Scholar]
- Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Häfner R, Chamaillard M, Zouali H, Thomas G, Hugot JP. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Onta T, Sashida M, Fujii N, Sugawara S, Rikiishi H, Kumagai K. Induction of acute arthritis in mice by peptidoglycan derived from gram-positive bacteria and its possible role in cytokine production. Microbiol Immunol. 1993;37:573–582. doi: 10.1111/j.1348-0421.1993.tb01679.x. [DOI] [PubMed] [Google Scholar]
- Royet J, Dziarski R. Peptidoglycan Recognition Proteins: pleiotropic sensors and effectors of antimicrobial defenses. Nat Microbiol Rev. 2007;5:264–277. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- Simelyte E, Rimpilainen M, Zhang X, Toivanen P. Role of peptidoglycan subtypes in the pathogenesis of bacterial cell wall arthritis. Ann Rheum Dis. 2003;62:976–982. doi: 10.1136/ard.62.10.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer TW, Rosenthal RS, Fleming TJ, O’Connor BL. Histological examination of experimental arthritis induced by gonococcal peptidoglycan. Br J Exp Pathol. 1987;68:793–802. [PMC free article] [PubMed] [Google Scholar]
- Stimpson SA, Lerch RA, Cleland DR, Yarnall DP, Clark RL, Cromartie WJ, Schwab JH. Effect of acetylation on arthropathic activity of group A streptococcal peptidoglycan-polysaccharide fragments. Infect Immun. 1987;55:16–23. doi: 10.1128/iai.55.1.16-23.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in antitype II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119:195–202. doi: 10.1111/j.1365-2567.2006.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara A, Yang S, Fujimoto Y, Fukase K, Kusumoto S, Shibata K, Sugawara S, Takada H. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005;7:53–61. doi: 10.1111/j.1462-5822.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Gupta D, Li X, Dziarski R. Peptidoglycan recognition protein 2 (N-acetylmuramoyl-L-Ala amidase) is induced in keratinocytes by bacteria through the p38 kinase pathway. Infect Immun. 2005;73:7216–7225. doi: 10.1128/IAI.73.11.7216-7225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liu L-H, Wang S, Li X, Lu X, Gupta D, Dziarski R. Human peptidoglycan recognition proteins require zinc to kill both Gram-positive and Gram-negative bacteria and are synergistic with antibacterial peptides. J Immunol. 2007;178:3116–3125. doi: 10.4049/jimmunol.178.5.3116. [DOI] [PubMed] [Google Scholar]
- Wang Z-M, Li X, Cocklin RR, Wang M, Wang M, Fukase K, Inamura S, Kusumoto S, Gupta D, Dziarski R. Human peptidoglycan recognition protein-L is an N-acetylmuramoyl-L-alanine amidase. J Biol Chem. 2003;278:49044–49052. doi: 10.1074/jbc.M307758200. [DOI] [PubMed] [Google Scholar]
- Xu M, Wang Z, Locksley RM. Innate immune responses in peptidoglycan recognition protein L-deficient mice. Mol Cell Biol. 2004;24:7949–7957. doi: 10.1128/MCB.24.18.7949-7957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidman-Remy A, Herve M, Poidevin M, Pili-Floury S, Kim MS, Blanot D, Oh BH, Ueda R, Mengin-Lecreulx D, Lemaitre B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, van der Fits L, Voerman JS, Melief M-J, Laman JD, Wang M, Wang H, Wang M, Li X, Walls CD, et al. Identification of serum N-acetylmuramoyl-L-alanine amidase as liver peptidoglycan recognition protein 2. Biochim Biophys Acta. 2005;1752:34–46. doi: 10.1016/j.bbapap.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Zhou X, Gao XP, Fan J, Liu Q, Anwar KN, Frey RS, Malik AB. LPS activation of Toll-like receptor 4 signals CD11b/CD18 expression in neutrophils. Am J Physiol. 2005;288:L655–662. doi: 10.1152/ajplung.00327.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


