Abstract
Atopic dermatitis (AD) is a chronic dermatosis bearing clinical, histological, and immunologic similarities to chronic allergic contact dermatitis (ACD). AD shows a Th2 cell-dominant inflammatory infiltrate, elevated serum IgE levels, a permeability barrier abnormality, and Staphylococcus aureas colonization. Repeated hapten challenges reportedly produce a Th2-like hypersensitivity reaction (Th2-like HR). Here, 9–10 challenges with oxazolone (Ox) to hairless mice also produced a chronic Th2-like HR. Permeability barrier function and expression of differentiation proteins, filaggrin, loricrin, and involucrin, became abnormal. CRTH-positive Th2-dominant inflammatory infiltrate, with increased IL-4 expression, and a large increase in serum IgE levels were observed. The barrier abnormality was associated with decreased stratum corneum (SC) ceramide content and impaired lamellar body secretion, resulting in abnormal lamellar membranes, as in human AD. Furthermore, as in human AD, epidermal serine protease activity in SC increased and expression of two lamellar body-derived antimicrobial peptides, CRAMP and mBD3, declined after Ox challenges, paralleling the decrease of their human homologues in AD. Thus, multiple Ox challenges to normal murine skin produce a chronic Th2-like HR, with multiple features of human AD. Because of its reproducibility, predictability, and low cost, this model could prove useful for evaluating both pathogenic mechanisms and potential therapies for AD.
INTRODUCTION
Over the last decade, a variety of murine models have been developed and characterized that display certain features of atopic dermatitis (AD). These models include: (1) spontaneous mutants, such as the Nc/Nga mouse (Matsuda et al., 1997; Aioi et al., 2001); (2) transgenic mice that either over-or under-express selective cytokines, such as IL-4, IL-18, or thymic stromal lymphopoietic factor (Chan et al., 2001b; Konishi et al., 2002; Chen et al., 2005); (3) mice genetically-engineered to over-express stratum corneum (SC) chymotryptic enzyme (kallikrein 7) (Hansson et al., 2002); and (4) mice challenged repeatedly with applications of soluble or aerosol haptens, such as ovalbumen (Savinko et al., 2005), mite antigens (Matsuoka et al., 2003; Gao et al., 2004; Kang et al., 2006), or trinitrochlorobenzene (Matsumoto et al., 2004). While repeated hapten challenges elicit an AD-like dermatosis with some features of AD (Matsumoto et al., 2004), an analogous process occurs only rarely in otherwise normal humans (Werfel et al., 1997), perhaps because barrier function is superior in human epidermis. The importance of a primary inherited defect in the barrier, with subsequent hapten ingress, for the pathogenesis of AD is becoming increasingly accepted, because inherited mutations in the gene that encodes the SC structural protein, filaggrin, have been reported in a plurality of European kindreds with AD (Marenholz et al., 2006; Palmer et al., 2006; Sandilands et al., 2006; Smith et al., 2006; Weidinger et al., 2006).
To date, hapten-induced, chronic ACD models, although having the unique advantages of being convenient, reproducible, and inexpensive, have not been sufficiently characterized to ascertain whether they are true analogues of AD. While the trinitrochlorobenzene model displays certain clinical and immunologic features of human AD (Matsumoto et al., 2004; Matsukura et al., 2005; Tagami and Kikuchi, 2006), whether epidermal structural, functional, and lipid biochemical abnormalities, characteristic of human AD, occur in this model, is not known. Without these types of essential data, hapten-induced models remain of limited use for further studies on either AD pathogenesis, or for the evaluation of barrier-based, therapeutic interventions of potential utility in AD. We describe here another hapten-induced chronic Th2-like hypersensitivity reaction (HR) model, in which simple applications of the sensitizer, oxazolone (Ox), previously utilized to elicit ACD (Sheu et al., 2002; Fowler et al., 2003), was utilized to generate a chronic Th2-like hypersensitivity reaction (Th2-like HR) model. Following 10 Ox challenges, a dermatosis emerges with not only the immunologic but also with epidermal structural, functional, and biochemical abnormalities that parallel human AD. Availability of this thoroughly characterized model should facilitate future studies on both AD pathogenesis, as well as the evaluation of emerging therapies for AD.
RESULTS
Repeated Ox applications provoke a pruritic dermatosis with increased epidermal hyperplasia and aberrant epidermal differentiation
While a single Ox challenge to hairless mouse skin produces an acute ACD-like, erythematous and edematous dermatosis over 6–12 hours (Figure S1; see also Sheu et al., 2002; Fowler et al., 2003), 9–10 Ox challenges over 22–25 days provoked a persistent, chronic dermatosis with evidence of moderate-to-severe pruritus (Figure S1).
Whereas single Ox challenges produce mild epidermal hyperplasia, this feature became more prominent with successive challenges (Figure S2), so that by 10 applications mean epidermal thickness was 2.06±0.05 vs 0.67±0.03 in vehicle-treated controls (P<0.0001). The development of epidermal hyperplasia could be attributed to a marked increase in DNA synthesis, demonstrated by increased numbers of proliferating cell nuclear antigen+ cells in the basal and first suprabasal epidermal layers (Figure S2). Finally, epidermal hyperplasia was accompanied by a prominent inflammatory infiltrate, even after one Ox challenge, but inflammation became more apparent after 10 Ox challenges (Figure S2). In parallel with the emergence of epidermal hyperplasia and inflammation, expression of three structural protein markers of differentiation (loricrin, involucrin, filaggrin) either declined in the outer nucleated layers, or extended proximally into suprabasal cell layers with repeated Ox challenges (Figure S3).
Abnormal epidermal function appears only after repeated hapten applications
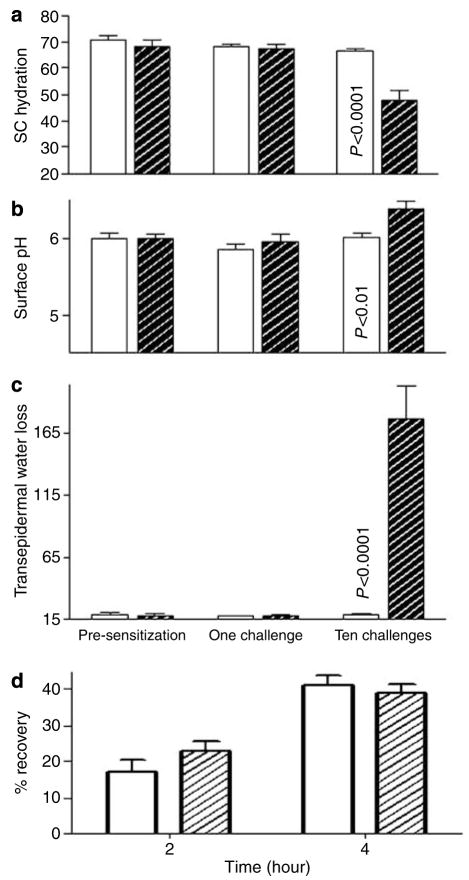
We next assessed whether progression from acute to chronic ACD is associated with alterations in epidermal functional parameters, including changes in permeability barrier homeostasis, SC hydration, and SC acidification. While all of these functions remain normal after a single Ox challenge, basal permeability barrier homeostasis, assessed as rates of transepidermal water loss (TEWL) increased markedly after 10 Ox challenges (Figure 1c, >10-fold elevation; P<0.0001). At this time point, SC hydration also declined significantly (Figure 1a; P<0.01), while skin surface pH increased by about a unit (Figure 1b; P<0.01). Finally, barrier recovery kinetics, assessed as the rate of decline in TEWL over time, either accelerated modestly or remained unchanged in repeatedly Ox-challenged skin sites (Figure 1d), as in human AD (Seidenari and Giusti, 1995).
Figure 1. Functional abnormalities emerge after multiple Ox Challenges.
(a) SC hydration assessed as electrical capacitance, in absolute units. (b) Surface pH assessed with a flat surface electrode. (c) Permeability barrier function, assessed as changes in basal TEWL with an electrolytic water analyzer (as mg/cm2/hour). (d) Kinetics of permeability barrier recovery after acute barrier disruption by cellophane tape stripping (initial TEWL is increased to 20- to 30-fold above basal levels).
SC lipid abnormalities correlate with the barrier abnormality in Ox-challenged mice
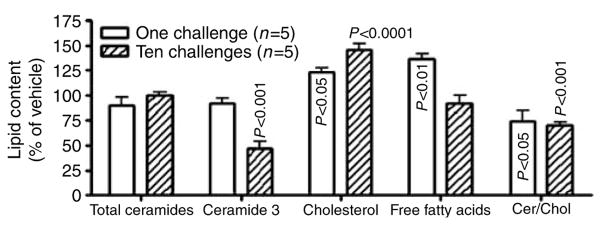
Human AD is characterized by a decline in two key SC lipids, ceramides (Cers), and free fatty acids, with a further, selective decline in certain Cer species, most notably Cer 3 (Di Nardo et al., 1998; Macheleidt et al., 2002). Although total Cer content of SC in 10 Ox-challenged mice did not reduced, total free fatty acid content declined and that of cholesterol increased, as in human AD (Figure 2). Moreover, one of the five individual Cer species (murine SC displays only five Cer species because of lesser variation in sphingoid base and N-acyl fatty acid hydroxylation), Cer 3 declined significantly versus vehicle-treated controls (Figure 2; P<0.001), as observed repeatedly in human AD (Di Nardo et al., 1998; Macheleidt et al., 2002).
Figure 2. Repeated Ox challenges alter stratum corneum lipid content/distribution.
Cohorts of hairless mice (n=5 each) were treated with vehicle alone, or Ox (1 or 10 applications). Lipids were extracted from isolated stratum corneum sheets with Bligh–Dyer solvents, fractionated, quantified, and the content of each fraction was expressed as μg/mg dry SC weight. Data were expressed as % change from vehicle-treated±SEM.
Ox-challenged mice display abnormalities in lamellar body secretion and post-secretory, lamellar membrane organization
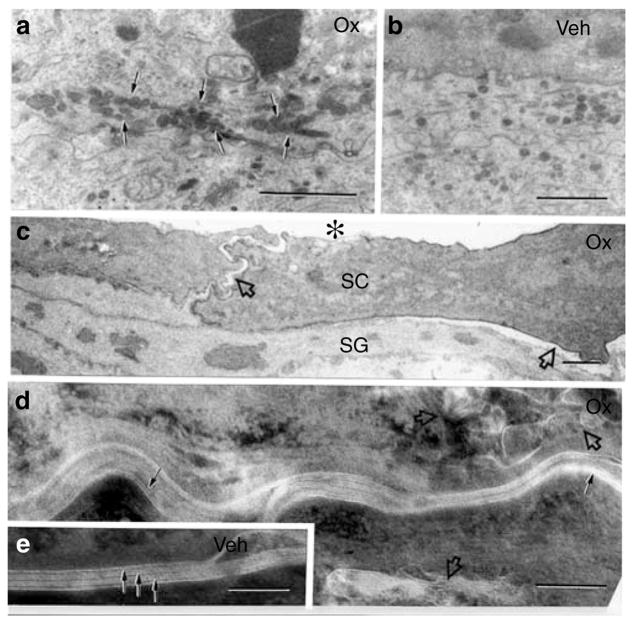
The epidermis of human AD displays abnormalities in both lamellar body secretion (Fartasch et al., 1992) and extracellular lamellar bilayer structure (Chamlin et al., 2002), findings that correlate with reported lipid abnormalities (Chamlin et al., 2002). While both lamellar body density and internal contents appeared normal in repeatedly Ox-challenged mice, secretion was impaired, as evidenced by retention of organelles in the peripheral cytosol (Figure 3a), coupled with reduced secretion at the stratum granulosum–SC interface (Figure 3c). Since considerable lamellar body contents become entombed within the corneocyte cytosol (Figure 3d, open arrows), rather than secreted, these contents would be unavailable to form lamellar membranes; hence, the decreased numbers of lamellar bilayers in repeated Ox-challenged mice (Figure 3d), as in human AD.
Figure 3. Repeat Ox-challenged SC displays entombed lamellar bodies, lamellar membrane disorganization, and corneocyte detachment.
(a) Lamellar bodies (LBs) accumulate in the peripheral cytosol of granular cells in Ox-challenged mice (arrows), while (b) LB are more widely dispersed in preparation for secretion in vehicle (Veh)-treated mice. (c) Ox-challenged corneocytes (SC) focally detach from underlying stratum granulosum (SG) and lose contact between themselves (open arrows), sometimes leading to large cleavage planes (asterisk). Decreased quantities and poor organization of extracellular lamellar membranes are seen in Ox-challenged (d, single arrows) versus vehicle-treated (e, layered arrows) mice. Note entombed organelle remnants in corneocyte cytosol of Ox-challenged mice, indicating incomplete LB secretion (d, open arrows). (a–c) Osmium tetroxide post-fixation; (d, e) ruthenium tetroxide post-fixation. Bars=1 μm (a–c); 0.1 μm (d, e).
Ultrastructural evidence of impaired SC cohesion in Ox-challenged mice
The desquamation abnormality in repeatedly Ox-challenged mice could result, in part, from epidermal hyperplasia (Figure S2), but it also correlated here with multiple foci in the lower SC where corneocytes appeared to detach prematurely (Figure 3c). Extensive separation occurred both between adjacent corneocytes (Figure 3c, asterisks), and between the SC and the underlying nucleated cell layers (Figure 3c, open arrows). These ultrastructural findings are consistent with the visible scale in these mice, increased serine protease (SP) activity in these mice (see below), and the decreased SC cohesion in human AD (Cork et al., 2006).
SP activity increases in Ox-challenged mice
The elevation in surface pH that follows repeated Ox challenges (Figure 1b) could result in increased SP activity (Hachem et al., 2003, 2005a). Such pH-induced changes in SP activity could, in turn, account for the abnormal corneocyte cohesion in Ox-challenged mice through degradation of corneodesmosomes. To assess this potential pathomechanism, we next assessed changes in SP activity and localization after Ox challenges by in situ zymography. SP activity was low both under basal conditions (Figure S4) and after single Ox challenges (Figure S4), and was restricted to a narrow region of the SC. In contrast, after 10 Ox challenges, SP activity increased throughout the SC, further impinging on the outer nucleated layers (Figure S4). The increase in SP activity correlates with the observed increase in pH of SC (c.f., Figure 1), providing a mechanistic basis for the alterations in desquamation in these mice.
Ox-challenged mice develop abnormalities in innate and adaptive immunity
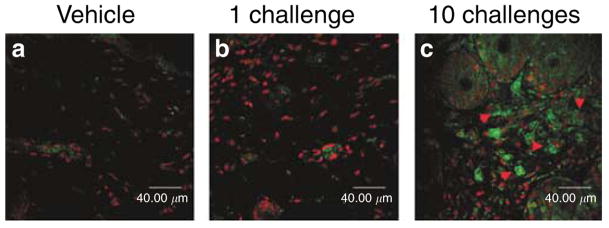
We next determined whether repeatedly Ox-challenged mice develop an immunophenotype that is similar to human AD. After a single Ox challenge, the dermis displayed a modest inflammatory infiltrate (Figure S2), dominated by postglandin D receptor (CRTH2)-negative lymphocytes (Figure 4b) and most became CRTH2 positive (Figure 4c), with an increased density of mast cells, but few eosinophils (Figure S2). Over subsequent challenges, the lymphocyte-dominated infiltrate increased progressively, with some lymphocytes appearing to invade the overlying epidermis (Figure S2). The density of eosinophils in the dermis of 10 Ox-challenged mice also increased significantly (139.04±28.13 vs 8.02±5.71 in vehicle-treated, and 29.41±18.46 after a single challenge; P<0.005 for 10 vs 1 challenge). In addition, immunostaining for IL-4 also markedly increased in the dermis of 10× Ox-challenged mice (Figure S5). Finally, the increase in Th2 immunophenotype was paralleled by a progressive increase in serum IgE levels. While mean serum IgE levels were 29.7±5.7 after one Ox challenge, they increased to 3,460±734 vs 20.2±3.5 in 10× vehicle-treated mice (P<0.002; n=5 each). These results show that repeated Ox challenges yield a predominant Th2 phenotype, prominent eosinophils, and highly elevated IgE levels, mirroring humans with “extrinsic” AD.
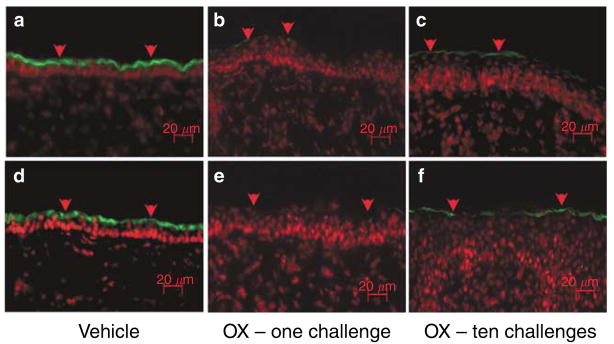
Figure 4. Th2 immunophenotype predominates after repeated Ox challenges.
(a) CRTH2 immunostaining of Th2 lymphocytes is virtually absent in dermis after (a) vehicle treatment alone or (b) one Ox challenge, but density of CRTH2-positive cells increases significantly after 10 challenges (c, arrows). Merged images with propidium iodide secondary staining in 5 μm frozen sections. Bar=40 μm.
In addition to the above-described changes in adaptive immunity, Ox-challenged mice rapidly developed alterations in antimicrobial peptide expression. While both the cathelicidin carboxyfragment, LL-37, and the human β-defensin 2, hBD2, typically upregulate during inflammation and/or wound healing (Sorensen et al., 2003; Butmarc et al., 2004), both of these peptides fail to upregulate in human AD (Ong et al., 2002b). Hence, we next assessed changes in expression of the murine analogues of LL-37 and hBD2 (i.e., cathelin-related antimicrobial peptide (CRAMP) and mBD3, respectively) after one vs 10 Ox challenges. Basal protein levels of both peptides were demonstrable in the outer nucleated layers in untreated murine epidermis (Figure 5a and d), but both CRAMP and mBD3 immunostainable proteins declined markedly after a single Ox challenge (Figure 5b and e), a decrease that was sustained after 10 Ox challenges (Figure 5c and f). Thus, repeated hapten challenges induce changes in two innate immunity peptides that parallel human AD (Ong et al., 2002b).
Figure 5. Antimicrobial peptide expression declines after one Ox challenge, and remains reduced after 10 challenges.
Immunofluorescent staining for (a–c) cathelicidin-related antimicrobial peptide (CRAMP) and (d–f) mouse β-defensin 3 (mBD3). (a, d) With vehicle treatment; (b, e) with one Ox challenge; (c, f) with 10 Ox challenges. Propidium iodide counterstaining. Bars=20 μm.
DISCUSSION
The striking increase in the incidence of atopic disorders, including AD, observed in recent decades, has been ascribed to the migration of populations from rural to urban areas, where lack of early exposure to a variety of microbes purportedly results in reduced immune tolerance. Although the “hygiene hypothesis” may have some validity for mucosal atopy, the increased prevalence of AD in industrialized, urban areas instead results from increased exposure to indoor aeroallergens, such as dust mites (Sager et al., 1992; Matsuoka et al., 2003; Kiank et al., 2006), and life-style changes coupled with frequent bathing, which could further degrade the barrier (Cork et al., 2006). Moreover, aeroantigens, responsible for mucosal atopy, can also gain access across the (defective) skin barrier in AD (Sager et al., 1992). Furthermore, early and frequent use of topical glucocorticoids, as well as increased psychologic stress, which increases endogenous GC, may further degrade the barrier (Denda and Tsuchiya, 2000; Kao et al., 2003; Choi et al., 2005a, 2006), thereby increasing disease susceptibility.
Until recently, research on the etiopathogenesis of AD has focused primarily on immunologic abnormalities, including the potential provocative role of sensitized dendritic cells (reviewed in Novak and Bieber (2005), increased IgE production and/or receptor expression (Boguniewicz et al., 2006), as well as the Th2 immunophenotype (Grewe et al., 1998; Leung et al., 2003, 2004), accompanied by production of cytokines that favor allergic responses and secondary pathogen colonization (Homey et al., 2006). Without discounting the role of immunologic influences in the development of AD, evidence is accumulating that the primary abnormality in AD could result from inherited defects in SC structure and function (reviewed in Segre (2006). Inherited barrier abnormalities may predispose to AD by facilitating antigen egress. Hence, the focus has shifted, quite appropriately, from a primarily immunologic (“inside-outside”) to a more-probable, “outside-inside” paradigm for AD pathogenesis (eg, Elias et al., 1996, 1996a, 1996b, 1999; Elias and Feingold, 1999). Accordingly, it becomes increasingly important to identify and characterize animal models that allow further investigations into the role of immunologic and skin barrier abnormalities in AD pathogenesis (Table S1).
The best-characterized murine model, the Nc/Nga mouse, develops a full panoply of atopic features, including a permeability barrier abnormality linked to Cer deficiency (Aioi et al., 2001). AD-like disease only develops when mice are housed under conventional, ambient (i.e., “dirty”), rather than sun protection factor conditions, but both environmental and topical haptens can provoke chronic Th2-like HR, even when Nc/Nga mice are housed in a sun protection factor facility (Matsuoka et al., 2003; Gao et al., 2004; Kang et al., 2006). Although the specific abnormality in Nc/Nga mice remains unknown, they display mutations on chromosome 9, linked to increased IgE production, as well as Th2-type T cells, bearing CCR-4, within chronic Th2-like HR lesions (Matsuoka et al., 2003; Gao et al., 2004; Kang et al., 2006). Coupled with further disadvantages of high costs, special housing requirements, and undependable elicitation of skin lesions, these mice have distinct disadvantages as an AD model. Likewise, published transgenic models, such as Klk7 (Brattsand and Egelrud, 1999) and IL-4 (Chan et al., 2001a, b) overexpressing, and IL-18 transgenic mice (Konishi et al., 2002), while potentially useful for dissecting specific etiopathogenic steps, are of limited use for assessing alternate therapeutic approaches for AD, and none of these 3 models is commercially-available.
Prompted by the urgent need to develop a convenient, reproducible model, relevant for studies on both AD pathogenesis and assessment of alternate therapeutic approaches, we turned to a hapten-induced model, already employed to assess pathogenesis and potential therapies of acute allergic contact dermatitis (AACD), the Ox-sensitized mouse (Nakae et al., 2001; Sheu et al., 2002; Fowler et al., 2003). We show here that with repeated hapten challenges over a 2- to 3-week period, this model shifts from typical delayed-type hypersensitivity, into a more chronic dermatosis, with multiple features of AD (Table S1), including the characteristic barrier (Seidenari and Giusti, 1995) and lipid abnormalities (Imokawa et al., 1991; Bleck et al., 1999). The short time required for disease induction, low costs, and reproducibility of disease expression are yet additional advantages of this model. Both the timing of disease development, and the cutaneous features of our model, are strikingly similar to the dermatosis that follows repeated applications of trinitrochlorobenzene (Kitagaki et al., 1995; Matsukura et al., 2005). Although such trinitrochlorobenzene mice demonstrate a shift from a Th1 to Th2 immunophenotype at sites of hapten application, neither epidermal structure, function, nor lipid biochemistry have been assessed in this model. Our studies show that initial hapten challenge, sufficient to elicit AACD, occurs while key epidermal functions still remain normal, emphasizing that inflammation alone does not suffice to produce epidermal functional abnormalities. Further hapten challenges, however, skew the AACD to a chronic ACD dermatosis, with a full panoply of features of AD, accompanied by the development of significant barrier dysfunction, likely allowing still more allergen to traverse the SC. The lesser competence of the permeability barrier in AD skin likewise presumably accounts for hapten ingress, sufficient to skew ACD toward chronic ACD, with features of AD. Although these results further underscore the potential importance of inherited abnormalities of the SC structural protein, filaggrin (Palmer et al., 2006), and/or SP/anti-protease polymorphisms (Walley et al., 2001; Vasilopoulos et al., 2004) as primary provocateurs of AD, repeated hapten challenge can lead to Th2 cell activation, which could also reduce barrier function (Hatano et al., 2005), linking immunologic changes to skin barrier abnormalities.
MATERIALS AND METHODS
Materials
Female hairless mice (hr/hr), aged 6–8 weeks old, were purchased from Charles River laboratories (Wilmington, MA) and fed mouse diet (Ralston-Purina Co., St Louis, MO) and water ad libitum. Ethanol and petroleum ether were purchased from Fisher Scientific (Fairlane, NJ). Ox, EDTA and trypsin were purchased from Sigma Chemical Co. (St Louis, MO). Affinity-purified, rabbit anti-mouse antibodies to loricrin, involucrin, and filaggrin were purchased from BabCo (Richmond, CA), and rabbit anti-mouse antibody against the postglandin D receptor, CRTH2/DP2, was from Cayman Chemical (Ann Arbor, MI). CRAMP antibody was a gift from Dr Richard Gallo, UCSD, and the mBD3 antibody was from Alpha Diagnostic (San Antonio, TX). Biotinylated goat anti-rabbit IgG antibody was purchased from Vector Lab (Burlingame, CA).
Experimental protocols and functional studies
All animal procedures were approved by the Animal Studies Subcommittee of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. One group of animals was sensitized by one topical treatment with 10 μl of 5% Ox, while the ethanol-treated vehicle group served as the control. A week later, the Ox-treated group was treated topically with 60 μl of 0.1% Ox on both flanks, once every other day for an additional 2 weeks, with ethanol alone serving again as vehicle control. Both before challenge and at the end of the treatment period, basal TEWL, measured with an electrolytic water analyzer (Meeco, Warrington, PA), and SC hydration, assessed as capacitance, with a Corneometer CM820 (Courage & Khazaka, Germany), as described previously (Choi et al., 2005b).
Immunohistochemistry and immunofluorescence
Immunohistochemical staining for assessment of changes in epidermal differentiation was performed as described earlier (Demerjian et al., 2006). Briefly, 5 μm paraffin sections were incubated with the primary antibodies overnight at 4°C. After washes ×3, sections were incubated with the secondary antibody for 30 minutes. Staining was detected with ABC-peroxidase kit from Vector Lab, and sections were then counterstained with hematoxylin. For immunohistochemistry for proliferating cells, changes in overall morphology were visualized after hematoxylin and eosin staining of 5 μm paraffin-enabled sections, and proliferating cells were detected by proliferating cell nuclear antigen staining. Briefly, 5 μm paraffin sections were incubated with biotinylated monoclonal antibody against proliferating cell nuclear antigen (CalTag Laboratories, Burlingame, CA) overnight at 4°C, and staining was detected by the ABC-peroxidase method (Vector).
Immunofluorescence was used to evaluate changes in T helper cell subpopulation expression. 5 μm frozen were incubated with the rabbit anti-mouse CRTH2/DP2 antibody overnight at 4°C, followed by incubation with fluorescence conjugated goat anti-rabbit antibody for 30 minutes at room temperature. Both CRAMP and mBD3 protein levels were assessed to determine whether antimicrobial peptide expression changes in epidermis, as described for human AD (Ong et al., 2002a, b). For immunohistochemical staining for IL-4, we employed the method of Fan et al. (2001). Sections were examined with either a Zeiss fluorescence microscope (Jena, Germany), or on a confocal microscope, and digital images were captured with AxioVision software (Carl Zeiss Vision, Munich, Germany).
Eosinophil density measurement
The areas of dermis in hematoxylin and eosin-stained sections first were quantitated in a digital microscope equipped with AxioVision software. The depth of the measured areas extended to≈170 μm below the basement membrane. The density of eosinophils was then expressed as the number of eosinophil per mm2 (n=15 for 10× challenged, n=12 for single challenge, and n=11 for vehicle-treated samples).
SP activity
SP activity was assessed in freshly obtained skin samples by in situ zymography, as previously described (Hachem et al., 2003, 2005b). 5 μm frozen sections were incubated with BODIPY-FI0-casain for 2 hours at 37°C. After 3× washing with 1% Tween 20 solution, sections were counter-stained with propidium iodide for 1 minute. Sections then were examined with the Zeiss or confocal microscopes, as above.
Serum IgE measurements
Blood samples were collected from mice tails after one Ox challenge, 10 Ox challenges, as well as in controls after one and 10 vehicle treatments alone. Serum IgE concentration was determined with a mouse IgE ELISA quantitation kit from Bethyl Laboratories (Montgomery, TX), following instructions provided by the manufacturer.
SC lipid content
After 2 weeks of treatment with Ox or vehicle, fresh SC sheets were collected after separation of dermis and epidermis by 10mM EDTA preincubations, followed by trypsinization, as described (Mao-Qiang et al., 1996). SC lipids were extracted by Bligh–Dyer solvents, and quantitated both as total lipids per mg protein and mg dry SC weight. Lipid extracts were further fractionated into cholesterol, free fatty acid, and Cer subfractions by high-performance thin layer chromatography, as described previously (Uchida and Hamanaka, 2006).
Electron microscopy
Skin biopsies of both vehicle and Ox-treated mice were fixed in Karnovsky’s fixative overnight, and post-fixed with either 0.25% ruthenium tetroxide or 1% aqueous osmium tetroxide, containing 1.5% potassium ferrocyanide, as described previously (Hou et al., 1991). Ultrathin sections were examined using an electron microscope (Zeiss 10A, Carl Zeiss, Thornwood, NY) operated at 60 kV.
Data are expressed as means ± SEM. A two-tailed Student’s t-test was used to determine significant differences, and a further analysis of variance analysis was performed when three or more groups were compared.
Supplementary Material
Figure S1. Repeatedly Ox-challenged mice develop a pruritic dermatosis.
Figure S2. Repeatedly Ox challenges cause epidermal hyperplasia and chronic inflammation.
Figure S3. Alterations in epidermal differentiation after multiple Ox challenges.
Figure S4. SP activity increases and extends into nucleated layers after 10 Ox challenges.
Figure S5. IL-4 increase levels in repeatedly Ox-challenged mice.
Table S1. Described pathophysiological features of human AD and AD model mice.
Acknowledgments
This study was supported by National Institutes of Health grants AR 050629, AR 19098 and PO39448, the Medical Research Service, Department of Veterans Affairs Medical Center.
Abbreviations
- ACD
allergic contact dermatitis
- AD
atopic dermatitis
- Cers
ceramides
- HR
hypersensitivity reaction
- SC
stratum corneum
- SP
serine protease
- TEWL
transepidermal water loss
- Th2-like HR
Th2-like hypersensitivity reaction
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Aioi A, Tonogaito H, Suto H, Hamada K, Ra CR, Ogawa H, et al. Impairment of skin barrier function in NC/Nga Tnd mice as a possible model for atopic dermatitis. Br J Dermatol. 2001;144:12–8. doi: 10.1046/j.1365-2133.2001.03946.x. [DOI] [PubMed] [Google Scholar]
- Bleck O, Abeck D, Ring J, Hoppe U, Vietzke JP, Wolber R, et al. Two ceramide subfractions detectable in Cer(AS) position by HPTLC in skin surface lipids of non-lesional skin of atopic eczema. J Invest Dermatol. 1999;113:894–900. doi: 10.1046/j.1523-1747.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M, Schmid-Grendelmeier P, Leung DY. Atopic dermatitis. J Allergy Clin Immunol. 2006;118:40–3. doi: 10.1016/j.jaci.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Brattsand M, Egelrud T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J Biol Chem. 1999;274:30033–40. doi: 10.1074/jbc.274.42.30033. [DOI] [PubMed] [Google Scholar]
- Butmarc J, Yufit T, Carson P, Falanga V. Human beta-defensin-2 expression is increased in chronic wounds. Wound Repair Regen. 2004;12:439–43. doi: 10.1111/j.1067-1927.2004.12405.x. [DOI] [PubMed] [Google Scholar]
- Chamlin SL, Kao J, Frieden IJ, Sheu MY, Fowler AJ, Fluhr JW, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- Chan JC, Duszczyszyn DA, Castellino FJ, Ploplis VA. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Pathol. 2001a;159:1681–8. doi: 10.1016/S0002-9440(10)63015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001b;117:977–83. doi: 10.1046/j.0022-202x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Martinez O, Venkataramani P, Lin SX, Prabhakar BS, Chan LS. Correlation of disease evolution with progressive inflammatory cell activation and migration in the IL-4 transgenic mouse model of atopic dermatitis. Clin Exp Immunol. 2005;139:189–201. doi: 10.1111/j.1365-2249.2004.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EH, Brown BE, Crumrine D, Chang S, Man MQ, Elias PM, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005a;124:587–95. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- Choi EH, Demerjian M, Crumrine D, Brown BE, Mauro T, Elias PM, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1657–62. doi: 10.1152/ajpregu.00010.2006. [DOI] [PubMed] [Google Scholar]
- Choi EH, Man MQ, Wang F, Zhang X, Brown BE, Feingold KR, et al. Is endogenous glycerol a determinant of stratum corneum hydration in humans? J Invest Dermatol. 2005b;125:288–93. doi: 10.1111/j.0022-202X.2005.23799.x. [DOI] [PubMed] [Google Scholar]
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene–environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. quiz 22–23. [DOI] [PubMed] [Google Scholar]
- Demerjian M, Man MQ, Choi EH, Brown BE, Crumrine D, Chang S, et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol. 2006;15:154–60. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Denda M, Tsuchiya T. Barrier recovery rate varies time-dependently in human skin. Br J Dermatol. 2000;142:881–4. doi: 10.1046/j.1365-2133.2000.03466.x. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- Elias PM. The stratum corneum revisited. J Dermatol. 1996a;23:756–8. doi: 10.1111/j.1346-8138.1996.tb02698.x. [DOI] [PubMed] [Google Scholar]
- Elias PM. Stratum corneum architecture, metabolic activity and interactivity with subjacent cell layers. Exp Dermatol. 1996b;5:191–201. doi: 10.1111/j.1600-0625.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Ansel JC, Woods LD, Feingold KR. Signaling networks in barrier homeostasis. The mystery widens. Arch Dermatol. 1996;132:1505–6. [PubMed] [Google Scholar]
- Elias PM, Feingold KR. Skin as an organ of protection. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, Fitzpatrick TB, editors. Fitzpatrick’s Dermatology in General Medicine. Philadelphia: McGraw-Hill; 1999. pp. 164–74. [Google Scholar]
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–26. [PubMed] [Google Scholar]
- Fan WY, Kouda K, Nakamura H, Takeuchi H. Effects of dietary restriction on spontaneous dermatitis in NC/Nga mice. Exp Biol Med. 2001;226:1045–50. doi: 10.1177/153537020122601112. [DOI] [PubMed] [Google Scholar]
- Fartasch M, Bassukas ID, Diepgen TL. Disturbed extruding mechanism of lamellar bodies in dry non-eczematous skin of atopics. Br J Dermatol. 1992;127:221–7. doi: 10.1111/j.1365-2133.1992.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–55. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- Gao XK, Nakamura N, Fuseda K, Tanaka H, Inagaki N, Nagai H. Establishment of allergic dermatitis in NC/Nga mice as a model for severe atopic dermatitis. Biol Pharm Bull. 2004;27:1376–81. doi: 10.1248/bpb.27.1376. [DOI] [PubMed] [Google Scholar]
- Grewe M, Bruijnzeel-Koomen CA, Schopf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–61. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Behne M, Aronchik I, Demerjian M, Feingold KR, Elias PM, et al. Extracellular pH Controls NHE1 expression in epidermis and keratinocytes: implications for barrier repair. J Invest Dermatol. 2005a;125:790–7. doi: 10.1111/j.0022-202X.2005.23836.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–53. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005b;125:510–20. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- Hansson L, Backman A, Ny A, Edlund M, Ekholm E, Ekstrand Hammarstrom B, et al. Epidermal overexpression of stratum corneum chymotryptic enzyme in mice: a model for chronic itchy dermatitis. J Invest Dermatol. 2002;118:444–9. doi: 10.1046/j.0022-202x.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- Hatano Y, Terashi H, Arakawa S, Katagiri K. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005;124:786–92. doi: 10.1111/j.0022-202X.2005.23651.x. [DOI] [PubMed] [Google Scholar]
- Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006;118:178–89. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Hou SY, Mitra AK, White SH, Menon GK, Ghadially R, Elias PM. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and X-ray diffraction. J Invest Dermatol. 1991;96:215–23. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–6. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- Kang JS, Lee K, Han SB, Ahn JM, Lee H, Han MH, et al. Induction of atopic eczema/dermatitis syndrome-like skin lesions by repeated topical application of a crude extract of Dermatophagoides pteronyssinus in NC/Nga mice. Int Immunopharmacol. 2006;6:1616–22. doi: 10.1016/j.intimp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456–64. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- Kiank C, Holtfreter B, Starke A, Mundt A, Wilke C, Schutt C. Stress susceptibility predicts the severity of immune depression and the failure to combat bacterial infections in chronically stressed mice. Brain Behav Immun. 2006;20:359–68. doi: 10.1016/j.bbi.2005.10.151. [DOI] [PubMed] [Google Scholar]
- Kitagaki H, Fujisawa S, Watanabe K, Hayakawa K, Shiohara T. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J Invest Dermatol. 1995;105:749–55. doi: 10.1111/1523-1747.ep12325538. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tsutsui H, Murakami T, Yumikura-Futatsugi S, Yamanaka K, Tanaka M, et al. IL-18 contributes to the spontaneous development of atopic dermatitis-like inflammatory skin lesion independently of IgE/stat6 under specific pathogen-free conditions. Proc Natl Acad Sci USA. 2002;99:11340–5. doi: 10.1073/pnas.152337799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–7. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY, Jain N, Leo HL. New concepts in the pathogenesis of atopic dermatitis. Curr Opin Immunol. 2003;15:634–8. doi: 10.1016/j.coi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166–73. doi: 10.1046/j.1523-1747.2002.01833.x. [DOI] [PubMed] [Google Scholar]
- Mao-Qiang M, Jain M, Feingold KR, Elias PM. Secretory phospholipase A2 activity is required for permeability barrier homeostasis. J Invest Dermatol. 1996;106:57–63. doi: 10.1111/1523-1747.ep12327246. [DOI] [PubMed] [Google Scholar]
- Marenholz I, Nickel R, Ruschendorf F, Schulz F, Esparza-Gordillo J, Kerscher T, et al. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. J Allergy Clin Immunol. 2006;118:866–71. doi: 10.1016/j.jaci.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–6. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- Matsukura S, Aihara M, Hirasawa T, Ikezawa Z. Effects of TNCB sensitization in DS-Nh mice, serving as a model of atopic dermatitis, in comparison with NC/Nga mice. Int Arch Allergy Immunol. 2005;136:173–80. doi: 10.1159/000083326. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizukoshi K, Oyobikawa M, Ohshima H, Tagami H. Establishment of an atopic dermatitis-like skin model in a hairless mouse by repeated elicitation of contact hypersensitivity that enables to conduct functional analyses of the stratum corneum with various non-invasive biophysical instruments. Skin Res Technol. 2004;10:122–9. doi: 10.1111/j.1600-0846.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Maki N, Yoshida S, Arai M, Wang J, Oikawa Y, et al. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy. 2003;58:139–45. doi: 10.1034/j.1398-9995.2003.23790.x. [DOI] [PubMed] [Google Scholar]
- Nakae S, Naruse-Nakajima C, Sudo K, Horai R, Asano M, Iwakura Y. IL-1 alpha, but not IL-1 beta, is required for contact-allergen-specific T cell activation during the sensitization phase in contact hypersensitivity. Int Immunol. 2001;13:1471–8. doi: 10.1093/intimm/13.12.1471. [DOI] [PubMed] [Google Scholar]
- Novak N, Bieber T. The role of dendritic cell subtypes in the pathophysiology of atopic dermatitis. J Am Acad Dermatol. 2005;53:S171–6. doi: 10.1016/j.jaad.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Ong PY, Hamid QA, Travers JB, Strickland I, Al Kerithy M, Boguniewicz M, et al. Decreased IL-15 may contribute to elevated IgE and acute inflammation in atopic dermatitis. J Immunol. 2002a;168:505–10. doi: 10.4049/jimmunol.168.1.505. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002b;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Sager N, Feldmann A, Schilling G, Kreitsch P, Neumann C. House dust mite-specific T cells in the skin of subjects with atopic dermatitis: frequency and lymphokine profile in the allergen patch test. J Allergy Clin Immunol. 1992;89:801–10. doi: 10.1016/0091-6749(92)90434-4. [DOI] [PubMed] [Google Scholar]
- Sandilands A, O’Regan GM, Liao H, Zhao Y, Terron-Kwiatkowski A, Watson RM, et al. Prevalent and rare mutations in the gene encoding filaggrin cause ichthyosis vulgaris and predispose individuals to atopic dermatitis. J Invest Dermatol. 2006;126:1770–5. doi: 10.1038/sj.jid.5700459. [DOI] [PubMed] [Google Scholar]
- Savinko T, Lauerma A, Lehtimaki S, Gombert M, Majuri ML, Fyhrquist-Vanni N, et al. Topical superantigen exposure induces epidermal accumulation of CD8+ T cells, a mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. J Immunol. 2005;175:8320–6. doi: 10.4049/jimmunol.175.12.8320. [DOI] [PubMed] [Google Scholar]
- Segre JA. Epidermal differentiation complex yields a secret: mutations in the cornification protein filaggrin underlie ichthyosis vulgaris. J Invest Dermatol. 2006;126:1202–4. doi: 10.1038/sj.jid.5700367. [DOI] [PubMed] [Google Scholar]
- Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995;75:429–33. doi: 10.2340/0001555575429433. [DOI] [PubMed] [Google Scholar]
- Sheu MY, Fowler AJ, Kao J, Schmuth M, Schoonjans K, Auwerx J, et al. Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models. J Invest Dermatol. 2002;118:94–101. doi: 10.1046/j.0022-202x.2001.01626.x. [DOI] [PubMed] [Google Scholar]
- Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–42. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol. 2003;170:5583–9. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- Tagami H, Kikuchi K. Diseases that affect barrier function. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 447–68. [Google Scholar]
- Uchida Y, Hamanaka S. Stratum corneum ceramides: function, origins, and therapeutic applications. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 43–64. [Google Scholar]
- Vasilopoulos Y, Cork MJ, Murphy R, Williams HC, Robinson DA, Duff GW, et al. Genetic association between an AACC insertion in the 3′UTR of the stratum corneum chymotryptic enzyme gene and atopic dermatitis. J Invest Dermatol. 2004;123:62–6. doi: 10.1111/j.0022-202X.2004.22708.x. [DOI] [PubMed] [Google Scholar]
- Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, Lawrence R, et al. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29:175–8. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–9. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Werfel T, Hentschel M, Kapp A, Renz H. Dichotomy of blood- and skin-derived IL-4-producing allergen-specific T cells and restricted V beta repertoire in nickel-mediated contact dermatitis. J Immunol. 1997;158:2500–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Repeatedly Ox-challenged mice develop a pruritic dermatosis.
Figure S2. Repeatedly Ox challenges cause epidermal hyperplasia and chronic inflammation.
Figure S3. Alterations in epidermal differentiation after multiple Ox challenges.
Figure S4. SP activity increases and extends into nucleated layers after 10 Ox challenges.
Figure S5. IL-4 increase levels in repeatedly Ox-challenged mice.
Table S1. Described pathophysiological features of human AD and AD model mice.