Abstract
OBJECTIVE:
The present study was a randomized, parallel, double-blind comparison between controlled-release (CR) tramadol and sustained-release (SR) diclofenac in patients with chronic pain due to osteoarthritis of the hips and/or knees.
METHODS:
Patients with at least moderate pain intensity, and having received analgesics over the past three months, underwent a two-to seven-day washout of current analgesics before initiation of 200 mg CR tramadol or 75 mg SR diclofenac. During the eight-week study, patients returned to the clinic biweekly. CR tramadol doses were titrated to a maximum of 200 mg, 300 mg or 400 mg per day. SR diclofenac doses were titrated to 75 mg or 100 mg once daily, or 75 mg twice a day based on pain relief and the presence of side effects. For rescue analgesic, patients took acetaminophen as needed, up to 650 mg three times a day.
RESULTS:
Forty-five patients on CR tramadol and 52 patients on SR diclofenac were evaluable. Significant improvements from prestudy treatment were shown for visual analogue scale pain (P=0.0001), stiffness (P<0.0005) and physical function (P=0.0001) scores for both treatments. There were no significant differences between the two treatments in the Western Ontario and McMaster Universities subscales, overall pain, pain and sleep, or the clinical effectiveness evaluation. Overall incidence of adverse events was similar in both groups, with more opioid-related adverse events with CR tramadol, and two serious adverse events occurring with the use of SR diclofenac.
CONCLUSIONS:
CR tramadol is as effective as SR diclofenac in the treatment of pain due to knee or hip osteoarthritis, with the potential for fewer of the serious side effects that characterize nonsteroidal anti-inflammatory drug administration.
Keywords: Chronic pain, Controlled-release, Diclofenac, NSAID, Osteoarthritis, Tramadol
Abstract
OBJECTIF :
La présente étude était une comparaison aléatoire et parallèle à double insu entre le tramadol à libération contrôlée (TLC) et le diclofénac à libération continue (DLC) chez les patients souffrant de douleur chronique causée par l’arthrose des hanches ou des genoux.
MÉTHODOLOGIE :
Des patients présentant une intensité au moins modérée de la douleur et qui avaient pris des analgésiques au cours des trois mois précédents ont subi un sevrage de leurs analgésiques habituels pendant deux à sept jours avant d’entreprendre un traitement de 200 mg de TLC ou de 75 m de DLC. Pendant l’étude de huit semaines, les patients sont allés à la clinique deux fois par semaine. Les doses de TLC ont été titrées à un maximum de 200 mg, 300 mg ou 400 mg par jour, tandis que celles de DLC l’étaient à 75 mg ou 100 mg une fois par jour, ou à 75 mg deux fois par jour, d’après l’analgésie et la présence d’effets secondaires. Les patients prenaient de l’acétaminophène comme analgésique de rattrapage au besoin, jusqu’à concurrence de 650 mg trois fois par jour.
RÉSULTATS :
Quarante-cinq patients prenant du TLC et 52 patients prenant du DLC pouvaient être évalués. Avec les deux traitements et selon l’échelle visuelle analogique, les auteurs ont constaté des améliorations considérables par rapport au traitement d’avant l’étude pour ce qui est de la douleur (P=0,0001), de la raideur (P<0,0005) et de la fonction physique (P=0,0001). Ils n’ont toutefois remarqué aucune différence significative entre les deux traitements selon les sous-échelles hebdomadaires moyennes de l’université de Western Ontario et de l’université McMaster, de la douleur globale, de la douleur et du sommeil ou de l’évaluation d’efficacité clinique. L’incidence globale d’effets secondaires était similaire au sein des deux groupes, plus d’effets secondaires reliés aux opiacés s’étant produits au sein du groupe prenant du TLC et deux effets secondaires graves ayant découlé de l’utilisation du DLC.
CONCLUSIONS :
Le TLC est aussi efficace que le DLC dans le traitement de la douleur causée par l’arthrose des genoux ou des hanches, mais il pourrait provoquer moins d’effets secondaires graves caractéristiques des anti-inflammatoires non stéroïdiens.
Osteoarthritis (OA) affects approximately 18% to 20% of the population aged 16 years and older in Canada (1). Pain is a major determinant of quality of life for patients with OA. Inadequate treatment of pain can have many negative consequences, including decreased immune response and adverse psychological effects, such as anxiety, depression, hopelessness, anger, hostility, poor interpersonal relations and suffering (2). The weakness, fatigue and stiffness as a result of progressive OA also contribute to decreased quality of life (2).
Typically, patients are treated with acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs), such as cyclo-oxygenase 2 (COX-2)-specific inhibitors, naproxen, ibuprofen or diclofenac. Although acetaminophen has been shown to provide similar analgesic efficacy to naproxen in OA patients (3), it does not provide any anti-inflammatory effects, and there is a preference for NSAID treatment (4,5). In a study of OA of the knee (6), an equal number of patients responded to acetaminophen and placebo, although mean change in pain intensity was greater for the acetaminophen group. In addition, while chronic therapeutic use of acetaminophen shows excellent tolerability, overdose has been associated with hepatic toxicity (7). Epidemiological studies have demonstrated a dose-dependent association between chronic renal failure and the long-term, daily ingestion of acetaminophen or acetylsalicylic acid (8). Patients receiving long-term NSAID therapy may experience severe gastrointestinal symptoms. NSAID-related ulceration and bleeding is estimated to result in up to 20,000 deaths each year in the United States (9). COX-2-specific inhibitors, such as celecoxib, may spare gastric mucosal prostaglandin synthesis, and consequently cause less gastrointestinal injury. However, a recent 12-month study comparing celecoxib, ibuprofen and diclofenac found no difference in the incidence of complicated ulcers between the three treatments; the incidence of symptomatic ulcers was the same for celecoxib and diclofenac (10). Both COX-2-specific inhibitors and NSAIDs may affect fluid and electrolyte balance in some patients, resulting in fluid retention, edema and hypertension. There are controversies concerning the effects of COX-2-specific inhibitors on cardiovascular disease and bone fracture healing (11). A meta-analysis of randomized trials regarding COX-2 inhibitors found increased risk of cardiovascular events with rofecoxib and diclofenac (12). The most recent recommendations of the American Heart Association include tramadol, not NSAIDs or COX-2-specific inhibitors, as first-line therapy for musculoskeletal symptoms in patients with cardiovascular disease or risk factors (13). Thus, alternatives to NSAIDs and COX-2-specific inhibitors are needed.
Tramadol has an atypical pharmacological profile, involving both opioid and monoaminergic mechanisms. While it possesses analgesic effectiveness comparable with low doses of strong opioids, such as morphine, at therapeutic doses tramadol lacks typical opioid adverse effects, such as respiratory depression (14). The analgesic effects of tramadol are exerted by complementary mechanisms, including the activation of μ-opioid receptors, and differential inhibition of serotonin and norepinephrine reuptake by the two isomers (15). The (+) enantiomer is more potent than the (−) enantiomer in inhibiting serotonin reuptake (15). Conversely, the (−) enantiomer is more potent in inhibiting norepinephrine reuptake and increasing presynaptic release. The interaction between the two enantiomers may be synergistic (16). As a result of its serotonin-like activity, tramadol has been associated with an increased potential risk of seizures. Several large cohort and case-controlled studies with more than 9200 adults have provided some perspective on the risk of seizures in patients receiving tramadol (17–19). In general, fewer than 1% of tramadol users claimed to have had a presumed incident of seizure. Most cases were associated with cofactors, such as the use of above-maximum therapeutic doses, concomitant use of products associated with increased seizure risk, or a history of seizures or substance abuse. The risk of idiopathic seizures due to tramadol alone, with no risk factors present, was considered low.
Although very rare, there have been some case reports suggesting that serotonin toxicity may be induced in uncommon circumstances, such as the combination of high doses of tramadol with selective serotonin reuptake inhibitors and/or other products with serotonergic effects (20). Such combinations should therefore be used with caution, and concomitant use of monoamine oxidase inhibitors with tramadol is contraindicated.
Tramadol has been shown to be effective in the treatment of OA pain (16,21–23), and is generally well-tolerated. The American Pain Society recommends tramadol, alone or in combination with acetaminophen or NSAIDs, for the management of OA pain when NSAIDs alone produce inadequate pain relief (24). Unlike NSAIDs, tramadol does not irritate the gastrointestinal mucosa, or exacerbate hypertension or congestive heart failure, making it potentially useful for the elderly (2). For patients awaiting total joint replacement surgery, tramadol may be a good analgesic option if NSAIDs or COX-2-specific inhibitors are not tolerated, or provide suboptimal pain relief.
Tramadol has been extensively evaluated in the treatment of chronic pain, such as low back pain (25), cancer pain, painful diabetic neuropathy (26,27), polyneuropathy (28) and fibromyalgia (29). Although tramadol may produce physical dependence during chronic use, little evidence of abuse has been found (30–34). Tolerance and dependence during long-term treatment is uncommon (35,36) and withdrawal, when present, is not considered to be as severe as that produced by other opioids. The ability of tramadol to inhibit the neuronal uptake of monoamines in the same concentration range at which it binds to μ-opioid receptors is distinct from typical opioids, and may explain its low potential for abuse in the treatment of chronic pain (26).
Compared with other centrally acting analgesics, tramadol has no clinically relevant cardiovascular effects (37) and insignificant effects on respiration (14). While μ-opioid agonists have undesirable effects on gastrointestinal function, resulting in nausea, emesis, abdominal cramps and especially constipation, these effects are less severe with tramadol, and attenuate over time (38). Tramadol has only a minor delaying effect on colonic transit, and no effect on upper gastrointestinal transit or gut smooth muscle tone (38).
An immediate-release (IR) formulation of tramadol was compared with NSAIDs in two randomized, double-blind trials (21,22) in patients with OA pain of at least moderate intensity. Although both treatments in these short trials produced equal pain relief, the incidence of side effects was greater with tramadol. An IR tramadol product containing acetaminophen was recently introduced into the Canadian market (39), although it has been restricted to short-term use for management of acute pain. A new controlled-release (CR) tramadol formulation has previously been shown to be comparable in efficacy with IR tramadol in the treatment of chronic OA pain (23). CR preparations not only provide an extended duration of action and reduced dosing frequency, but because of reduced fluctuations in plasma concentrations, they may provide a favourable efficacy and side effect profile, as well as improved compliance.
In a recently published double-blind crossover comparison of IR and CR tramadol in patients with chronic noncancer pain (40), CR tramadol was shown to have superior pain control (versus IR tramadol given as needed), full 24 h efficacy and higher patient-rated treatment efficacy (40). This same formulation was found to have superior pain control and functional improvements over placebo in a double-blind, randomized crossover trial (41). The present randomized, double blind, parallel study was designed to compare this CR tramadol formulation with sustained-release (SR) diclofenac in patients with OA pain of moderate or greater intensity.
METHODS
Patients
One hundred twenty-nine patients were enrolled into the study. Men and nonpregnant women between the ages of 35 and 75 years with primary OA were enrolled. Primary OA was defined by the presence of pain of at least moderate severity, stiffness, disability and bony crepitus of the hip and/or knee joint. Eligible patients had one hip or knee joint requiring use of NSAIDs, acetaminophen or opioid analgesics, on a scheduled or as-needed basis, for at least three months before the study. As well, they needed radiographic evidence of joint degeneration, from no more than six months before enrolment, in the medial and/or lateral tibiofemoral compartment, or in the hip, with a minimum severity of grade 2, as illustrated in the Standard Atlas of Radiographs of Arthritis (42). Grade 2 changes are defined by the presence of an osteophyte and joint space narrowing. Patients with more advanced radiographic grades were also eligible, as long as they were not awaiting surgery. Patients with intolerance to any opioid or NSAID, or a history of drug or alcohol abuse were excluded from the study. Patients with the following medical conditions were also excluded: renal or hepatic impairment, secondary OA, significant pain of alternate etiology, shortened gastrointestinal transit time, peptic ulcer disease, inflammatory disease of the gastrointestinal tract, a history of seizures or a recognized risk of seizures. Patients receiving corticosteroids, viscosupplementation, monoamine oxidase inhibitors, carbamazepine, quinidine, antidepressants, neuroleptics, cyclobenzaprine or promethazine were excluded from the study. Research ethics boards at the participating centres approved the protocol and informed consent, and each patient gave written informed consent before participating in the study.
Medications
Oral CR tramadol (Zytram XL, Purdue Pharma, Canada) in 200 mg, 300 mg and 400 mg tablets or SR diclofenac (Voltaren SR, Novartis, Switzerland) in 75 mg and 100 mg tablets was administered once daily in the morning, or 75 mg SR diclofenac was administered twice daily. All patients were randomly assigned an initial dose of either active CR tramadol 200 mg and placebo SR diclofenac 75 mg each morning, or active SR diclofenac 75 mg and placebo CR tramadol 200 mg each morning. The doses of active medication and placebo were titrated at weekly clinic visits (200 mg, 300 mg or 400 mg CR tramadol, or 75 mg, 100 mg or 150 mg SR diclofenac) up to the maximum daily dose, unless adequate pain control was achieved or side effects prevented the dose from being titrated further. Study medication was prepackaged with an assigned randomization number, according to a computer-generated code, in blocks of four. Blinding was maintained using the double-dummy technique. Breakthrough pain was managed with 325 mg to 650 mg acetaminophen (Tylenol, McNeil Consumer Healthcare, Canada) every 4 h to 6 h, as required.
Study design
The present study was a randomized, double-blind, parallel-group comparison of the efficacy, safety and clinical effectiveness of CR tramadol and SR diclofenac over a six-week treatment period. Following the pretreatment visit (visit 1), all analgesics were withdrawn for a two- to seven-day washout period before randomization to active treatment.
On the evening before the first dose of active treatment, and every morning during the treatment evaluation, patients recorded their pain intensity in a diary using the pain subscale of the Western Ontario and McMaster Universities (WOMAC) OA index, and an overall pain intensity 100 mm visual analogue scale (VAS) (anchors: no pain, extreme pain) (43,44). Patients were asked to rate their pain intensity experience while walking on a flat surface, going up or down stairs, at night while in bed, sitting or lying, and standing upright. The overall daily pain intensity was also assessed with a 100 mm VAS (anchors: no pain, extreme pain) by asking the patient, “What was your average pain over the past 24 hours?”
At visit 1 and each subsequent visit, patients completed the entire WOMAC questionnaire, including the pain, stiffness and physical function subscales, and provided an overall VAS score for pain intensity over the past two weeks. Severity of stiffness was assessed after first awakening in the morning, and after sitting, lying or resting later in the day (anchors: no stiffness, extreme stiffness). Physical function was assessed by asking the patients about their degree of difficulty with descending stairs, ascending stairs, rising from sitting, standing, bending to the floor, walking on flat surfaces, getting in and out of a car, going shopping, putting on socks or stockings, rising from bed, taking off socks or stockings, lying in bed, getting in and out of the bath, sitting, getting off and on the toilet, heavy domestic duties and light domestic duties (anchors: no difficulty, extreme difficulty).
The impact of pain on quantity and quality of sleep was assessed at visit 1 and each clinic visit using the Pain and Sleep Questionnaire, which consists of eight items related to the impact of pain on sleep. Seven items were rated on a 100 mm VAS (anchors: never to always), and one item was based on the number of hours of sleep. The scores for items 1 through 5 of the Pain and Sleep Questionnaire (‘trouble falling asleep due to pain’, ‘needed pain medication to sleep’, ‘needed sleeping medication to sleep’, ‘awakened by pain at night’ and ‘awakened by pain in the morning’) were summed to determine a composite score.
At the completion of the study, the patient and investigator each provided a global assessment of clinical effectiveness, using a seven-point categorical scale (markedly improved, moderately improved, slightly improved, no change, slightly worse, moderately worse, markedly worse). The propensity for abuse was assessed by completion of the Drug Liking Index, a nine-point scale (1 = ‘I dislike the drug effect very much’ and 9 = ‘I like the drug effect very much’).
Adverse events spontaneously reported by patients and adverse events observed by the investigator were recorded at each visit.
Data analysis
The primary measures of efficacy were the daily overall pain intensity VAS score and the daily WOMAC VAS pain sub-scale score. Because the dose was titrated upwards during the six-week treatment period, efficacy data from weeks 4 to 6 served as the primary basis for comparing the two treatments. All randomly assigned patients were included in the intent-to-treat (ITT) population, with the exception of one patient who did not meet the eligibility criteria. All patients who completed the study were included in the per protocol population, with the exception of one patient with a protocol violation. Safety data are presented using the ITT population and efficacy data are presented using the per protocol population.
The characteristics of the two treatment groups were compared for both demographic and efficacy variables. Change from prestudy treatment was tested using the paired t test, and 95% confidence limits were calculated for the difference among treatments in change scores. Categorical outcome measures were analyzed using Pearson’s χ2 to determine differences between groups, and McNemar’s χ2 to determine differences between treatment visits among patients. Analysis of covariance, using visit 1 as a covariate, was used to compare treatment effects for continuous variables. Compliance data and acetaminophen use were analyzed by repeated measures ANOVA, comparing treatment groups at treatment weeks 2, 4 and 6.
The sample size was estimated based on type 1 and type 2 error rates of α=0.05 (two-tailed) and β=0.20 for a parallel group study, assuming a 15 mm difference in pain intensity score to be clinically meaningful, as proposed by Bellamy et al (45). All data are reported as mean ± SD.
RESULTS
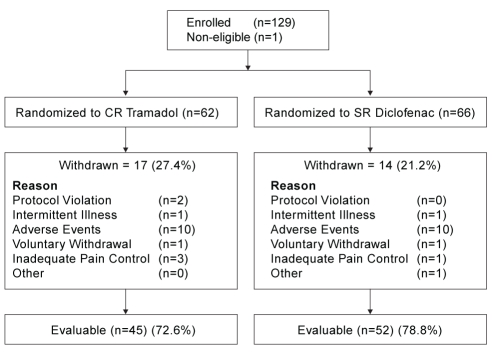
One hundred twenty-nine patients were enrolled. However, one patient was excluded from all analyses due to lack of evidence of OA, leaving 128 patients in the ITT population. Sixty-two patients were randomly assigned to the CR tramadol group (42 women and 20 men) and 66 patients were randomly assigned to the SR diclofenac group (44 women and 22 men). Ninety-seven patients were evaluated for efficacy; 45 in the CR tramadol group (mean age 59.4±9.5 years) and 52 in the SR diclofenac group (mean age 64.7±6.9 years). The reasons for patient withdrawal were similar in both groups and are shown in Figure 1.
Figure 1).
Patient disposition. CR Controlled-release; SR Sustained-release
The mean duration of OA was 9.3±8.4 years and 12.0±10.0 years for the CR tramadol and SR diclofenac groups, respectively (P=0.3576). A description of the joints involved is shown in Table 1. The mean number of joints involved was 2.6±1.0 and 2.8±1.3 for the CR tramadol and SR diclofenac groups, respectively (P=0.6261). Ninety-four per cent of patients in the CR tramadol group, and 88% of patients in the SR diclofenac group had an OA grade of 2 or 3 for their worst joint.
TABLE 1.
Joints affected by osteoarthritis
| Joint | Number of patients, n (%)
|
|
|---|---|---|
| CR tramadol | SR diclofenac | |
| Left knee | 37 (82) | 40 (77) |
| Right knee | 38 (84) | 44 (85) |
| Left hip | 12 (37) | 20 (38) |
| Right hip | 14 (31) | 24 (46) |
| Other* | 14 (33) | 18 (35) |
The mean number of joints involved was 2.6±1.0 and 2.8±1.3 for the CR tramadol and SR diclofenac groups, respectively (P=0.6261).
Other joints included backs, hands, feet, fingers, toes, shoulders, wrists, ankles and necks. CR Controlled-release; SR Sustained-release
During the last two weeks of treatment (visits 4 and 5), the mean daily dose of CR tramadol (370.2±51.9 mg at each visit) and SR diclofenac (142.6±23.8 mg and 141±23.6 mg for visits 4 and 5, respectively) remained stable. The maximum daily dose of CR tramadol and SR diclofenac was attained by 73% and 83% of patients, respectively. Patients could use up to six 325 mg tablets of acetaminophen per day for unrelieved pain. The mean weekly numbers of rescue tablets consumed for week 6 were 1.6±2.1 and 1.7±2.0 for CR tramadol and SR diclofenac, respectively. There was no significant difference in the amount of rescue medication consumed between the treatment groups or between the weeks of treatment for either group.
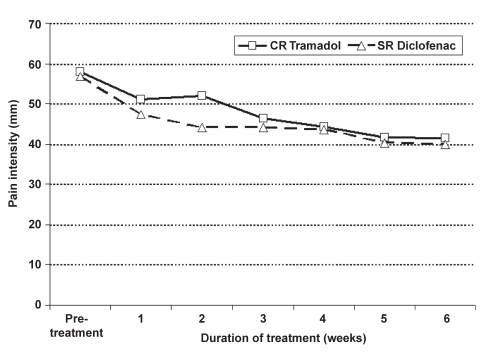
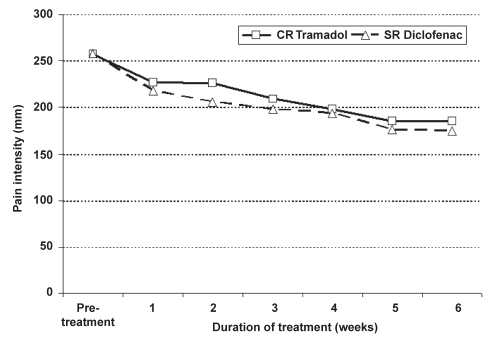
There was no significant difference between the overall VAS pain scores and the WOMAC pain subscale averaged weekly for the two treatments. However, pain scores decreased significantly from pretreatment assessed at visit 1 for both treatments, starting at week 1 (Figure 2 and Figure 3). The mean pretreatment scores for the WOMAC pain subscale were 257.1±98.7 and 257.6±116.4 for CR tramadol and SR diclofenac, respectively. Mean change from visit 1 to the last week of treatment (week 6) for the WOMAC pain subscale was 73.2±99.9 (P=0.0001) for CR tramadol and 80.2±108.1 (P=0.0001) for SR diclofenac. Mean change from visit 1 for the overall VAS pain score was 17.3±22.6 (P=0.0001) for CR tramadol, and 16.4±24.4 (P=0.0001) for SR diclofenac.
Figure 2).
Pain intensity (100 mm visual analogue scale) by week during controlled-release (CR) tramadol and sustained-release (SR) diclofenac treatment
Figure 3).
Western Ontario and McMaster Universities pain subscale by week during controlled-release (CR) tramadol and sustained-release (SR) diclofenac treatment
The mean difference between treatments, in change from prestudy treatment, for overall VAS pain intensity was 0.39, with an upper 95% confidence level of 8.5. For the WOMAC pain subscale (standardized to 100 mm), the corresponding mean difference was −1.4, with an upper 95% confidence level of 5.8. For both measures, the upper 95% confidence level was below 10 mm.
Both treatment groups improved significantly from pre-treatment for physical functioning (mean change of 257.0±354.4 for CR tramadol, P=0.0005, and 247.4±379.5 for SR diclofenac, P=0.0001) and stiffness (mean change of 34.3±61.4 for CR tramadol, P=0.0005, and 36.8±57.4 for SR diclofenac, P=0.0001) as measured by the WOMAC subscales completed at the first clinic visit and at the end of the present study. There was no significant difference between the two treatment groups (Table 2).
TABLE 2.
Western Ontario and McMaster Universities physical function and stiffness subscales completed at clinic visits
| Time (weeks) | Physical function
|
Stiffness
|
||
|---|---|---|---|---|
| CR tramadol | SR diclofenac | CR tramadol | SR diclofenac | |
| 0 (visit 1) | 890.8±313.0 | 854.5±392.1 | 124.0±48.0 | 115.8±50.5 |
| 2 | 793±427.8* | 696.3±434.7* | 107.2±52.1* | 86.4±51.3* |
| 4 | 685±404.6* | 651.7±449.8* | 92.2±47.4* | 82.4±57.9* |
| 6 | 633.9±406.7* | 607.1±456.2* | 89.7±52.4* | 79±56.2* |
P<0.05 for change from baseline. CR Controlled-release; SR Sustained-release
Pain and Sleep Index scores for the two treatment groups were similar at visit 1 and at the end of treatment (Table 3). For each of the categories in the Pain and Sleep Index, the mean response decreased compared with visit 1, indicating improved sleep. There was no statistically significant difference between the treatment groups at visit 1 or visit 5. No significant difference was found between the treatment groups for the ‘pain and sleep’ score and the ‘quality of sleep’ score, but a significant result was found for the covariate, indicating a significant change from prestudy treatment at the end of the present study.
TABLE 3.
Impact of pain on sleep at baseline and visits 4 and 5 of each treatment
| Question*† | CR tramadol
|
SR diclofenac
|
||
|---|---|---|---|---|
| Baseline | Visits 4 and 5 | Baseline | Visits 4 and 5 | |
| Hours of sleep | 6.7±1.5 | 6.9±1.4 | 6.3±1.5 | 6.4±1.4 |
| Quality of sleep | 50.8±26.7 | 60.9±26.1 | 47.6±32.5 | 55.7±29.6 |
| Trouble falling asleep | 35.9±30.9 | 21.0±23.3 | 43.8±32.5 | 32.0±34.8 |
| Need pain meds to sleep | 39.5±35.3 | 22.4±31.0 | 43.1±39.3 | 27.2±35.1 |
| Need sleeping meds to sleep | 17.5±28.3 | 10.5±23.8 | 17.6±29.1 | 15.0±27.6 |
| Awakened by pain at night | 40.9±32.5 | 27.8±31.5 | 44.0±32.3 | 32.0±32.2 |
| Awakened by pain in morning | 35.0±31.6 | 25.1±29.0 | 39.6±33.9 | 27.3±29.5 |
| Awakened by partner | 20.5±24.2 | 20.7±28.0 | 28.4±32.5 | 14.8±23.7 |
| Overall pain and sleep | 180.0±113.0 | 117.3±120.7 | 203.0±139.3 | 140.1±143.6 |
All scales are 100 mm visual analogue scales, except for hours of sleep and the overall pain and sleep (composite) score;
Lower scores indicate a lower impact of pain on sleep, except for ‘hours of sleep’ and ‘quality of sleep’, where higher scores are better. CR Controlled-release; Meds Medication; SR Sustained-release
Patients were asked to rate how much they liked or disliked the nonanalgesic effects of the drug at the end of the study. There was no significant difference between treatment groups in the scores for the Drug Liking Index. Of the 45 patients in the CR tramadol group, six disliked the drug effect, 22 neither liked nor disliked the drug effect and 17 liked the drug effect. Of the 52 patients in the SR diclofenac group, two disliked the drug effect, 31 neither liked nor disliked the drug effect and 19 liked the drug effect. Therefore, the majority of the patients neither liked nor disliked the drug effect (49% for CR tramadol and 60% for SR diclofenac, P=0.8000).
For global effectiveness, 67% and 54% of the evaluated patients reported moderate to marked improvement with CR tramadol and SR diclofenac, respectively (P=0.6590). Investigators also indicated patients’ pain improved moderately or markedly with CR tramadol (49%) and SR diclofenac (54%) (P=0.4290). No significant difference was found between the treatments for global effectiveness.
The overall incidence of adverse events related to the study drug was similar for both treatments, with 78% of CR tramadol patients reporting 165 adverse events and 59% of SR diclofenac patients reporting 101 adverse events. Early discontinuations from the study due to adverse events occurred in 16% and 15% of patients in the CR tramadol and SR diclofenac groups, respectively. The most commonly reported adverse events related to the study medication for both groups are given in Table 4. Ten patients from each treatment group withdrew due to similar adverse events, such as gastrointestinal upset and dizziness.
TABLE 4.
Incidence of most common adverse events related to study medication
| Event | Incidence, n (%)
|
|
|---|---|---|
| CR tramadol | SR diclofenac | |
| Dizziness | 15 (24.2) | 12 (18.2) |
| Nausea | 15 (24.2) | 7 (10.6) |
| Constipation | 13 (21.0) | 10 (15.2) |
| Somnolence | 11 (17.7) | 5 (8.1) |
| Vomiting | 9 (14.5) | 3 (4.5) |
| Headache | 7 (11.3) | 1 (1.5) |
| Sweating | 9 (14.5) | 0 (0) |
| Abdominal pain | 2 (3.2) | 6 (9.1) |
CR Controlled-release; SR Sustained-release
As patients in the present study were titrated to the most effective dose over time, adverse events were examined posthoc by the final dose of CR tramadol. Patients were subdivided into those who were stabilized on 400 mg (n=34), 300 mg to 350 mg (n=17) and 200 mg to 250 mg (n=10) of CR tramadol. One patient’s dose was unknown due to noncompliance. While constipation was stable across all dose ranges (20.0%, 23.5% and 20.6% for 200 mg to 250 mg, 300 mg to 350 mg and 400 mg, respectively), there were declines in other adverse events, such as dizziness (60.0%, 41.2% and 11.8% for 200 mg to 250 mg, 300 mg to 350 mg and 400 mg, respectively) and nausea (30.0%, 41.2% and 17.6% for 200 mg to 250 mg, 300 mg to 350 mg and 400 mg, respectively) for the three dose ranges.
Two patients treated with SR diclofenac experienced serious adverse events; one patient was hospitalized for gastrointestinal bleeding after 45 days of treatment and the other patient was hospitalized for severe pancreatitis after 14 days of treatment. Both of these events were judged to be related to SR diclofenac. There were no serious adverse events in patients treated with CR tramadol.
DISCUSSION
The results of the present trial demonstrated that CR tramadol is as effective as SR diclofenac in the treatment of pain due to OA of the hip and/or knee. Patients in both groups reported improved pain relief at the end of the treatment period, compared with prestudy treatment. Furthermore, patients reported improvements in sleep quality and physical function, which are important determinants of quality of life in patients with chronic musculoskeletal disease. Because lack of sleep can exacerbate pain, improvement of sleep is an important goal of pain management (46). Patients were titrated to stable and effective doses of CR tramadol and SR diclofenac within four to six weeks of initiation, showing statistically significant improvements in pain control and physical functioning.
In a recent meta-analysis of randomized controlled trials (47), it was found that treatment of OA with tramadol led to decreases in pain intensity and improvements in function. In the discussion, the authors concluded that the maximum expected decrease in pain intensity with tramadol treatment was 12.5 mm on a 100 mm VAS scale. The authors rated this level of pain relief as similar to that of acetaminophen, and inferior to that of NSAIDs. In a comparison of CR tramadol with placebo, using the same methodology as the present study, VAS reductions of 5.7 mm were observed for the placebo arm (48). In the present study, however, decreases of 17.4 mm and 16.4 mm were seen for CR tramadol and SR diclofenac, respectively, indicating that properly dosed tramadol can be equally efficacious as an NSAID product.
In a titration-to-effect crossover study by Beaulieu et al (40), once-daily dosing of CR tramadol was compared with IR tramadol given as needed. Interestingly, the patients who self-administered IR tramadol as needed considerably underdosed their medication compared with the patients taking scheduled CR tramadol, resulting in poorer pain control. In the present study, scheduled, once-daily CR tramadol and SR diclofenac were used to a maximum dose of 400 mg/day and 150 mg/day, respectively, to differentiate from the Pavelka study (49). This difference in the dosing schedule was associated with a much higher average total daily dose of CR tramadol, compared with the average total daily dose of IR tramadol relative to the Beaulieu et al study (370.2 mg versus 164.8 mg for tramadol) (40). In an earlier study by Pavelka et al (49), IR tramadol was compared with IR diclofenac in OA patients. Both medications were given as needed, to a maximum dose of 300 mg/day for tramadol and 150 mg/day for diclofenac. Correspondingly, patients experienced greater functional improvement in the overall WOMAC pain, stiffness and function scores in the present study, compared with the study of Pavelka et al (26.8% versus 19.2% with tramadol, and 27.5% versus 24.8% with diclofenac) (49). In a recent comparison with CR tramadol, placebo response on the WOMAC function score was 19.2% (48). The incidence of adverse events was also lower in the study of Pavelka et al (20% and 3% of patients during tramadol and diclofenac treatment, respectively, compared with 78% and 65% in the present study) (49), which is consistent with the lower doses used when analgesics were prescribed on an as-needed basis.
Adverse events usually occur at the beginning of tramadol therapy and diminish with continued treatment (50). High initiation doses and rapid titration of IR tramadol were reported to lead to intolerable adverse events and discontinuation of treatment in more than 30% of patients, possibly as many as 50% (51,52). In the present study, CR tramadol titrations were made at weekly intervals from a starting dose of 150 mg daily. This resulted in good tolerability, with 16% of discontinuations due to adverse events. There were no indications of an increase in adverse events at higher doses of tramadol. This was similar to the SR diclofenac group, in which 15% of withdrawals were due to adverse events. A majority of patients, on either product, attained the maximum daily dose permitted: 400 mg of CR tramadol and 150 mg of SR diclofenac.
Previous studies have shown that tramadol is as effective as NSAIDs (21,49), and more effective than dextropropoxyphene (53) in the treatment of chronic OA pain. However, patients receiving long-term NSAID therapy risk severe gastrointestinal symptoms, including ulceration and bleeding. In most patients, and especially high-risk groups such as elderly patients, concurrent cytoprotective agents are recommended, increasing the cost of treatment (54). The results of a large meta-analysis (9) of published and unpublished data supported the association between NSAID use and serious upper gastrointestinal complications, and in another meta-analysis (12), diclofenac had a relative risk of serious cardiovascular events of 1.40 (95% CI 1.16 to 1.70). In the present study, one patient in the diclofenac treatment group experienced gastrointestinal bleeding and another patient experienced pancreatitis. For OA patients exhibiting gastrointestinal intolerance of NSAIDs, a trial course of tramadol would represent an alternative therapy without similar risks for gastrointestinal toxicities. There were no serious adverse events experienced by patients during treatment with CR tramadol. This adverse event profile suggests that CR tramadol, and not acetaminophen-codeine combination products, is the suitable alternative for patients intolerant of, or unsuitable for, NSAID therapy.
The three-step ladder, proposed for cancer pain relief by the World Health Organization (WHO), is now widely used for all types of pain. Step 1 includes nonopioid analgesics, step 2, weak opioids, and step 3, strong opioids. It was suggested that tramadol should be retained in step 2 with codeine (55), the prodrug for morphine that is commonly used in modest doses for the treatment of OA pain, usually in combination with either acetaminophen or acetylsalicylic acid. Stronger opioids, such as CR oxycodone, are also effective in the treatment of moderate to severe pain due to OA (56–58). They may be reserved for OA pain refractory to other treatments, as is consistent with the American Pain Society and the WHO analgesic ladder (34).
The present study showed a trend toward slightly higher incidences of adverse events with CR tramadol, compared with SR diclofenac (52 and 49 patients, respectively), although there was no significant difference in the number of patients withdrawing due to adverse events. More patients in the CR tramadol treatment group reported nausea than patients in the SR diclofenac treatment group (15 and seven patients, respectively), whereas more patients in the SR diclofenac group reported abdominal pain than in the CR tramadol group (six and two patients, respectively).
Although tramadol exerts its analgesic effect through a weak binding affinity for the μ-opioid receptor, it also binds weakly to the serotonin presynaptic receptor, causing it to act as an atypical opioid analgesic. Consistent with this atypical profile, and as recently summarized by WHO, no significant respiratory or cardiac side effects have been associated with tramadol when the drug is given at the recommended oral doses (14,31,34). Tolerance, dependence, diversion and abuse also appear to be lower with tramadol than other opioids (31,34). In the present study, assessment of patient approval of tramadol was not significantly different from that of diclofenac, which is consistent with the results of two post-marketing surveillance studies that confirm the low abuse potential of tramadol. In an American postmarketing surveillance study (32), the rate of abuse was low, with only two cases per 100,000 patients in the first 18 months of availability, and a decline to one case per 100,000 patients in the succeeding 18 month period. Most of the cases occurred in individuals with a history of substance abuse (32). In a second study of health care professionals (33), the incidence of tramadol abuse or dependence was only 6.9 per 1000 patients per year.
CONCLUSION
CR tramadol, a once-daily formulation marketed as Zytram XL, is as effective as SR diclofenac in the treatment of pain due to knee or hip OA, with the potential for fewer of the serious side effects that characterize NSAID administration.
Footnotes
SUPPORT: This study was supported by a research grant from Purdue Pharma, Canada.
REFERENCES
- 1.Badley EM. The effect of osteoarthritis on disability and health care use in Canada. J Rheumatol Suppl. 1995;43:19–22. [PubMed] [Google Scholar]
- 2.Roth SH, Reder RF. The role of opioids in the treatment of osteoarthritis. Resid Staff Physician. 1998;44:31–6. [Google Scholar]
- 3.Williams HJ, Ward JR, Egger MJ, et al. Comparison of naproxen and acetaminophen in a two-year study of treatment of osteoarthritis of the knee. Arthritis Rheum. 1993;36:1196–206. doi: 10.1002/art.1780360904. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Zhao S, Lane N. Preference for nonsteroidal anti-inflammatory drugs over acetaminophen by rheumatic disease patients: A survey of 1,799 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Arthritis Rheum. 2000;43:378–85. doi: 10.1002/1529-0131(200002)43:2<378::AID-ANR18>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Pincus T, Koch G, Lei H, et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): Two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis. 2004;63:931–9. doi: 10.1136/ard.2003.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miceli-Richard C, Le Bars M, Schmidely N, Dougados M. Paracetamol in osteoarthritis of the knee. Ann Rheum Dis. 2004;63:923–30. doi: 10.1136/ard.2003.017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham GG, Scott KF, Day RO. Tolerability of paracetamol. Drug Saf. 2005;28:227–40. doi: 10.2165/00002018-200528030-00004. [DOI] [PubMed] [Google Scholar]
- 8.Fored CM, Ejerblad E, Lindblad P, et al. Acetaminophen, aspirin, and chronic renal failure. N Engl J Med. 2001;345:1801–8. doi: 10.1056/NEJMoa010323. [DOI] [PubMed] [Google Scholar]
- 9.Ofman JJ, MacLean CH, Straus WL, et al. A meta-analysis of severe upper gastrointestinal complications of nonsteroidal anti-inflammatory drugs. J Rheumatol. 2002;29:804–12. [PubMed] [Google Scholar]
- 10.Important safety information for patients taking Celebrex (celecoxib). <http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/_2002/2002_40_e.html> (Version current at March 26, 2008).
- 11.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963–76. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 12.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclo-oxygenase: A systematic review of the observational studies of selective and nonselective inhibitors of cyclo-oxygenase 2. JAMA. 2006;296:1633–44. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 13.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal anti-inflammatory drugs: An update for clinicians: A scientific statement from the American Heart Association. Circulation. 2007;115:1634–42. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 14.Houmes RJ, Voets MA, Verkaaik A, Erdmann W, Lachmann B. Efficacy and safety of tramadol versus morphine for moderate and severe postoperative pain with special regard to respiratory depression. Anesth Analg. 1992;74:510–4. doi: 10.1213/00000539-199204000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–85. [PubMed] [Google Scholar]
- 16.Roth SH. Efficacy and safety of tramadol HCl in breakthrough musculoskeletal pain attributed to osteoarthritis. J Rheumatol. 1998;25:1358–63. [PubMed] [Google Scholar]
- 17.Gardner JS, Blough D, Drinkard CR, et al. Tramadol and seizures: A surveillance study in a managed care population. Pharmacotherapy. 2000;20:1423–31. doi: 10.1592/phco.20.19.1423.34854. [DOI] [PubMed] [Google Scholar]
- 18.Gasse C, Derby L, Vasilakis-Scaramozza C, Jick H. Incidence of first-time idiopathic seizures in users of tramadol. Pharmacotherapy. 2000;20:629–34. doi: 10.1592/phco.20.7.629.35174. [DOI] [PubMed] [Google Scholar]
- 19.Jick H, Derby LE, Vasilakis C, Fife D. The risk of seizures associated with tramadol. Pharmacotherapy. 1998;18:607–11. [PubMed] [Google Scholar]
- 20.Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95:434–41. doi: 10.1093/bja/aei210. [DOI] [PubMed] [Google Scholar]
- 21.Dalgin P, for the TPS-OA study group Comparison of tramadol and ibuprofen for the chronic pain of osteoarthritis Arthritis Rheum 199740Suppl 9S86(Abst) [Google Scholar]
- 22.Pavelka K. Treatment of pain in osteoarthritis. Eur J Pain. 2000;4(Suppl A):23–30. [PubMed] [Google Scholar]
- 23.Adler L, McDonald C, O’Brien C, Wilson M. A comparison of once-daily tramadol with normal release tramadol in the treatment of pain in osteoarthritis. J Rheumatol. 2002;29:2196–9. [PubMed] [Google Scholar]
- 24.Simon L, Lipman A, Jacox A. Guideline for the Management of Pain in Osteoarthritis, Rheumatoid Arthritis and Juvenile Chronic Arthritis. 2nd ed. Glenview: American Pain Society; 2002. [Google Scholar]
- 25.Schnitzer TJ, Gray WL, Paster RZ, Kamin M. Efficacy of tramadol in treatment of chronic low back pain. J Rheumatol. 2000;27:772–8. [PubMed] [Google Scholar]
- 26.Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50:1842–6. doi: 10.1212/wnl.50.6.1842. [DOI] [PubMed] [Google Scholar]
- 27.Harati Y, Gooch C, Swenson M, et al. Maintenance of the long-term effectiveness of tramadol in treatment of the pain of diabetic neuropathy. J Diabetes Complications. 2000;14:65–70. doi: 10.1016/s1056-8727(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 28.Sindrup SH, Andersen G, Madsen C, Smith T, Brøsen K, Jensen TS. Tramadol relieves pain and allodynia in polyneuropathy: A randomised, double-blind, controlled trial. Pain. 1999;83:85–90. doi: 10.1016/s0304-3959(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 29.Russell IJ, Kamin M, Sager DS, et al. Efficacy of tramadol in treatment of pain in fibromyalgia. Arthritis Rheum. 1997;40(Suppl):S521. [Google Scholar]
- 30.Keup W. Mißbrauchsmuster bei Abhängigkeit von Alkohol, Medikamenten und Drogen: Frühwarnsystem-daten für die Bundesrepublik Deutschland 1976–1990. Lambertus: Freiburg im Breisgau; 1993. [Google Scholar]
- 31.Preston KL, Jasinski DR, Testa M. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991;27:7–17. doi: 10.1016/0376-8716(91)90081-9. [DOI] [PubMed] [Google Scholar]
- 32.Cicero TJ, Adams EH, Geller A, et al. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend. 1999;57:7–22. doi: 10.1016/s0376-8716(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 33.Knisely JS, Campbell ED, Dawson KS, Schnoll SH. Tramadol post-marketing surveillance in health care professionals. Drug Alcohol Depend. 2002;68:15–22. doi: 10.1016/s0376-8716(02)00107-2. [DOI] [PubMed] [Google Scholar]
- 34.WHO Expert Committee on Drug Dependence. Thirty-fourth report. <http://whqlibdoc.who.int/trs/WHO_TRS_942_eng.pdf> (Version current March 26, 2008).
- 35.Richter W, Barth H, Flohé L, Giertz H. Clinical investigation on the development of dependence during oral therapy with tramadol. Arzneimittelforschung. 1985;35:1742–4. [PubMed] [Google Scholar]
- 36.Osipova NA, Novikov GA, Beresnev VA, Loseva NA. Analgesic effect of tramadol in cancer patients with chronic pain: A comparison with prolonged-action morphine sulfate. Curr Ther Res. 1991;50:812–21. [Google Scholar]
- 37.Chrubasik J, Buzina M, Schulte-Mönting J, Atanassoff P, Alon E. Intravenous tramadol for post-operative pain – comparison of intermittent dose regimens with and without maintenance infusion. Eur J Anaesthesiol. 1992;9:23–8. [PubMed] [Google Scholar]
- 38.Wilder-Smith CH, Bettiga A. The analgesic tramadol has minimal effect on gastrointestinal motor function. Br J Clin Pharmacol. 1997;43:71–5. doi: 10.1111/j.1365-2125.1997.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 39.Janssen-Ortho Inc. Product Monograph Tramacet <http://www.janssen-ortho.com/JOI/pdf_files/tramacet_E.pdf> (Version current at March 26, 2008).
- 40.Beaulieu AD, Peloso P, Bensen W, et al. A randomized, double-blind, 8-week crossover study of once-daily controlled-release tramadol versus immediate-release tramadol taken as needed for chronic noncancer pain. Clin Ther. 2007;29:49–60. doi: 10.1016/j.clinthera.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Beaulieu A, Callaghan D, O’Mahony W, et al. A randomized, double-blind, crossover comparison of the efficacy and safety of oral controlled release tramadol with placebo in patients with painful osteoarthritis Pain Res Manage 200611108(Abst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Symposium on Population Studies in Relation to Chronic Rheumatic Diseases, Rome, 1961 . Atlas of Standard Radiographs of Arthritis. Vol. 2. Philadelphia: FA Davis; 1963. The Epidemiology of Chronic Rheumatism. [Google Scholar]
- 43.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 44.Bellamy N. Pain assessment in osteoarthritis: Experience with the WOMAC osteoarthritis index. Semin Arthritis Rheum. 1989;18(Suppl 2):14–7. doi: 10.1016/0049-0172(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 45.Bellamy N, Carette S, Ford PM, et al. Osteoarthritis antirheumatic drug trials. III. Setting the delta for clinical trials – results of a consensus development (Delphi) exercise. J Rheumatol. 1992;19:451–7. [PubMed] [Google Scholar]
- 46.Holman GH. Chronic nonmalignant pain. Clin Geriatr. 1997;5:21–4. 38, 41. [Google Scholar]
- 47.Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis: A systematic review and meta-analysis. J Rheumatol. 2007;34:543–55. [PubMed] [Google Scholar]
- 48.Thorne C, Beaulieu AD, Callaghan DJ, et al. A randomized, double-blind, crossover comparison of the efficacy and safety of oral controlled-release tramadol and placebo in patients with painful osteoarthritis. Pain Res Manage. 2008;13:93–102. doi: 10.1155/2008/165421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pavelka K, Peliskova Z, Stehlikova H, Ratcliffe S, Repas C. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clin Drug Investig. 1998;16:421–9. doi: 10.2165/00044011-199816060-00002. [DOI] [PubMed] [Google Scholar]
- 50.Sorge J, Stadler TH. Comparison of the analgesic efficacy and tolerability of tramadol 100 mg sustained-release tablets and tramadol 50 mg capsules for the treatment of chronic low back pain. Clin Drug Investig. 1997;14:157–64. [Google Scholar]
- 51.Petrone D, Kamin M, Olson W. Slowing the titration rate of tramadol HCl reduces the incidence of discontinuation due to nausea and/or vomiting: a double-blind randomized trial. J Clin Pharm Ther. 1999;24:115–23. doi: 10.1046/j.1365-2710.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 52.Ruoff GE. Slowing the initial titration rate of tramadol improves tolerability. Pharmacotherapy. 1999;19:88–93. doi: 10.1592/phco.19.1.88.30515. [DOI] [PubMed] [Google Scholar]
- 53.Jensen EM, Ginsberg F. Tramadol versus dextropropoxyphene in the treatment of osteoarthritis. A short term double-blind study. Drug Invest. 1994;8:211–8. [Google Scholar]
- 54.Rostom A, Dubé C, Jolicoeur E, Boucher M, Joyce J. Gastroduodenal ulcers associated with the use of non-steroidal anti-inflammatory drugs: A systematic review of preventive pharmacological interventions. Ottawa: Canadian Coordinating Office for Health Technology Assessment; 2004. [Google Scholar]
- 55.Reig E. Tramadol in musculoskeletal pain – a survey. Clin Rheumatol. 2002;21(Suppl 1):S9–11. doi: 10.1007/s100670200030. [DOI] [PubMed] [Google Scholar]
- 56.Roth SH, Fleischmann RM, Burch FX, et al. Around-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: Placebo-controlled trial and long-term evaluation. Arch Intern Med. 2000;160:853–60. doi: 10.1001/archinte.160.6.853. [DOI] [PubMed] [Google Scholar]
- 57.Caldwell JR, Hale ME, Boyd RE, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal anti-inflammatory drugs: A double blind, randomized, multicenter, placebo controlled trial. J Rheumatol. 1999;26:862–9. [PubMed] [Google Scholar]
- 58.Markenson JA, Croft J, Zhang PG, Richards P. Treatment of persistent pain associated with osteoarthritis with controlled-release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain. 2005;21:524–35. doi: 10.1097/01.ajp.0000146215.86038.38. [DOI] [PubMed] [Google Scholar]